Abstract
Postoperative adhesions remain one of the more challenging issues in surgical practice. Although peritoneal adhesions occur after every abdominal operation, the density, time interval to develop symptoms, and clinical presentation are highly variable with no predictable patterns. Numerous studies have investigated the pathophysiology of postoperative adhesions both in vitro and in vivo. Factors such as type and location of adhesions, as well as timing and recurrence of adhesive obstruction remain unpredictable and poorly understood. Although the majority of postoperative adhesions are clinically silent, the consequences of adhesion formation can represent a lifelong problem including chronic abdominal pain, recurrent intestinal obstruction requiring multiple hospitalizations, and infertility. Moreover, adhesive disease can become a chronic medical condition with significant morbidity and no effective therapy. Despite recent advances in surgical techniques, there is no reliable strategy to manage postoperative adhesions. We herein review the pathophysiology and clinical significance of postoperative adhesions while highlighting current techniques of prevention and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative adhesions remain one of the more challenging issues in surgical practice. Although peritoneal adhesions occur after every abdominal operation, the density, time interval to develop symptoms, and clinical presentation are highly variable with no predictive patterns.1,2 Adhesion formation following an invasive intervention was first recognized more than 1500 years ago, when lung adhesions were described as an injury response to lung puncture.3 In 1889, von Dembrowski published the first data on induction of adhesions in an animal model. Since then, numerous studies have investigated the pathophysiology of postoperative adhesions both in vitro and in vivo.4 However, factors such as type and location of adhesions, as well as timing and recurrence of adhesive obstruction remain unpredictable and poorly understood. Furthermore, only minimally invasive surgical techniques have been shown to reduce adhesion formation and associated morbidity and mortality.5
Although the majority of postoperative adhesions are clinically silent, the consequences of adhesion formation can represent a lifelong problem including chronic abdominal pain, recurrent intestinal obstruction requiring multiple hospitalizations, and infertility.6,7 Moreover, adhesive disease can become a chronic medical condition with significant morbidity and no effective therapy. As an unavoidable consequence of any abdominal operation, discussion of these adverse outcomes should be part of preoperative informative consent.8 Adhesions are a particular bane of pelvic surgery and a major cause of complications following gynecological surgeries.9 In addition, despite recent advances in surgical techniques, there is no reliable strategy to manage postoperative adhesions.10 Herein, we review the pathophysiology and clinical significance of postoperative adhesions while highlighting current techniques on prevention and treatment.
Pathogenesis of Peritoneal Adhesions
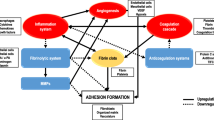
The uncertainty in the biologic processes involved with adhesion formation is a main hindrance to identifying effective treatment strategies. By definition, peritoneal adhesions are pathologic bands connecting adjacent structures. The physical properties of these bands can vary significantly from a thin membrane of connective tissue to a thick fibrous band that may contain blood vessels and nerves, or a tight connection between two organ surfaces.5,11 Typically, tissue injury initiates an inflammatory response, and the subsequent healing process stimulates fibrous tissue formation. However, frequently the inflammatory process involves organs that may not have been directly injured.11 Several investigators have tried to explain why the normal healing process creates adhesions in some patients, but not in others. One theory postulates that adhesion formation is a result of the disequilibrium between fibrin deposition and degradation (Fig. 1). Fibrin formation is the end product of the coagulation cascade in the peritoneal cavity, when thrombin triggers conversion of fibrinogen into fibrin.12 The systemic coagulopathy results in the fibrin deposits, which are a matrix for development of fibrocollagenous tissue and formation of an extracellular matrix (ECM).12 The ECM may stay in place even after degradation of the fibrin deposits.
The activation of the fibrinolytic system during first 5–7 days following peritoneal injury is critical to prevent adhesion formation. The conversion of plasminogen into plasmin is the main trigger of fibrin degradation into fibrin-split products. The tissue-type (tPA) and urokinase-type (uPA) plasminogen activators are the most well-known stimulators of plasminogen conversion into plasmin, which are produced and stored in endothelial cells, mesothelial cells, and macrophages.13 tPA, a serine protease, is the most potent plasminogen activator with a high affinity for fibrin. The presence of fibrin markedly enhances the activation rate of plasminogen.13,14 In turn, there is typically higher plasminogen activation at sites at risk of adhesions. In the peritoneal cavity, tPA is responsible for up to 95% of the plasminogen activity.15 uPA also is equally effective in the degradation of the fibrin matrix.16 However, due to lower fibrin affinity, uPA stimulates lower levels of plasminogen activation compared with tPA. uPA also appears to have an alternative role in fibrinolysis, while it plays a more important role in tissue remodeling.17
Plasminogen activation is inhibited by plasminogen activator inhibitors (PAI)-1 and PAI-2, through formation of inactive complexes. The glycoprotein PAI-1 is the most potent inhibitor of both tPA and uPA, while PAI-2 seems to be less effective. Nevertheless, PAI-2 still plays a role in peritoneal tissue repair.18 Both PAI-1 and PAI-2 are produced by various cell types, including endothelial and mesothelial cells, monocytes, macrophages, and fibroblasts. PAI-3 and protease nexin 1 are two other plasminogen activator inhibitors, yet their biologic role requires further investigation. Furthermore, plasmin might be inhibited directly by several protease inhibitors such as α2-macroglobulin, α1-antitrypsin, and α2-antiplasmin. The role of direct plasmin inhibitors in peritoneal fibrinolysis are, however, also not well defined.5 Of note, the levels of PAI-1 and tPA/PAI complex are increased in peritoneal samples from patients with higher propensity to form severe adhesions, and hence might be used as biomarkers to identify high-risk patients.12
The balance between plasminogen activators and inhibitors appears to be the major determinant of peritoneal tissue repair process (normal healing vs. adhesion formation). When fibrinolysis fails to occur, the initially formed temporary fibrin matrix persists and can progressively become organized with collagen-secreting fibroblasts. Additionally, the secretion of angiogenic factors by fibroblasts creates new blood vessels.19 Peritoneal adhesions are formed after these newly vascularized tissues are covered with a layer of peritoneum.20,21
Epidemiology
Adhesions occur in nearly all patients undergoing intra-abdominal operations.22–24 Postoperative adhesions are the primary cause for small bowel obstruction (SBO) and comprise 70% of admissions for bowel obstruction.1,5,25,26 Approximately 3% of all laparotomies and 1% of all surgical admissions are related to adhesions.26 The majority of epidemiologic data regarding adhesive bowel obstruction is derived from data of national registries and retrospective cohorts of elective abdominal surgery. Because of the nature of these studies, it is difficult to determine specific operative factors that may influence the development of bowel obstruction. The epidemiological data of the Surgical and Clinical Adhesions Research (SCAR) group reported that adhesions were more common following procedures that involve small intestine, colon, appendix, or the uterus.19,27–29 The procedures with highest risk of adhesion-related hospital readmission were total proctocolectomy (15.4%), total colectomy (8.8%), and ileostomy (10.6%).29 In contrast, procedures in the upper abdomen involving the stomach, gallbladder, or pancreas were associated with lower rates of adhesion formation.30 Similarly, age was inversely correlated to adhesion-related readmissions with individuals younger than 60 years to be at highest risk. Inflammation due to peritonitis, Crohn’s disease, and colon procedures performed for colon cancer also have been demonstrated to increase adhesion-related complications.29
The LAPAD (LAParotomy or LAParoscopy and ADhesions) trial was the largest study designed to investigate risk factors associated with adhesive SBO. Patients who underwent elective open or laparoscopic abdominal surgery for either benign or malignant conditions were included in the study. Intra-operative factors such as incision type, the presence and severity of adhesions, and adhesiolysis time were among numerous variables that were assessed. The results demonstrated that procedures of the lower gastrointestinal tract (odds ratio 4.57, P < 0.01), as well as severity of adhesions in the operative area (odds ratio 2.37, P = 0.04) were independent risk factors for adhesive SBO.31 The presence of midline incision at the index procedure was correlated with iatrogenic bowel injury, whereas the number of previous laparotomies was irrelevant. Using these data, Broek et al. developed a nomogram to predict iatrogenic bowel injury during adhesiolysis.32 Emergency surgery has also been identified as a risk factor for adhesion formation. Sisodia et al. reported that patients who have had an emergent operation demonstrated higher incidence of adhesion-related complications compared with patients who underwent elective surgery.33
Postoperative adhesions may present within a wide clinical spectrum of disease, from a single band causing a closed loop obstruction to asymptomatic extensive dense adhesions. Therefore, the size of the adhesion bands is not a good predictor of their sequela. Identification of factors associated with adhesion formation and, more importantly, adhesion-related complications are pivotal to distinguish high-risk patients (Table 1). Considering highly variable and sometimes contradictory results of the current data, future studies with rigorous methodology are required.
Economic Burden of Peritoneal Adhesions
Several studies have evaluated the economic burden of postoperative adhesions on the healthcare system. The major limitation of most studies has been the lack of standard definitions for adhesion-related complications. Moreover, there is no consistent recording of many complications in health records. This is partly attributed to the fact that a different surgeon than the index procedure often treats patients suffering adhesion-related complications. As a result, usually the primary surgeon is unaware of the complication.2
Ellis et al. estimated that 33% of patients, who underwent abdominal or pelvic surgery, were readmitted for adhesion-related complications with an average of 2 admissions during a 10-year follow-up.19 In another study over a 24-year period, intestinal obstruction was responsible for 0.9% of all admissions, 3.3% of major laparotomies, and 28.8% of large or small bowel obstructions.27 In 1988 in the USA, admissions for adhesiolysis accounted for nearly 950,000 days of inpatient care.25,34 Updated data in 1994 indicated that more than 300,000 admissions took place for complications of peritoneal adhesions.35 Furthermore, lysis of adhesions was associated with a prolonged operative time, 6% incidence of iatrogenic bowel injury, and increased risk of postoperative complications.5,26,30 Likewise, the length of hospital stay following surgery for peritoneal adhesions was increased, mainly due to a prolonged recovery period.36,37 In the USA, the total cost of hospital and surgical expenditures for the management of adhesion-related complications has increased from $1.3 billion in 1994 to $5 billion in 2001 (Table 2).29 Efforts to decrease the incidence of postoperative adhesive disease would likely have a dramatic economic influence. In fact, it has been estimated that anti-adhesion agents, with an average cost of $200 and potential effectiveness of less than 25%, might reduce the healthcare expenditures by approximately $55 million over 10 years.38
Prevention of Peritoneal Adhesions
The best approach to limit the morbidity and decrease the economic burden of adhesion-related complications is prevention of the formation of postoperative adhesions. Current preventative measures can be categorized into two main strategies: alterations in surgical technique to minimize tissue injury and physical barriers (Table 3).42,43 These strategies are summarized in Fig. 2.
Technical Measures
Appropriate surgical technique is an important factor in helping to avoid adhesion formation. Gentle handling of tissues and delicate dissection technique are crucial for limiting tissue injury, inflammation, and preventing serosal damage.39 Furthermore, minimizing exposure and desiccation of bowel surface as well as removal of debris can reduce the risk of adhesion formation.39,44 It is important to avoid unnecessary introduction of foreign bodies such as talc, lint, or fibrinogenic suture materials. For instance, suture materials such as silk or chromic catgut stimulate more tissue reactivity than polyglactin.45,46 Another experimental study demonstrated that absorbable sutures such as polyglactin and mixes of lactic and glycolic acids were associated with a lower incidence of postoperative adhesion formation compared with non-absorbable fixation methods such as polypropylene sutures and titanium tackers.47 Similarly, polyglactin sutures were associated with a lower incidence of peritoneal adhesions in both sterile and contaminated settings compared with other absorbable sutures such as Polydioxanone and Poliglecaprone 25, a benefit that was less profound in the contaminated setting, perhaps suggesting the crucial role of inflammation in adhesion formation.48 Finally, several factors related to the surgical environment such as air-handlers, powder-free gloves, and “lint-free” surgical supplies have also been reported to reduce peritoneal adhesion formation.39
The closure of the parietal peritoneum during closure of the abdominal wall was previously thought to prevent adhesion formation. However, a recent systematic review demonstrated conflicting evidence regarding the long-term benefits of peritoneal closure on adhesion reduction among patients undergoing cesarean section.49 In a prospective cohort of 173 patients who underwent repeat cesarean delivery, Lyell et al. reported that peritoneal closure was associated with increased risk of postoperative adhesions. The closure of the rectus muscles resulted in fewer combined filmy and dense adhesions overall (27.5 vs. 46%; P = 0.04).41
Abdominal wall reconstruction with synthetic or bioprosthetic mesh also carries a significant risk of adhesion formation. While bioprosthetic mesh appears to elicit fewer and lower-grade adhesions compared with synthetic mesh, further studies are required.50 Prostheses coated with permanent anti-adhesive barriers (Bard™ Composix™ E/X, Bard™ Composix™ L/P, and DUALMESH (®) Biomaterial) have been correlated with improved adhesion characteristics compared with uncoated meshes, likely attributed to the inflammatory reaction that is caused by the chemical composition of the barrier or the conditions required for resorption and metabolism of the barrier components.51 Moreover, on repeated laparotomies, the adhesions were easier to separate, translating into less operative time and effort.52
In a meta-analysis of 17 studies, Ten Broek et al. demonstrated that no specific open surgical technique significantly reduced the incidence of adhesion-related complications.39 Leaving the peritoneum open with the abdominal closure (relative risk (RR) 0.36; 95% CI 0.21–0.63) and laparoscopy (RR 0.14; 95% CI 0.03–0.61) were associated with reduced incidence of adhesions.39 Several studies have validated the benefit of laparoscopy in reduction of postoperative adhesions.40,53,54 Furthermore, laparoscopic adhesiolysis may result in a reduction, but not elimination, of the frequency, extent, severity, and type of abdominal adhesion reformation between organs and the abdominal wall. Of note, the incidence of visceral adhesions is not minimized by laparoscopy.55,56 In addition, some investigators have failed to note an advantage of laparoscopy over open surgery in the prevention of adhesive SBO (Table 4).6,57–60 For example, in one retrospective study of 700 patients who underwent laparoscopic or open colorectal surgery, SBO occurred in 11.2% of patients in the laparoscopic group versus 9.8% in the open surgery arm.53 Although the difference between the two groups did not reach statistical significance, conversion to an open procedure and stoma formation were independent risk factors for SBO development.
Minimally invasive (MIS) radical prostatectomy is another procedure that has been studied with regard to its relation to adhesion formation. MIS prostatectomy is performed within the peritoneal cavity and hence may have an increased incidence of SBO compared with open radical extra-peritoneal prostatectomy. In a retrospective study performed by Loeb et al., 14,147 patients with prostate cancer were treated with either open extra-peritoneal (n = 10,954) or MIS (n = 3193) radical prostatectomy.61 With a median follow-up of 45 and 76 months, respectively, the overall incidence of SBO was 3.7% for minimally invasive and 5.3% for open radical prostatectomy (p = 0.0005). Adhesiolysis occurred in 1.1 and 2.0% of MIS and open prostatectomy patients (p = 0.0003).61 However, multivariable analysis did not show any significant difference in the incidence of SBO or adhesiolysis between MIS and open prostatectomy.61
In women undergoing gynecological surgery, 60–90% develop adhesions and adhesions can be the etiology of 15–20% of secondary infertility.42 Comparing the incidence of adhesions between gynecological laparotomy and laparoscopic surgery, patients incur similar risk of adhesion-related readmissions.43 In contrast, an early randomized controlled trial including 105 patients with tubal pregnancy reported that patients who had a laparotomy formed significantly more adhesions than patients undergoing laparoscopy (p < 0.001); however, tubal patency did not differ between groups.62 Among patients with adhesions and infertility, adhesiolysis can be an important infertility treatment leading to improved ability to become pregnant and decreased pregnancy loss.63
Physical Barriers
Despite recent advances in the development of adhesion barriers, major drawbacks have limited the effectiveness of these products. Solid or liquid physical barriers are applied to cover the injured peritoneal tissues and keep the tissues separated until completion of the reepithelialization process.64,65 However, these barriers are unable to cover all visceral and peritoneal surfaces, limiting their efficacy. Although studies have shown that physical barriers minimize the formation of postoperative adhesions, there is no significant improvement in clinical outcomes of patients due to adhesion-related complications (e.g., reoperation rates, chronic abdominal pain, or infertility).66 Increased risk of intraabdominal abscesses and anastomotic leak, particularly if the barriers are used near anastomosis, prolonged operative time, and higher cost are some of the disadvantages of physical barriers.66–68
A range of physical barriers have been approved for clinical application in the USA (Table 3).5 Gore Preclude is a polytetrafluoroethylene membrane that prevents the development of pelvic adhesions, especially after second look surgeries. However, a strip of Gore-Tex remains within the pelvis permanently.69 Gynecare Interceed is an oxidized regenerated cellulose that precludes formation of de novo and recurrent adhesions. The major drawback of this barrier is that it becomes ineffective in the presence of blood.67 Both Gore Preclude and Gynecare Interceed barriers have been reported to reduce the incidence and extent of adhesions compared with good surgical technique alone.67,70 Oxidized regenerated cellulose is not inferior to polytetrafluoroethylene membrane in terms of safety and efficacy.67,69
Seprafilm is a chemically modified sodium hyaluronate/carboxymethylcellulose absorbable barrier. Seprafilm reduced the incidence of adhesions and adhesive SBO requiring reoperation, but it did not change the overall SBO incidence.71,72 A prospective, randomized controlled study of patients who underwent abdominopelvic surgery due to inflammatory bowel disease demonstrated that Seprafilm was safe and efficient in decreasing adhesion formation.73 Application of Seprafilm on the suture or staple line of a fresh intestinal anastomosis should be avoided, as it may increase the risk of anastomotic leak and fistula formation.72,73 In a long-term follow-up of 35 patients who underwent Hartman’s procedure, van der Wal et al. reported that Seprafilm placement did not prevent SBO. The incidence of chronic abdominal complaints were, however, lower in the Seprafilm group compared with controls (35.3 vs. 77.8%, respectively; P = 0.018).73–75 In contrast, a multicenter randomized controlled trial reported that Seprafilm did not prevent adhesion formation following cesarean delivery.76 Finally, a multicenter study of patients undergoing colectomy and ileal pouch-anal anastomosis with diverting-loop ileostomy demonstrated that Seprafilm peritoneal placement before closure was related with macroscopically less adhesion formation.77
At the time of laparoscopic surgery, the use of barriers alone has led to a 50–60% macroscopic improvement in adhesion formation. The combination of broad peritoneal cavity protection, by insufflating a low-temperature and humidified gas mixture of CO2, N2O, and O2, with local application of a barrier has been demonstrated to be almost 100% effective in preventing postoperative adhesion.60
Application of liquid barriers such as polyethylene glycol (Spraygel), which is approved only in Europe, have also been proven to be effective in reducing adhesion formation.78
Intestinal Inflammation
As noted, activation of the inflammatory cascade plays an important role in the pathogenesis of postoperative adhesion formation. In turn, intestinal microflora may play a role in adhesion formation. Specifically, bacterial intestinal colonization has been associated with a fourfold decrease in peritoneal and peri-anastomotic adhesion formation risk.79,80 A possible underlying mechanism of the inflammatory contribution of bacterial translocation is the activation of the cell-mediated immune response. More specifically, bacterial translocation activates the expression of macrophages and interleukin-6 (pro-inflammatory cytokine),81 that in turn can lead to exacerbation of peritoneal adhesion formation.82
Intestinal bacterial burden reduction, either medically (antibiotics) or mechanically (bowel preparation), may have an impact on the incidence of postoperative adhesions. More specifically, intravenous administered of broad-spectrum antibiotics to decrease the bacterial burden may also reduce the risk and severity83 of postoperative peritoneal adhesions, as has been demonstrated in experimental animal models.84 Similarly, bowel preparation may induce long-acting changes in intestinal microbiota homeostasis, a potential mechanism to reduce bacterial burden and translocation that in turn could exacerbate peritoneal adhesion formation.85,86
Ongoing Research
Despite promising results in animal studies, new systemic agents have not shown similar efficiency in humans.42 Nonsteroidal anti-inflammatory drugs, corticosteroids, calcium channel blockers, antihistamines, antibiotics, fibrinolytic agents (streptokinase and urokinase), colchicine, and vitamins have had disappointing results in human trials.87–90 Pioglitazone, a PPAR-γ agonist, has been demonstrated to reduce postoperative adhesion formation without compromising anastomotic healing in a mouse model. However, the safety and efficacy of PPAR-γ agonists in humans has yet to be determined.91 The local administration of simvastatin in a rat model also was reported to reduce the incidence and severity of peritoneal and small bowel adhesions.92
Currently, products that utilize the barrier method for separating traumatized tissues represent the only successful strategy in postoperative adhesion prevention. Brochhausen et al. introduced scanning electron microscopy (SEM) and subsequent morphometry as a useful tool to analyze the performance of barrier materials. Striking morphological differences in the peritoneal lesion surface organization was observed between the different barriers, depending on the degree of barrier colonization by mesothelial cells.93 Chaturvedi et al. reported that an ultrapure alginate-based antiadhesive barrier gel decreased the incidence of postoperative adhesion formation in a rat model with cecal abrasion and peritoneal sidewall excision.94 Recently, Fredriksson et al. demonstrated that using carbazate-activated polyvinyl alcohol (PVAC)-impregnated sutures in a rat model was associated with reduced intraperitoneal adhesions compared to controls (P = 0.04). However, intra-peritoneal instillation of PVAC had no effect.95
Conclusion
Adhesions are an inevitable consequence of intra-abdominal surgeries. Complications associated with postoperative adhesions continue to challenge the health care system, without any single, implementable solution. Meticulous and minimally invasive surgical techniques as well as physical barriers are currently the only widely accepted methods of postoperative adhesion prevention. Although the understanding of the pathogenesis of peritoneal adhesions has undoubtedly grown over the past decade, much more research needs to be done. In particular, innovation of novel adhesion-reducing adjuvants, further high-quality studies with better methodology and larger number of patients are required.
References
Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Digestive surgery. 2001;18(4):260–73. doi:50149.
Schreinemacher MH, ten Broek RP, Bakkum EA, van Goor H, Bouvy ND. Adhesion awareness: a national survey of surgeons. World journal of surgery. 2010;34(12):2805–12. doi:10.1007/s00268-010-0778-8.
Wiseman DM. Disorders of adhesions or adhesion-related disorder: monolithic entities or part of something bigger--CAPPS? Seminars in reproductive medicine. 2008;26(4):356–68. doi:10.1055/s-0028-1082394.
Bruggmann D, Tchartchian G, Wallwiener M, Munstedt K, Tinneberg HR, Hackethal A. Intra-abdominal adhesions: definition, origin, significance in surgical practice, and treatment options. Deutsches Arzteblatt international. 2010;107(44):769–75. doi:10.3238/arztebl.2010.0769.
Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World journal of gastroenterology. 2011;17(41):4545–53. doi:10.3748/wjg.v17.i41.4545.
Taylor GW, Jayne DG, Brown SR, Thorpe H, Brown JM, Dewberry SC et al. Adhesions and incisional hernias following laparoscopic versus open surgery for colorectal cancer in the CLASICC trial. The British journal of surgery. 2010;97(1):70–8. doi:10.1002/bjs.6742.
van Goor H. Consequences and complications of peritoneal adhesions. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2007;9 Suppl 2:25–34. doi:10.1111/j.1463-1318.2007.01358.x.
Rajab TK, Kimonis KO, Ali E, Offodile AC, Brady M, Bleday R. Practical implications of postoperative adhesions for preoperative consent and operative technique. Int J Surg. 2013;11(9):753–6. doi:10.1016/j.ijsu.2013.07.019.
Herrmann A, De Wilde RL. Adhesions are the major cause of complications in operative gynecology. Best Pract Res Clin Obstet Gynaecol. 2016;35:71–83. doi:10.1016/j.bpobgyn.2015.10.010.
Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Human reproduction update. 2001;7(6):556–66.
Duron JJ. Postoperative intraperitoneal adhesion pathophysiology. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2007;9 Suppl 2:14–24. doi:10.1111/j.1463-1318.2007.01343.x.
Ivarsson ML, Bergstrom M, Eriksson E, Risberg B, Holmdahl L. Tissue markers as predictors of postoperative adhesions. The British journal of surgery. 1998;85(11):1549–54.
Ichinose A, Takio K, Fujikawa K. Localization of the binding site of tissue-type plasminogen activator to fibrin. The Journal of clinical investigation. 1986;78(1):163–9. doi:10.1172/JCI112546.
Norrman B, Wallen P, Ranby M. Fibrinolysis mediated by tissue plasminogen activator. Disclosure of a kinetic transition. European journal of biochemistry. 1985;149(1):193–200.
Holmdahl L, Eriksson E, Al-Jabreen M, Risberg B. Fibrinolysis in human peritoneum during operation. Surgery. 1996;119(6):701–5.
Lu HR, Wu Z, Pauwels P, Lijnen HR, Collen D. Comparative thrombolytic properties of tissue-type plasminogen activator (t-PA), single-chain urokinase-type plasminogen activator (u-PA) and K1K2Pu (a t-PA/u-PA chimera) in a combined arterial and venous thrombosis model in the dog. Journal of the American College of Cardiology. 1992;19(6):1350–9.
Vassalli JD, Pepper MS. Tumour biology. Membrane proteases in focus. Nature. 1994;370(6484):14–5. doi:10.1038/370014a0.
Andreasen PA, Georg B, Lund LR, Riccio A, Stacey SN. Plasminogen activator inhibitors: hormonally regulated serpins. Molecular and cellular endocrinology. 1990;68(1):1–19.
Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353(9163):1476–80. doi:10.1016/S0140-6736(98)09337-4.
Rout UK, Diamond MP. Role of plasminogen activators during healing after uterine serosal lesioning in the rat. Fertility and sterility. 2003;79(1):138–45.
Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. The European journal of surgery = Acta chirurgica. 1999;165(11):1012–9. doi:10.1080/110241599750007810.
ten Broek RP, Schreinemacher MH, Jilesen AP, Bouvy N, Bleichrodt RP, van Goor H. Enterotomy risk in abdominal wall repair: a prospective study. Ann Surg. 2012;256(2):280–7.
ten Broek RP, Strik C, Issa Y, Bleichrodt RP, van Goor H. Adhesiolysis-related morbidity in abdominal surgery. Annals of surgery. 2013;258(1):98–106. doi:10.1097/SLA.0b013e31826f4969.
Van Der Krabben AA, Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, Schaapveld M, Van Goor H. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87(4):467–71.
Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. The European journal of surgery Supplement : = Acta chirurgica Supplement. 1997 (577):5–9.
Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;577:5–9.
Lower AM, Hawthorn RJ, Clark D, Boyd JH, Finlayson AR, Knight AD et al. Adhesion-related readmissions following gynaecological laparoscopy or laparotomy in Scotland: an epidemiological study of 24 046 patients. Human reproduction. 2004;19(8):1877–85. doi:10.1093/humrep/deh321.
Parker MC, Ellis H, Moran BJ, Thompson JN, Wilson MS, Menzies D et al. Postoperative adhesions: ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Diseases of the colon and rectum. 2001;44(6):822–29; discussion 9-30.
Parker MC, Wilson MS, Menzies D, Sunderland G, Clark DN, Knight AD et al. The SCAR-3 study: 5-year adhesion-related readmission risk following lower abdominal surgical procedures. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2005;7(6):551–8. doi:10.1111/j.1463-1318.2005.00857.x.
ten Broek RP, Issa Y, van Santbrink EJ, Bouvy ND, Kruitwagen RF, Jeekel J et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. Bmj. 2013;347:f5588. doi:10.1136/bmj.f5588.
Strik C, Stommel MW, Schipper LJ, van Goor H, Ten Broek RP. Long-term impact of adhesions on bowel obstruction. Surgery. 2016;159(5):1351–9. doi:10.1016/j.surg.2015.11.016.
ten Broek RP, Strik C, van Goor H. Preoperative nomogram to predict risk of bowel injury during adhesiolysis. The British journal of surgery. 2014;101(6):720–7. doi:10.1002/bjs.9479.
Sisodia V, Sahu SK, Kumar S. Clinical Profile of Patients with Postoperative Adhesive Intestinal Obstruction and its Association with Intraoperative Peritoneal Adhesion Index. Chirurgia. 2016;111(3):251–8.
Ray NF, Larsen JW, Jr., Stillman RJ, Jacobs RJ. Economic impact of hospitalizations for lower abdominal adhesiolysis in the United States in 1988. Surgery, gynecology & obstetrics. 1993;176(3):271–6.
Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. Journal of the American College of Surgeons. 1998;186(1):1–9.
Coleman MG, McLain AD, Moran BJ. Impact of previous surgery on time taken for incision and division of adhesions during laparotomy. Diseases of the colon and rectum. 2000;43(9):1297–9.
Beck DE, Ferguson MA, Opelka FG, Fleshman JW, Gervaz P, Wexner SD. Effect of previous surgery on abdominal opening time. Diseases of the colon and rectum. 2000;43(12):1749–53.
Wilson MS. Practicalities and costs of adhesions. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2007;9 Suppl 2:60–5. doi:10.1111/j.1463-1318.2007.01360.x.
Ten Broek RP, Kok-Krant N, Bakkum EA, Bleichrodt RP, van Goor H. Different surgical techniques to reduce post-operative adhesion formation: a systematic review and meta-analysis. Human reproduction update. 2013;19(1):12–25. doi:10.1093/humupd/dms032.
Burns EM, Currie A, Bottle A, Aylin P, Darzi A, Faiz O. Minimal-access colorectal surgery is associated with fewer adhesion-related admissions than open surgery. The British journal of surgery. 2013;100(1):152–9. doi:10.1002/bjs.8964.
Lyell DJ, Caughey AB, Hu E, Blumenfeld Y, El-Sayed YY, Daniels K. Rectus muscle and visceral peritoneum closure at cesarean delivery and intraabdominal adhesions. American journal of obstetrics and gynecology. 2012;206(6):515 e1–5. doi:10.1016/j.ajog.2012.02.033.
Liakakos T, Thomakos N, Fine PM, Dervenis C and Young RL ( 2001 ) Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg 18, 260–273.
Lower AM, Hawthorn RJ, Clark D, Boyd JH, Finlayson AR, Knight AD, Crowe AM; Surgical and Clinical Research (SCAR) Group. Adhesion-related readmissions following gynaecological laparoscopy or laparotomy in Scotland: an epidemiological study of 24 046 patients. Hum Reprod. 2004;19(8):1877–85.
Corona R, Verguts J, Koninckx R, Mailova K, Binda MM, Koninckx PR. Intraperitoneal temperature and desiccation during endoscopic surgery. Intraoperative humidification and cooling of the peritoneal cavity can reduce adhesions. American journal of obstetrics and gynecology. 2011;205(4):392 e1–7. doi:10.1016/j.ajog.2011.06.091.
O'Leary DP, Coakley JB. The influence of suturing and sepsis on the development of postoperative peritoneal adhesions. Annals of the Royal College of Surgeons of England. 1992;74(2):134–7.
Elkins TE, Stovall TG, Warren J, Ling FW, Meyer NL. A histologic evaluation of peritoneal injury and repair: implications for adhesion formation. Obstetrics and gynecology. 1987;70(2):225–8.
Schreinemacher MH, van Barneveld KW, Peeters E, Miserez M, Gijbels MJ, Greve JW et al. Adhesions to sutures, tackers, and glue for intraperitoneal mesh fixation: an experimental study. Hernia : the journal of hernias and abdominal wall surgery. 2014;18(6):865–72. doi:10.1007/s10029-013-1192-6.
Ishikawa K, Sadahiro S, Tanaka Y, Suzuki T, Kamijo A, Tazume S. Optimal sutures for use in the abdomen: an evaluation based on the formation of adhesions and abscesses. Surgery today. 2013;43(4):412–7. doi:10.1007/s00595-012-0249-y.
Walfisch A, Beloosesky R, Shrim A, Hallak M. Adhesion prevention after cesarean delivery: evidence, and lack of it. American journal of obstetrics and gynecology. 2014;211(5):446–52. doi:10.1016/j.ajog.2014.05.027.
Blumenthal-Barby JS, Kostick KM, Delgado ED, Volk RJ, Kaplan HM, Wilhelms LA et al. Assessment of patients’ and caregivers’ informational and decisional needs for left ventricular assist device placement: Implications for informed consent and shared decision-making. Journal of Heart and Lung Transplantation. 2015;34(9):1182–9. doi:10.1016/j.healun.2015.03.026.
Deeken CR, Faucher KM, Matthews BD. A review of the composition, characteristics, and effectiveness of barrier mesh prostheses utilized for laparoscopic ventral hernia repair. Surgical endoscopy. 2012;26(2):566–75. doi:10.1007/s00464-011-1899-3.
Gonzalez R, Rodeheaver GT, Moody DL, Foresman PA, Ramshaw BJ. Resistance to adhesion formation: a comparative study of treated and untreated mesh products placed in the abdominal cavity. Hernia : the journal of hernias and abdominal wall surgery. 2004;8(3):213–9. doi:10.1007/s10029-004-0213-x.
Smolarek S, Shalaby M, Paolo Angelucci G, Missori G, Capuano I, Franceschilli L et al. Small-Bowel Obstruction Secondary to Adhesions After Open or Laparoscopic Colorectal Surgery. JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2016;20(4):e2016.00073. doi:10.4293/JSLS.2016.00073.
Takase-Sanchez MM, Brooks HM, Hale DS, Heit MH. Obliterative Surgery for the Treatment of Pelvic Organ Prolapse: A Patient Survey on Reasons for Surgery Selection and Postoperative Decision Regret and Satisfaction. Female Pelvic Medicine and Reconstructive Surgery. 2015;21(6):325–31. doi:10.1097/spv.0000000000000198.
Sidana A, Hernandez DJ, Feng Z, Partin AW, Trock BJ, Saha S et al. Treatment decision-making for localized prostate cancer: what younger men choose and why. Prostate. 2012;72(1):58–64. doi:10.1002/pros.21406.
Kavic SM, Kavic SM. Adhesions and adhesiolysis: the role of laparoscopy. JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2002;6(2):99–109.
Lower AM, Hawthorn RJ, Clark D, Boyd JH, Finlayson AR, Knight AD et al. Adhesion-related readmissions following gynaecological laparoscopy or laparotomy in Scotland: an epidemiological study of 24 046 patients. Human reproduction (Oxford, England). 2004;19(8):1877–85. doi:10.1093/humrep/deh321.
Scholin J, Buunen M, Hop W, Bonjer J, Anderberg B, Cuesta M et al. Bowel obstruction after laparoscopic and open colon resection for cancer: results of 5 years of follow-up in a randomized trial. Surgical endoscopy. 2011;25(12):3755–60. doi:10.1007/s00464-011-1782-2.
Pados G, Venetis CA, Almaloglou K, Tarlatzis BC. Prevention of intra-peritoneal adhesions in gynaecological surgery: theory and evidence. Reproductive biomedicine online. 2010;21(3):290–303. doi:10.1016/j.rbmo.2010.04.021.
Mais V. Peritoneal adhesions after laparoscopic gastrointestinal surgery. World Journal of Gastroenterology : WJG. 2014;20(17):4917–25. doi:10.3748/wjg.v20.i17.4917.
Loeb S, Meyer CP, Krasnova A, Curnyn C, Reznor G, Kibel AS et al. Risk of Small Bowel Obstruction After Robot-Assisted vs Open Radical Prostatectomy. Journal of endourology. 2016;30(12):1291–5. doi:10.1089/end.2016.0206.
Lundorff P, Hahlin M, Källfelt B, Thorburn J, Lindblom B. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55(5):911–5.
Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7(6):567–76.
Risberg B. Adhesions: preventive strategies. The European journal of surgery Supplement : = Acta chirurgica Supplement. 1997(577):32–9.
diZerega GS. Use of adhesion prevention barriers in pelvic reconstructive and gynecologic surgery. In: diZerega, editor. Peritoneal Surgery. New York: Springer; 2000. p. 379–99.
ten Broek RP, Stommel MW, Strik C, van Laarhoven CJ, Keus F, van Goor H. Benefits and harms of adhesion barriers for abdominal surgery: a systematic review and meta-analysis. Lancet (London, England). 2014;383(9911):48–59. doi:10.1016/s0140-6736(13)61687-6.
Ahmad G, O'Flynn H, Hindocha A, Watson A. Barrier agents for adhesion prevention after gynaecological surgery. The Cochrane database of systematic reviews. 2015(4):CD000475. doi:10.1002/14651858.CD000475.pub3.
Zeng Q, Yu Z, You J, Zhang Q. Efficacy and safety of Seprafilm for preventing postoperative abdominal adhesion: systematic review and meta-analysis. World journal of surgery. 2007;31(11):2125–31; discussion 32. doi:10.1007/s00268-007-9242-9.
Al-Jabri S, Tulandi T. Management and prevention of pelvic adhesions. Seminars in reproductive medicine. 2011;29(2):130–7. doi:10.1055/s-0031-1272475.
Wiseman DM, Trout JR, Franklin RR, Diamond MP. Metaanalysis of the safety and efficacy of an adhesion barrier (Interceed TC7) in laparotomy. The Journal of reproductive medicine. 1999;44(4):325–31.
Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Diseases of the colon and rectum. 2006;49(1):1–11. doi:10.1007/s10350-005-0268-5.
Robb WB, Mariette C. Strategies in the prevention of the formation of postoperative adhesions in digestive surgery: a systematic review of the literature. Dis Colon Rectum. 2014;57(10):1228–40. doi:10.1097/DCR.0000000000000191.
Beck DE, Cohen Z, Fleshman JW, Kaufman HS, van Goor H, Wolff BG et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Diseases of the colon and rectum. 2003;46(10):1310–9. doi:10.1097/01.DCR.0000089117.62296.E4.
van der Wal JB, Iordens GI, Vrijland WW, van Veen RN, Lange J, Jeekel J. Adhesion prevention during laparotomy: long-term follow-up of a randomized clinical trial. Annals of surgery. 2011;253(6):1118–21. doi:10.1097/SLA.0b013e318217e99c.
Chen TA, Yeh CY. Long-term Follow-up of Adhesion Prevention With Use of Hyaluronic Acid-carboxymethylcellulose Membrane (Seprafilm). Annals of surgery. 2017;265(4):e27. doi:10.1097/sla.0000000000000421.
Kiefer DG, Muscat JC, Santorelli J, Chavez MR, Ananth CV, Smulian JC et al. Effectiveness and short-term safety of modified sodium hyaluronic acid-carboxymethylcellulose at cesarean delivery: a randomized trial. American journal of obstetrics and gynecology. 2016;214(3):373 e1- e12. doi:10.1016/j.ajog.2015.10.012.
Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. Journal of the American College of Surgeons. 1996;183(4):297–306.
Dasiran F, Eryilmaz R, Isik A, Okan I, Somay A, Sahin M. The effect of polyethylene glycol adhesion barrier (Spray Gel) on preventing peritoneal adhesions. Bratislavske lekarske listy. 2015;116(6):379–82.
Bothin C, Midtvedt T. The role of the gastrointestinal microflora in postsurgical adhesion formation—a study in germfree rats. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 1992;24(5):309–12.
Bothin C, Okada M, Midtvedt T, Perbeck L. The intestinal flora influences adhesion formation around surgical anastomoses. The British journal of surgery. 2001;88(1):143–5. doi:10.1046/j.1365-2168.2001.01613.x.
Moris D, Felekouras E, Chrousos GP. No Cytokine Is an Island: IL-6 Alone Is not Sufficient to Predict Morbidity after a Major Abdominal Surgery. Annals of surgery. 2016. doi:10.1097/SLA.0000000000001977.
Cahill RA, Wang JH, Redmond HP. Enteric bacteria and their antigens may stimulate postoperative peritoneal adhesion formation. Surgery. 2007;141(3):403–10. doi:10.1016/j.surg.2006.09.010.
Oncel M, Kurt N, Remzi FH, Sensu SS, Vural S, Gezen CF et al. The effectiveness of systemic antibiotics in preventing postoperative, intraabdominal adhesions in an animal model. The Journal of surgical research. 2001;101(1):52–5. doi:10.1006/jsre.2001.6245.
Bothin C, Midtvedt T, Perbeck L. Orally delivered antibiotics which lower bacterial numbers decrease experimental intra-abdominal adhesions. Langenbeck's archives of surgery. 2003;388(2):112–5. doi:10.1007/s00423-003-0369-3.
Drago L, Toscano M, De Grandi R, Casini V, Pace F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. European journal of gastroenterology & hepatology. 2016;28(5):532–7. doi:10.1097/MEG.0000000000000581.
Fry DE. Antimicrobial Bowel Preparation for Elective Colon Surgery. Surgical infections. 2016;17(3):269–74. doi:10.1089/sur.2015.271.
Rodgers KE, Girgis W, Campeau JD, di Zerega GS. Reduction of adhesion formation by intraperitoneal administration of anti-inflammatory peptide 2. Journal of investigative surgery : the official journal of the Academy of Surgical Research. 1997;10(1–2):31–6.
Guvenal T, Cetin A, Ozdemir H, Yanar O, Kaya T. Prevention of postoperative adhesion formation in rat uterine horn model by nimesulide: a selective COX-2 inhibitor. Human Reproduction. 2001;16(8):1732–5.
Kirdak T, Uysal E, Korun N. Assessment of effectiveness of different doses of methylprednisolone on intraabdominal adhesion prevention. Ulusal travma ve acil cerrahi dergisi= Turkish journal of trauma & emergency surgery: TJTES. 2008;14(3):188–91.
Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World journal of gastroenterology. 2011;17(41):4545–53.
Hong GS, Schwandt T, Stein K, Schneiker B, Kummer MP, Heneka MT et al. Effects of macrophage-dependent peroxisome proliferator-activated receptor γ signalling on adhesion formation after abdominal surgery in an experimental model. Br J Surg. 2015;102(12):1506–16. doi:10.1002/bjs.9907.
Javaherzadeh M, Shekarchizadeh A, Kafaei M, Mirafshrieh A, Mosaffa N, Sabet B. Effects of Intraperitoneal Administration of Simvastatin in Prevention of Postoperative Intra-abdominal Adhesion Formation in Animal Model of Rat. Bull Emerg Trauma. 2016;4(3):156–60.
Brochhausen C, Schmitt VH, Rajab TK, Planck CN, Krämer B, Tapprich C et al. Mesothelial morphology and organisation after peritoneal treatment with solid and liquid adhesion barriers—a scanning electron microscopical study. J Mater Sci Mater Med. 2012;23(8):1931–9. doi:10.1007/s10856-012-4659-6.
Chaturvedi AA, Lomme RM, Hendriks T, van Goor H. Prevention of postsurgical adhesions using an ultrapure alginate-based gel. The British journal of surgery. 2013;100(7):904–10. doi:10.1002/bjs.9131.
Fredriksson F, Sellberg F, Bowden T, Engstrand T, Berglund D, Lilja HE. Sutures impregnated with carbazate-activated polyvinyl alcohol reduce intraperitoneal adhesions. J Pediatr Surg. 2017. doi:10.1016/j.jpedsurg.2017.01.058.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Moris, D., Chakedis, J., Rahnemai-Azar, A.A. et al. Postoperative Abdominal Adhesions: Clinical Significance and Advances in Prevention and Management. J Gastrointest Surg 21, 1713–1722 (2017). https://doi.org/10.1007/s11605-017-3488-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3488-9