Abstract
Background
The correlation between resected gastric volume (RGV) and neuro-humoral changes (ghrelin and GLP-1) after laparoscopic sleeve gastrectomy (LSG) and their effects on type 2 diabetes mellitus (T2DM) has been evaluated.
Materials
Ninety-eight patients were divided in two groups: RGV <1200 mL (group A: 53 pts) and RGV >1200 mL (group B: 45 pts). Insulin secretion (insulin area under the curve (AUC)), insulinogenic index (IGI) and insulin-resistance (homeostasis model assessment, HOMAIR) were assessed before and after surgery (at the 3rd day and 6, 12 and 24 months after LSG) using the oral glucose tolerance test (OGTT). At the same time, ghrelin and GLP-1 levels were determined.
Results
A significant difference in T2DM resolution rate was observed after 6, 12 and 24 months in favour of RGV >1200 mL. Group B performed better than group A at the 3rd day and at the 6th, 12th and 24th months with regard to AUC, IGI and HOMAIR. In both groups, OGTT resulted in decreased ghrelin values and a significant increase in GLP-1 values for group B at the 3rd day and at the 6th and 12th months with no difference at the 24th month.
Conclusion
Ghrelin and GLP-1 changes play a role in the regulation of glucose metabolism during the 1st year after LSG. RGV influences ghrelin and GLP-1 plasma levels after LSG, with a significant improvement in the T2DM control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and type 2 diabetes mellitus (T2DM) are closely related and represent the two most common metabolic disorders in the world.1,2 During the last decade, bariatric surgery has emerged as an effective treatment for obesity-associated T2DM, especially if compared to medical therapy alone.2–4
Among the various kinds of surgery, laparoscopic sleeve gastrectomy (LSG) proved to be a valuable procedure for treating obesity complicated by T2DM with similar results to those achieved with Roux-en-Y gastric bypass.3,5,7 The mechanism of T2DM resolution after LSG is not yet clearly defined. Various studies showed that LSG achieves diabetes improvement independently of the amount of weight loss and caloric restriction, suggesting that hormonal mechanisms could also be involved.6,7 The removal of gastric fundus and the rapid gastric emptying have been proposed to justify hormonal changes, thus highlighting the role of the stomach in the regulation of glucose metabolism.8,9
Several authors have recently documented a relationship between resected gastric volume (RGV) and weight loss and the resolution of comorbidities after sleeve gastrectomy. This evidence suggests a significant improvement in LSG results for an RGV greater than 1200 mL.10–12 No previous study has compared RGV with postoperative gut hormone levels involved in glycaemic control.
The aim of this study was to determine the effect of RGV on ghrelin and glucagon-like peptide-1 (GLP-1) plasma levels and the impacts on insulin secretion and insulin resistance in obese diabetic patients who have undergone LSG.
Materials and Methods
Study Design
This is a prospective observational study including morbidly obese patients who underwent LSG during the period from April 2012 to September 2013 at our University Hospital. According to the National Institute of Health, all patients with a body mass index (BMI) ≥40 or ≥35 kg/m2 with at least one coexisting obesity-related comorbidity were eligible for the study. Other inclusion criteria were diabetes duration less than 10 years13,14, diagnosis of poorly controlled DM2 after administration of hypoglycemic drugs for 6 months, glycated haemoglobin A1c (HbA1c) levels ≥6.5 %, age between 30 and 60 years and no immunosuppressive therapy.
Before surgery, each patient was evaluated for at least 6 months by a multidisciplinary team including a psychologist, an internist, a gastroenterologist and a nutritionist. All surgical operations were performed at the Surgical Unit of San Salvatore Hospital in L’Aquila (Italy), by a single surgeon experienced in bariatric surgery.
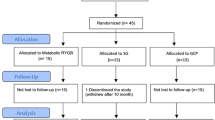
During the period considered, 98 patients met the inclusion criteria and completed 2 years of follow-up. Patients were divided into two groups, according to the RGV, measured at the end of surgery. In group A, 45 patients were enrolled with an RGV lower than 1200 mL; in group B, 53 patients with an RGV greater than 1200 mL (Fig. 1).
The end-points of the study were the evaluation of T2DM resolution, insulin secretion response, insulin resistance and changes in ghrelin and GLP-1 plasma concentrations, after LSG and according to the RGV.
Surgical Procedure and Postoperative Management
All procedures were performed laparoscopically, using a four-port technique. The greater omentum was sectioned close to the gastric wall using an ultrasonic dissector (Ethicon Endo-Surgery). Sleeve calibration was obtained by a 36-Fr gastric bougie, pushed toward and along the lesser curvature, and the stomach was transected with sequential firings of linear green and blue GIA reloads (Echelon® 60 mm, Ethicon Endo-Surgery). Gastric transection started 6 cm from the pylorus and ended 1 cm lateral to the esophago-gastric junction. The resected stomach was placed in a plastic bag and pulled out intact from the abdomen by enlarging the right subcostal incision.
In order to measure the RGV and pressure leak, a 16-Fr Foley catheter was inserted in the specimen through the gastric antrum, and a saline solution was manually injected using a 50-mL syringe until leakage was detected on the staple line. A purse string suture was used to close the hole around the catheter, and a manometric glass tube was used to evaluate the leak pressure in each specimen.
All patients were double-checked with a methylene blue test and upper gastrointestinal transit on the 2nd postoperative day, and, if no leakage was detected, a liquid diet was started on the 3rd postoperative day. The patients were discharged on the 5th postoperative day after eating mashed foods. The postoperative follow-up was conducted by a bariatric surgeon of our team 1, 3, 6, 12, 18 and 24 months after the operation and yearly thereafter.
Laboratory Analysis
An oral glucose tolerance test (OGTT) with 75 g of glucose (in a total volume of 400 mL) was administered in the morning after a 12-h overnight fast in the absence of antidiabetic drugs. The plasma glucose level (PGL) was measured using the glucose oxidase method (YSI 2300 Stat Plus, Yellow Springs, OH, USA). Patients with basal PGL over 200 mg/dL were excluded from the study. The OGTT was performed in all patients 48 h before surgery and 3 days and 6, 12 and 24 months postoperatively. After the test PGL, insulin secretion, HbA1c, ghrelin and GLP-1 were measured and compared in both groups. HbA1c levels were determined by a direct turbidimetric inhibition immunoassay that determines HbA1c as a percentage of total hemoglobin (%HbA1c) (Thermo Fisher Scientific). Assays were performed on an Indiko Plus (Thermo Fisher Scientific) automated biochemistry analyser, and results were reported as %HbA1c. Plasma insulin level (PIL) was measured as radioimmune insulin (IRI) with radioimmunoassay INSI-CTK (Dia Sorin Saluggia Italia). Insulin secretion, calculated by insulin total area under the curve (AUC), was assessed 1, 30, 60, 90 and 120 min after oral glucose load. The early insulin secretory response to specific glycaemic response was measured by “insulinogenic index” (IGI) [Insulin Radioimmune(IRI)30min − IRIfasting / PGL30min − PGLfasting],15 while insulin-resistance was measured with the “homeostasis model assessment” (HOMAIR) [IRIfasting (mU/mL) x PGLfasting (mmol/L)/22,5)].16 Ghrelin and GLP-1 levels were evaluated preoperatively and postoperatively before and 15 min after the OGTT. Plasma ghrelin was measured using a commercially available RIA kit (Phoenix Pharmaceuticals Inc., Phoenix, AZ, USA). GLP-1 was measured using a commercially available ELISA kit (Linco Research, St. Charles, MO, USA). These samples were collected in tubes containing DPP-IV inhibitor (Millipore, Billerica, MA, USA). The diabetologists on our team monitored the patients every 3 weeks. A PGL <100 mg/dL and HbA1c level <6.5 % without hypoglycaemic drugs were considered as resolution of diabetes.
Statistical Analysis
This study was designed as a prospective observational, parallel group trial to compare two clinical entities. Calculations were based on a power value of 1 − β = 0.90, alpha set at 0.05, a type II error of 0.10 and correlation between follow-up measurements of 0.700 using a two-tailed test (Software Stata/IC 12.1: sampsi—sample size and power for means and proportions). The data were analysed for normality of distribution with Shapiro-Wilk test. Because the data were distributed not normally, mean values of overall differences were compared among the groups, by a non-parametric analysis of variance, Kruskal-Wallis test and post hoc analysis for comparisons of pairs of mean values with Mann-Whitney test with Bonferroni adjustment for multiple comparisons, and thus significance for the univariate analyses was assessed at p < 0.0167. Categorical variables, expressed as percentage, were compared by chi square and t test. Comparisons between groups were analysed on an intention-to-treat basis.
Linear and logistic regression modelling was performed to determine the association between VGR and T2DM resolution at 24 months postsurgery, respectively. All regression models were adjusted for gender, age, initial obesity level (BMI) and RGV (Table 1)
Results
Patients’ demographic characteristics are shown in Table 2. Two patients were excluded because of a PGL greater than 200 mg/dL, and eight patients were lost at the follow-up. No statistical difference was found between groups in terms of age, preoperative BMI, pressure leak, sex and diabetes characteristics. RGV was not correlated to preoperative BMI and biochemical parameter of insulin sensitivity and resistance: the values of HbA1c, PGL, IGI and HOMAIR were comparable in both groups (Table 2). Diabetes treatments, as insulin and hypoglycaemic drugs, were similar between the groups: 48.8 and 51.1 % for insulin and 88.7 and 81.6 % for hypoglycaemic drugs respectively.
%Excess Weight Loss and Diabetes Remission
The overall %EWL on the 3rd day and at the 6th, 12th and 24th months was 8.4, 38.9, 45.6 and 51.8 %, respectively. A statistically significant difference in favour of group B was found at 24 months (Fig. 2).
No significant differences in PGL were reported at 48 preoperative hours. During the OGTT, PGL levels were significantly improved in group B compared with group A at the 3rd day and at the 6th and 12th months. No difference was recorded at 24 months (Fig. 3).
The mean preoperative %HbA1c level was 8.4 ± 1.5 without significant difference between groups (p = 0.17) (Table 2), while the mean rate at 6, 12 and 24 months was 6.4, 6.8 and 5.7 % respectively, with a significant reduction during the whole follow-up in favour of group B (Table 3A).
The resolution rate of DM2 at the 3rd day and at 6, 12 and 24 months in the two groups was 8.5, 44.7, 59.7 and 68.1 and 9.7, 58.5, 73.2 and 78.0 % respectively, with an overall resolution of 72.7 % at 24 months: a significant difference in the proportion of patients experiencing T2DM resolution was observed at the 6th, 12th and 24th months in favour of group B (Table 3B).Furthermore, the results from the multivariate logistic regression modelling of T2DM resolution, controlling for the effects of sex, age, initial BMI and RGV, are shown in Table 1.
Insulin Secretion and Insulin Resistance
No significant difference concerning AUC was found preoperatively. Therefore, during OGTT, PIL levels were significantly increased in group B compared with group A during the whole follow-up period (Fig. 4). The mean IGI before the operation was 0.021 ± 0.03, with no difference between groups (0.025 ± 0.012 vs. 0.019 ± 0.009, p = 0.183). The IGI increased significantly more in group B compared to group A at the 3rd postoperative day (0.073 vs. 0.032, p = 0.042), at the 6th month (0.193 vs. 0.092, p = 0.024) and at the 12th month after surgery (0.248 vs. 0.191, p = 0.035). At the 24th month of follow-up, our data showed similar results (0.252 vs. 0.248, p = 0.081) (Fig. 5a). Before the operation, the mean HOMAIR index was 9.4 ± 8.7, with no difference between groups (9.5 ± 9.2 vs. 9.2 ± 7.7, p = 0.074). During the postoperative period, the HOMAIR showed significantly better results in group B at the 3rd day (5.7 ± 5.6 vs. 7.4 ± 6.7, p = 0.046), at the 6th month (3.1 ± 3.8 vs. 5.8 ± 4.1, p = 0.032), at the 12th month (2.2 ± 3.6 vs. 3.4 ± 2.4, p = 0.041) and at the 24th month after surgery (1.4 ± 1.9 vs. 2.3 ± 2.6, p = 0.041) (Fig. 5b).
Ghrelin and GLP-1 Changes
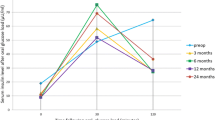
Basal ghrelin plasma (BGP) concentrations, similar in both groups preoperatively, decreased significantly more in group B at the 3rd day and at the 6th and 12th months after LSG (Fig. 6a).
During OGTT, ghrelin values diminished before and after LSG in both groups compared to basal ghrelin level (Fig. 6a, b). Ghrelin levels after 15 min (ghrelin-15) were significantly decreased in group B at the 3rd postoperative day (84.7 ± 39.8 vs. 108.5 ± 42.6 pg/ml, p = 0.031) and at the 6th (87.4 ± 27.9 vs. 111.5 ± 31.5 pg/ml, p = 0.026) and 12th months after surgery (92.2 ± 52.5 vs. 115.1 ± 51.3 pg/ml, p = 0.028) (Fig. 6b). Furthermore, BGP and ghrelin-15 were significantly increased at the 24th postoperative month compared to the values recorded on the 3rd day after LSG (Fig. 6a, b).
Basal GLP-1 plasma concentrations, similar in both groups preoperatively, increased significantly more in group B compared to group A after LSG. Figure 6c shows the increase between the 3rd postoperative day and the 12th month after surgery. During OGTT, at 15 min GLP-1 (GLP-1-15), values diminished before and after LSG in both groups compared to basal GLP-1 (Fig. 6d). The GLP-1 levels after 15 min (GLP-1-15) were significantly decreased in group B at the 3rd postoperative day (3.77 ± 2.9 vs. 3.40 ± 3.7 pg/ml, p = 0.043) and 6 (3.75 ± 4.1 vs. 3.31 ± 4.5 pg/ml, p = 0.046) and 12 months after surgery (3.68 ± 2.7 vs. 3.31 ± 5.3 pg/ml, p = 0.046). As for ghrelin, GLP-1 and GLP-1-15 were significantly increased at the 24th month compared to the values at the 3rd day after LSG (Fig. 6).
Discussion
The literature data regarding diabetes remission following LSG are extremely variable, ranging from 60 to 80 % depending on the patient population and the length of follow-up.8,17–21 Gill et al. in a recent meta-analysis concerning 673 patients reported 66.2 % of diabetes remission and 26.9 % of T2DM improvement.21 In comparison to purely restrictive bariatric procedures such as gastric banding, glycaemic control is often achieved before significant weight loss after LSG.13,22 This evidence suggests that the control of the glycaemic status may be a direct effect of metabolic surgery rather than a secondary effect of the weight loss.23,24
Several authors have explained this LSG weight-independent antidiabetic effect as stemming from reduced plasma ghrelin concentrations and increased levels of GLP-1, highlighting a role of the stomach in the regulation of glucose metabolism.10,13,17–27 The metabolic consequence of LSG is the decrease of plasma ghrelin concentrations closely related to the amount of ghrelin-secreting cells removed by the surgery.26 Moreover, GLP-1 is produced by the transit of nutrient materials in the distal ileum: a greater amount of food intake determines a greater secretion of GLP-1.27 According to the “hindgut theory”,26 the rapid transit of undigested nutrients to the distal gastrointestinal tract, caused by the increased gastric emptying after LSG, up-regulates the production of GLP-1 from intestinal L cells in the distal bowel.24,26,27
Ghrelin has been demonstrated to be suppressed by insulin and to inhibit glucose-induced insulin release. Furthermore, ghrelin infusion is reported to increase insulin resistance and, finally, the removal of the gastric fundus might contribute to improve insulin sensitivity.24 Increased GLP-1 production by enteroendocrine L cells, lining in the distal gastrointestinal tract, can normalize blood glucose and regulate insulin synthesis and pro-insulin gene expression, as well as the secretion of glucagon and somatostatin.22,27
Our results showed an overall increase of plasma ghrelin concentrations and an overall reduction of GLP-1 in both groups during follow-up (Fig. 6).
Moreover, we hypothesized that the amount of RGV might directly affect the plasma concentrations of gut hormones involved in postoperative weight-independent T2DM control. Two anatomical results of LSG could play an important role in this antidiabetic effect. First of all, a larger RGV could remove a higher number of oxyntic cells, thus reducing ghrelin plasma levels, and secondly, the increase of gastric emptying could up-regulate GLP-1 plasma levels.
In order to measure RGV, we manually injected a saline solution using a 16-Fr Foley catheter inserted in the gastric antrum, until leakage of the staple line was detected.11,28–30 We noted no difference between the leak pressures in both groups. Causey et al.31 reported similar results about the leak pressure generated by the saline solution injection, suggesting the accuracy of this method of measurement with no significant prevalence in the location of the leak.
The positive correlation between RGV and weight loss is still a matter of debate.10–12,31 Some authors found a positive relationship between RGV and weight loss,10,12,31 while for others weight loss results were independent of RGV.11 Weiner et al. reported that the resection of ≤500 mL of stomach volume was a prediction of weight loss failure or early weight regain.31 Our findings about %EWL after LSG were consistent with those reported in literature (Fig. 2). Contrary to Obeidat et al.,11 we recorded a significant positive correlation between VGR and %EWL starting 1 year after surgery and confirmed at 24 months of follow-up in patients with a higher RGV.
Our multivariate logistic regression modelling of T2DM resolution showed that gender, age and baseline BMI did not play a role in diabetic remission: only the RGV seems to be crucial in achieving significant results. This could be demonstrated by the evidence that during OGTT all indicators of insulin secretion (AUC and IGI) and insulin resistance (HOMAIR) were better in group B than in group A (Figs. 4 and 5). These results seem to justify the significant differences in the proportion of patients experiencing better glucose homeostasis and T2DM resolution in RGV >1200 mL during the follow-up period (Table 3b).
Moreover, our results suggest that RGV affects glucose homeostasis through two time-related pathways. During the 1st year after surgery, a higher RGV may promote a favourable change in hormonal plasma levels with positive effects on insulin secretion and insulin resistance, as shown by the early improvement in glycaemic control and insulin response at the 3rd day after LSG (Fig. 6). On the contrary, although during the 2nd postoperative year no difference in ghrelin and GLP-1 concentrations was found between groups, a better glucose metabolism control was achieved in group B. In our opinion, this result is not related to hormonal changes but rather to the greater weight loss recorded in patients with a greater RGV (Fig. 2). At a longer-term follow-up, the positive effect of weight loss that improves insulin sensitivity and decreases insulin resistance has already been reported11,23,25 and suggests that the fat mass participates in the modulation of glucose metabolism.32
This study involved several limitations, including the lack of randomisation and the impossibility of measuring the residual gastric volume after sleeve resection. There is no conclusive evidence regarding the way to determine the optimal size of the gastric sleeve with precision.10,11,31 As proposed by other authors, we assumed that the standardization of a technique performed by only one surgeon would yield similar results in terms of sleeve size.12,33
In conclusion, postoperative LSG metabolic effects result in a high T2DM remission rate. The positive effect of RGV seems to be related to increased insulin secretion and to the improvement of insulin resistance during the first 2 years of follow-up. We suggest that RGV could affect glucose homeostasis through two time-related pathways: during the first 12 months after surgery, the positive effects on glucose homeostasis occurred in the group with higher RGV due to a more favourable change in ghrelin and GLP-1 plasma levels; during the 2nd year, these effects seem to be related to improved insulin resistance, which results in improved weight control. Further studies with a larger cohort of patients are needed to confirm this “two-step hypothesis”.
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378 (9785): 31–40.
Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and metanalysis. Am J Med 2009;122(3):248–256.e5.
Cho JM, Kim HJ Menzo El, Park S, Szomstein S, Rosenthal RJ. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis. 2015 Mar 5. pii: S1550-7289(15)00056-8.
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004 Dec 23;351(26):2683–93.
BuchwaldH, OienDM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4): 427–36.
Yip S, Signal M, Smith G, Beban G, Booth M, Babor R, Chase JG, Murphy R.: Lower glycemic fluctuations early after bariatric surgery partially explained by caloric restriction. Obes Surg. 2014 Jan;24(1):62–70.
Plourde CE, Grenier-Larouche T, Caron-Dorval D, Biron S, Marceau S, Lebel S, Biertho L, Tchernof A, Richard D, Carpentier AC.: Bili-opancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity (Silver Spring). 2014 Aug;22(8):1838–46.
Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: Comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: A prospective randomized trial. Ann Surg. 2009 Aug;250(2):234–41.
Blaire Anderson, Noah J. Switzer, Ahmad Almamar, Xinzhe Shi, Daniel W. Birch, Shahzeer Karmali: The Impact of Laparoscopic Sleeve Gastrectomy on Plasma Ghrelin Levels: a Systematic Review. Obes Surg 2013; 23(9):1476–80.
L Pawanindra, A Vindal, M Midha, P Nagpal, A Manchanda, J Chander. Early post-operative weight loss after laparoscopic sleeve gastrectomy correlates with the volume of the excised stomach and not with that of the sleeve! Preliminary data from a multi-detector computed tomography-based study. Surg Endosc (2015) 29:2921–2927
FW Obeidat, HA Shanti, AA Mismar, MS Elmuhtaseb, MS Al-Qudah. Volume of resected stomach as a predictor of excess weight loss after sleeve gastrectomy. Obes Surg. 2014 Nov. 24(11):1904–8.
Sista F, Abruzzese V, Colozzi S, Schietroma M, Carlei F, Mattei A, Clementi M, Carandina S, Barrat C, Amicucci G. Does gastric resection volume influence sleeve gastrectomy results? A prospective study. Int J Surg. 2016:3(1):11–17.
Basso N, Capoccia D, Rizzello M, Abbatini F, Mariani P, Maglio C, Coccia F, Borgonuovo G, De Luca ML, Asprino R, Alessandri G, Casella G, Leonetti F. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc 2011; 25:3540–3550.
Laville M, Disse E Bariatric surgery for diabetes treatment: why should we go rapidly to surgery. Diabetes Metab. 2009 Dec;35(6 Pt 2):562–3.
Philips DIW, Clark PM, Hales CN, Osmond C: Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabetic Med 11, 286–292, 1994
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RL: Homeostasis model assessment insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419, 1985
Nocca D, Guillaume F, Noel P, Picot MC, Aggarwal R, El Kamel M, Schaub R, de Sequin de Hons C, Renard E, Fabre JM. Impact of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on HbA1c blood level and pharmacological treatment of type 2 diabetes mellitus in severe or morbidly obese patients: results of a multicentre prospective study at 1 year. Obes Surg 2011jun;21(6):738–43.
Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010 May;24(5):1005–10
de Gordejuela AG, Pujol Gebelli J, García NV, Alsina EF, Medayo LS, Masdevall Noguera C. Is sleeve gastrectomy as effective as gastric bypass for remission of type 2 diabetes in morbidly obese patients? Surg Obes Relat Dis. 2011 Jul-Aug;7(4):506–9.
Shah PS, Todkar JS, Shah SS. Effectiveness of laparoscopic sleeve gastrectomy on glycemic control in obese Indians with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010 Mar 4;6(2):138–41
Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. 2010 Nov-Dec;6(6):707–13
Evans S, Pamuklar Z, Rosko J, Mahaney P, Jiang N, Park C, Torquati A. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction .Surg Endosc. 2012 Apr;26(4):1086–94
Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010 May;147(5):664–9.
Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JO, Schmitz O Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Meta 2008; 93(2):438–444.
Pinkney JH, Sjostrom CD, Gale EA. Should surgeons treat diabetes in severely obese people? Lancet. 2001 Apr 28;357(9265):1357–9.
A. Maleckas, L. Venclauskas, V. Wallenius, H. Lönroth, L. Fändriks. Surgery in the treatment of type 2 diabetes mellitus. Scand J Surg. 2015 Mar;104(1):40–7
Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012 Aug;26(8):2231–9
Jagat Pal Singh, Om Tantia Tamonas Chaudhuri, Shashi Khanna, Prateek H. Patil. Is Resected Stomach Volume Related to Weight Loss After Laparoscopic Sleeve Gastrectomy? Obes Surg.2014 Oct;24(10):1656–61.
Firas W. Obeidat, Hiba A. Shanti, Ayman A. Mismar, M. S. Elmuhtaseb, Mohammad S. Al- Qudah Volume of Resected Stomach as a Predictor of Excess Weight Loss After Sleeve Gastrectomy. Obes Surg.2014 Nov ;24(10):1904–1908.
Causey MW, Fitzpatrick E, Carter P. Pressure tolerance of newly constructed staple lines in sleeve gastrectomy and duodenal switch. Am J Surg. 2013 May;205(5):571–4; discussion 574–5.
RA Weiner, S Weiner, I Pomhoff, Cjacobi W Makarewicz G Weigand. Laparoscopic sleeve gastrectomy- influence of sleeve size and resected gastric volume. Obesity Surg;17: 1297–1305.
Faraj M, Lu HL, Cianflone K. Diabetes, lipids, and adipocyte secretagogues. Biochem Cell Biol. 2004 Feb;82(1):170–90.
Yuval JB, Mintz Y, Cohen MJ, Rivkind AI, Elazary R. The effects of bougie caliber on leaks and excess weight loss following laparoscopic sleeve gastrectomy. Is there an ideal bougie size? Obes Surg. 2013 Oct;23(10):1685–91.
Acknowledgments
We would like to thank Professor Alessio Agnifili, who made substantial contributions to the conception and design of the study. The author considers him a mentor in the healthcare profession and in his everyday life.
Authors’ contributions
Sista F and Abruzzese V have made substantial contributions to the conception and design of the study, as well as drafting of the work and its critical revision for important intellectual content; Clementi M has made analysis and interpretation of literature data; and Carandina S and Amicucci G have given final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethical committee of the DISCAB Department of the University of L’Aquila approved the protocol of the study. All patients signed a consent form.
Financial support
None
Rights and permissions
About this article
Cite this article
Sista, F., Abruzzese, V., Clementi, M. et al. Effect of Resected Gastric Volume on Ghrelin and GLP-1 Plasma Levels: a Prospective Study. J Gastrointest Surg 20, 1931–1941 (2016). https://doi.org/10.1007/s11605-016-3292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3292-y