Abstract
Background
Total pancreatectomy is actually considered a viable option in selected patients even if large comparative studies between partial versus total pancreatectomy are not currently available. Our aim was to evaluate whether total pancreatectomy can be considered as feasible, safe, efficacious, and cost-effective as pancreaticoduodenectomy.
Methods
A single center, prospective, observational trial, regarding postoperative outcomes, long-term results, and cost-effectiveness, in a tertiary referral center was conducted, comparing consecutive patients who underwent elective total pancreatectomy and/or pancreaticoduodenectomy.
Results
Seventy-three consecutive elective total pancreatectomies and 184 pancreaticoduodenectomies were compared. There were no significant differences regarding postoperative outcomes and overall survival. The quality of life, evaluated in 119 patients according to the EQ-5D-5L questionnaire, showed that there were no significant differences regarding the five items considered. The mean EQ-5D-5L score was similar in the two procedures (total pancreatectomy = 0.872, range 0.345–1.000; pancreaticoduodenectomy = 0.832, range 0.393–1.000; P = 0.320). The impact of diabetes according to the Problem Areas in Diabetes (PAID) questionnaire did not show any significant differences except for question 13 (total pancreatectomy = 0.60; pancreaticoduodenectomy = 0.19; P = 0.022). The cost-effectiveness analysis suggested that the quality-adjusted life year was not significantly different between the two procedures (total pancreatectomy = 0.910, range 0.345–1.000; pancreaticoduodenectomy = 0.910, range −0.393–1.000; P = 0.320).
Conclusions
From this study, it seems reasonable to suggest that total pancreatectomy can be considered as safe, feasible, and efficacious as PD and acceptable in terms of cost-effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The decision to perform a total pancreatectomy in the setting of pancreatic disease continues to be a difficult one for many surgeons. Nevertheless, several studies1 – 4 have recently reported good perioperative results after total pancreatectomy (TP) and a recent systematic review of the National Cancer Data Base5 confirmed these results in a large cohort of patients who underwent TP for pancreatic cancer. In addition, the new formulation of intermediate and long-acting insulin and the development of modern pancreatic enzyme preparations allowed obtaining good long-term results and quality of life.6 – 8 Thus, the surgeon fear in performing TP is not justified, and actually, total pancreatectomy is considered the treatment of choice in those patients with multi-focal parenchymal diseases including intraductal papillary mucinous neoplasia (IPMNs), pancreatic neuroendocrine tumors (PNETs), renal cell metastases,9 and in those with extensive pancreatic cancer with the benefits to achieve complete tumor resection and negative margins. Moreover, some pancreatic surgeons have suggested elective total pancreatectomy over pancreaticoduodenectomy (PD) in high risk cases in order to avoid a potential postoperative pancreatic fistula (POPF) and subsequent mortality and morbidity after pancreaticoduodenectomy.10 – 12 Many authors13 – 16 proposed risk score systems, based on multiple parameters, to predict clinically relevant POPF and to select the high risk patients. However, large comparative studies between partial versus total pancreatectomy are not currently available. In relation to these considerations, the aim of the present study was to evaluate whether TP can be considered as feasible, safe, and efficacious as PD by comparing the two procedures as regards postoperative outcomes, long-term results, and cost-effectiveness analysis.
Methods
Study Design and Descriptive Data
This study was a single center, prospective, observational trial. With the approval of the Ethic Committee of S.Orsola-Malpighi Hospital and patient informed consent, all patients who underwent elective total pancreatectomy and/or pancreaticoduodenectomy for benign or malignant pancreatic diseases, in the tertiary referral university center of S.Orsola-Malpighi Hospital from January 2011 to January 2015, were compared as regards postoperative outcomes and long-term results. In addition, a cost-effectiveness analysis was carried out. The variables evaluated for each patient included sex, age at diagnosis, body mass index (BMI), American Society of Anesthesiologists (ASA) score, co-morbidities, preoperative diabetes, jaundice, other symptoms, insertion of a preoperative biliary stent, Wirsung duct size (>3 mm), operating time, vascular resection, and pathologic diagnosis.
Preoperative Decision-Making, Surgical Techniques and Postoperative Course
Preoperatively, all patients were evaluated by a multidisciplinary team of pancreatic surgeons, medical oncologists, gastroenterologists, and radiologists. Total pancreatectomy was preferred in multi-focal neoplastic lesions or when the disease involved the entire gland or in cases in which a vascular reconstruction was necessary. In frail patients (elderly >80 years; diabetic, with important co-morbidities) and in those patients with a high risk of pancreatic fistula calculated according our score system,15 the final choice between the two procedures was taken intraoperatively. The pancreaticoduodenectomies and total pancreatectomies were performed using the Whipple procedure. In the PDs, the pancreatic remnant was always monitored with a pancreaticojejunostomy without stenting. In both the PDs and the TPs, the biliary tract was treated with a hepaticojejunostomy with stenting if the bile duct was small in diameter. The procedures were performed by two experienced surgeons who had each performed more than 100 pancreatic resections. Postoperatively, in all patients, low molecular weight heparin was administered. In patients who underwent PD, somatostatin analogs were administered postoperatively from postoperative days 1–7, except in the presence of a pancreatic leak in which case they were continued. All patients with a post-operative diagnosis of diabetes were referred to the endocrinology team which provided diabetes-related education, discharge instructions, and follow-up care. Finally, all patients were followed up by the surgeons and oncological team.
Postoperative Outcomes and Long-Term Results
Postoperative mortality, morbidity, reoperation rate, readmission rate, intensive care unit (ICU) stay, length of hospital stay (LOS), and type of discharge were obtained to evaluate the postoperative outcomes.
The long-term results included overall survival and evaluation of the quality of life (QoL). The overall survival was evaluated separately in malignant and premalignant or benign tumors. The quality of life was evaluated in all patients still alive at 1 year after surgery. All patients were contacted by phone, and those who agreed to participate in the study were asked to complete two surveys by mail. In addition, data referring to diabetes as well as the presence of pre and postoperative diabetes and its therapy, and the daily dose of different types of insulin were also obtained. The survey instruments included the Euro Quality of Life Group Association (EQ-5D-5L) questionnaire17 and the Problem Areas in Diabetes (PAID) questionnaire.7 All patients who underwent either TP or PD and were still alive at 1 year after surgery responded to the EQ-5D-5L questionnaire while only the diabetic patients also responded to the PAID questionnaire.
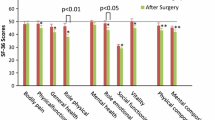
The EQ-5D-5L17 questionnaire assessed the quality of life of the patients and consisted of five items: (1) mobility; (2) self-care; (3) usual activities (e.g., work, study, housework, family, or leisure activities); (4) pain/discomfort; and (5) anxiety/depression. For each answer, there was a score from 1 to 5 in relation to the status of the patient where lower scores indicated a better quality of life. The final EQ-5D-5L score ranged from negative values to 1 where 1 was the best health status.
The PAID7 questionnaire measured the impact of diabetes and consisted of 20 questions with a score from 0 to 4 where a lower score indicated better satisfaction. The scores obtained had to be added and then multiplied by a coefficient (1.25) in order to obtain a final score (the PAID score).
Cost-Effectiveness Analysis
The cost-effectiveness analysis was carried out in accordance with the EVEREST guidelines18 and was carried out only for patients still alive at 1 year after surgery. The total cost of both surgical procedures regarding hospital stay, operating room costs, and ICU stay was obtained from the accounting office of our hospital. The daily cost for the treatment of the diabetic patients was calculated. The mean differential cost and the mean differential quality-adjusted life year (QALY) were calculated and plotted on a cost utility plane. The horizontal axis represented the differences in QALYs and the vertical axis the differences in costs. The incremental cost-effectiveness ratio (ICER) was properly computed as cost per QALY gained and reported as mean values. The ICER slope and 95 % confidence intervals (95 % CI) were plotted. Uncertainty regarding cost-effectiveness was also explored using the cost-effectiveness acceptability curve (CEAC) which shows the probability that an intervention is cost-effective as compared with the alternative, given the observed data, for a range of monetary values which a decision-maker might be willing to pay for a particular unit change in outcome (willingness-to-pay (WTP)).19 The incremental net benefit (INB) was calculated in order to obtain a confidence interval for producing the cost-effectiveness analysis acceptability curve.
Terminology and Definitions
Postoperative mortality was defined as the number of deaths occurring during hospitalization or within 30 days after surgery. The postoperative morbidity rate included all complications following surgery up to the day of discharge; they were classified according to the Clavien-Dindo classification.20 A postoperative pancreatic fistula was defined and graded according to the criteria of the International Study Group on Pancreatic Fistula.21 Post-pancreatectomy hemorrhage (PPH) was defined using the International Study Group of Pancreatic Surgery criteria.22 Readmission rate was defined as readmission within 30 days of hospital discharge. Length of hospital stay was calculated as the interval from the day of surgery to the date of discharge. Operating time was defined as the time interval from the incision to the suturing of the skin. The pathologic diagnosis was determined on the basis of the final pathology reports.
Statistical Analysis
Medians, standard deviations, and frequencies were used to describe the data. Mean values were used for the EQ-5D-5L and PAID questionnaires. The Fisher’s exact test, Pearson chi-square, and the Student t tests were applied to describe the variables. Survival was estimated using the Kaplan-Meier method, and the log-rank test was used for comparison between the two procedures. The results and costs were reported as mean difference and confidence interval (95 % CI). A confidence interval for the cost per QALY ratio was obtained using the non percentile bootstrap method, based on 2000 replications. Fieller’s method was used to establish the confidence interval.23 Cost-effectiveness analyses were carried out using STATATM 5.0 software (Stata Corporation, College Station, Texas, USA). Data analyses were carried out by running Microsoft Excel and IBM SPSS for Windows (version 22.0) on a personal computer. Two-tailed P values less than 0.05 were considered statistically significant.
Results
Descriptive Data
A total of 452 consecutive pancreatic resections were obtained in our prospective institutional database between January 2011 and January 2015. Seventy-three consecutive elective total pancreatectomies and 184 pancreaticoduodenectomies were compared regarding postoperative outcomes. The patients still alive 1 year after surgery who agreed to participate to the study (n = 119), 35 elective TPs and 84 PDs were compared regarding quality of life and cost-effectiveness (Fig. 1).
Flowchart of patients who underwent pancreatic resections for periampullary neoplasms (n = 452). Patients who underwent elective total pancreatectomy and pancreaticoduodenectomy were compared regarding postoperative outcomes (73 and 184 patients, respectively) and, if still alive at 1 year after surgery (n = 119), long-term results and cost-effectiveness (35 and 84, respectively)
Table 1 summarizes the descriptive data. Total pancreatectomy was significantly preferred with respect to PD in relation to pathology (P < 0.001), in patients with ASA score III (P = 0.040), preoperative diabetes (P = 0.002), in the presence of a dilated Wirsung duct (P < 0.001), and in patients in which a vascular resection was performed (P < 0.001). Regarding the pathology, total pancreatectomy was more frequently performed for pancreatic ductal adenocarcinoma (PDAC) (52.1 versus 41.3 %), intraductal papillary mucinous neoplasms (IPMNs) (20.5 versus 7.1 %), and metastatic tumors (4.1 versus 1.6 %). Operating time was significantly longer in TP than in PD (P < 0.001).
Postoperative Outcomes and Long-Term Results
Table 2 summarizes the postoperative outcomes of the two procedures. There were no significant differences between TP and PD regarding overall mortality, morbidity, LOS, ICU stay, reoperation rate, type of discharge, and readmission rate. Nine patients out of the 184 (4.9 %) who underwent PD were reoperated on for a postoperative pancreatic fistula. Moreover, the LOS range was longer in patients who underwent PD with respect to TP (2–177 versus 9–91 days).
The median overall survival was not significantly different between the two procedures (TP 28 months, range 6–57; PD, 27 months, range 18–36; P = 0.432) (Fig. 2a) or in patients with PDAC (TP 21 months, range 14–27; PD, 19 months, range 16–22; P = 0.867) (Fig. 2b). Survival at 1, 3, and 5 years was 74, 48, and 31 % for TP and 78, 45, and 37 % for PD, considering all patients and 68, 30, and 15 % for TP and 70, 24, and 13 % for PD, for patients affected by PDAC. The QoL was determined in 119 patients (TP = 35; PD = 84) according to the EQ-5D-5L questionnaire which showed that there were no significant differences between the two surgical procedures regarding the five items considered (Table 3). The mean EQ-5D-5L score was similar in the two procedures (TP mean value 0.872, range 0.345–1.000; PD mean value 0.832, range −0.393–1.000; P = 0.320).
Diabetes mellitus developed in all patients who underwent TP (n = 35), and all these patients required insulin therapy. Among the patients who underwent PD, 43 (51.2 %) out of 84 developed diabetes; 21 required insulin therapy and 22 oral therapy (mean total consumption = 222.6 mg/day, range 10–1500). The mean total consumption/day of insulin therapy was significantly greater in patients who underwent TP than in those who underwent PD (30 Unit/day, range 14–50; versus 12 Units/day, range 0–48, respectively, P < 0.001), namely rapid and short-acting (19 Units/day, range 4–40 versus 8 Units/day, range 0–35, respectively. P < 0.001), intermediate-acting (3 Units/day, range 0–19 versus 1 Unit/day, range 0–10, respectively. P = 0.017), and long-acting insulin (7 Units/day, range 0–18 versus 3 Units/day, range 0–34, respectively. P = 0.002). The impact of diabetes according to the PAID questionnaire (evaluated in 78 patients; TP = 35; PD = 43) did not show any significant differences between TP and PD except for question 13 (TP mean score = 0.60; PD mean score = 0.19; P = 0.022). The response to questions 2, 6, and 14 was not statistically significant but they showed a trend in favor of PD (P = 0.084, 0.083 and 0.087, respectively) (Table 4). The PAID score analysis showed a mean score of 5.571 (range 0–40) for TP and 3.023 (range 0–15) for PD and was not significantly different, even if it showed a trend in favor of PD (P = 0.081).
Costs-Effectiveness Analysis
A cost-effectiveness analysis was carried out for 119 patients (TP = 35; PD = 84). Mean total cost and health-related quality of life are reported in Table 5. Operating room costs were higher regarding TP with respect to PD (7400 versus 6710 €) but they were not statistically significant, even if they indicated a strong trend in favor of PD (P = 0.053). On the other hand, mean hospital stay cost was significantly reduced for TP with respect to PD (4560 € versus 5329 €; P = 0.049). The mean total cost for 1 year of treatment of diabetes was significantly higher in TP patients than in PD patients (247 € versus 14 €; P < 0.001). The QALY was not significantly different between the two procedures (TP = 0.910, range 0.345–1.000; PD = 0.910, range −0.393–1.00; P = 0.320) at 1 year after surgery. The incremental cost-effectiveness ratio was −17,922 € per additional QALY gain. Figure 3a shows the cost utility plane; from 2000 bootstrapped replications, 1300 observations (65 %) were found to be in the accept quadrant (southeast); 500 (25 %) in the uncertain quadrant (northeast), 120 (6 %) (southwest) and 80 (0.4 %) (northwest) in the reject quadrants. Figure 3b confirms that the mean ICER slope (−17,113 €) with a 95 % confidence interval from +16,963 to −18,254 € is in the accept quadrant. Figures 4a, b shows the incremental net benefit with a 95 % confidence interval and the cost-effectiveness acceptability curve. Total pancreatectomy was superior to PD for any cost established as willingness-to-pay. The acceptability curve showed that TP had a high probability (from 70 to 80 %) of being cost-effective with respect to PD when any willingness-to-pay for QALY was accepted.
a The cost utility plane: from 2000 bootstrapped replications, 1300 observations (65 %) were found to be in the accept quadrant (southeast); 500 (25 %) in the uncertain quadrant (northeast), 120 (6 %) (southwest), and 80 (0.4 %) (northwest) in the reject quadrants. ICER incremental cost-effectiveness ratio, QALY quality-adjusted life years. b The mean ICER slope was −17113 € with a 95 % confidence interval from +16,963 to −18,254 €, and it was in the accept quadrant
a Incremental net benefit (INB) with 95 % confidence interval. CI 95 %: lower and upper confidence interval. Total pancreatectomy resulted superior to pancreaticoduodenectomy for any costs established as willingness-to-pay (WTP). b The cost-effectiveness acceptability curve (CEAC) showed that a total pancreatectomy had a high probability (from 70 to 80 %) of being cost-effective respect on pancreaticoduodenectomy when any willingness-to-pay (WTP) for QALY was accepted
Discussion
Total pancreatectomy is currently considered a viable option in selected patients even if large comparative studies dealing with partial versus total pancreatectomy are not currently available. To our knowledge, in fact, there are only four studies comparing TP with PD in the current literature.24 – 27 First, in 2007, Muller MW et al.24 reported a matched-pair analysis comparing the perioperative outcomes and the long-term results of 87 elective TPs and 87 PDs. This study revealed no differences between the two surgical procedures regarding postoperative mortality, morbidity, hospital stay, and quality of life despite limitations caused by insulin-dependent diabetes mellitus in TP patients. On the contrary, Bhayani NH et al.,25 using the National Surgical Quality Improvement Project data from 2005 to 2011, showed better perioperative outcomes for PD with respect to TP, comparing 6314 PDs with 198 TPs. Epelboym et al.,26 comparing the long-term results of 17 TPs and 14 PDs, suggested that the overall quality of life is comparable with that of patients who undergo a partial pancreatic resection. Finally, Satoi S et al.27 reported an additional matched-pairs analysis of 45 TPs and 45 PDs, carried out only for pancreatic cancer patients which revealed similar perioperative outcomes and overall survival between the two surgical procedures. These studies have several limitations: (1) a retrospective design, (2) the comparison between the two procedures was principally carried out using a matched-pairs analysis, and (3) the evaluation of both postoperative outcomes and long-term results together was rarely included. Thus, the opinion regarding TPs with respect to PDs was often fragmentary and incomplete. In addition, to our knowledge, no studies reported a cost-effectiveness analysis between the two procedures.
The present study represents the first observational, prospective study comparing TP with PD. In addition, this study evaluated both perioperative outcomes and long-term results and, for the first time, a cost-effectiveness analysis was carried out.
According to our descriptive data, the indications for performing TP were clearly different from those to perform PD. Total pancreatectomy was principally performed for neoplastic diseases involving the entire gland as well as IPMNs or metastatic disease, or in PDACs in which the resection margin after PD was involved. In addition, total pancreaticoduodenectomy was performed more frequently than PD in diabetic and frail patients (ASA score III) and, finally, in those in which a vascular resection had to be carried out. Postoperative outcomes were similar between the two procedures, suggesting that TP was as feasible and safe as PD. Moreover, from our data, it was interesting to note that the reoperation rate due to a pancreatic fistula can be eliminated in the case of TP while it was approximately 5 % in patients who underwent PD. Bhayani NH et al.22 also reported this fact using the National Surgical Quality Improvement Project (NSQIP) data from 2005 to 2011, but the authors pointed out that TP is associated with increased major postoperative morbidity and mortality. Thus, they concluded that TP cannot be routinely recommended for reducing perioperative morbidity when PD is an appropriate surgical option. However, there are some limitations in this study: (1) there is no way to determine why TP was performed; (2) NSQIP is a multi-institutional database and includes both high-volume and low-volume centers for pancreatic surgery with probable different perioperative outcomes. In our experience, postoperative mortality and morbidity after TP were similar to PD. Thus, when PD represents an appropriate surgical option but the patient is frail (elderly >80 years of age with co-morbidities), diabetic, and with a high risk of postoperative pancreatic fistula because the pancreas remnant resulted friable, soft, and easily bleeding,15 TP could be considered a reasonable, alternative option to PD with the aim of avoiding pancreatic fistula and its life-threatening complications. For the same reason, in those cases in which a vascular reconstruction is necessary, the benefit of TP over PD could be explored.
Regarding the long-term results, the main question is: “Is TP as efficacious (considering overall survival and QoL) as PD?” Satoi S et al.24 reported that TP and PD have similar overall survival in malignant tumors. Our experience confirmed this fact. Thus, if it is evident that TP failed to improve the prognosis, it should be pointed out that TP was at least as efficacious as PD. Moreover, it should be noted that, in premalignant and benign tumors, the overall survival was similar in the two procedures, suggesting that the deaths for diabetes patients after TP did not significantly influence the overall survival. Regarding the QoL, first, Muller et al.24 achieved a comparable and satisfying QoL after TP with respect to PD. Second, in a small sample of patients, Epelboym I et al.26 reported a QoL and an impact of diabetes which was acceptable and comparable between the two procedures. The present study is the second one comparing TP and PD in which the quality of life and the impact of diabetes were both considered. The patients who underwent TP had a good QoL (EQ-5D-5L score near 1) without significant differences with respect to PD. In addition, pancreatogenic diabetes impacts poorly on the quality of life of these patients (low score) as does the diabetes of those patients who underwent PD. However, even if many of the differences in diabetes-related QoL do not reach statistical significance (except question 13), there are strong trends in favor of PD on some questions of PAID questionnaire including being scared of living with diabetes (question 2), feeling depressed about diabetes (question 6), not accepting diabetes (question 14), and overall diabetes-related QoL (PAID score). In summary, diabetes from TP is harder to control (and more expensive) than after PD, but it is not so hard and it is no lethal. Thus, TP was as efficacious as PD.
Regarding cost, there are no studies which have compared the crude costs of TP and of PD. In our experience, the analysis of crude costs showed that the total cost was similar between the two procedures (15,027 € versus 15,082 €; P = 0.509). However, there are some differences in the individual parameters considered. The operating room costs were higher for TP with respect to PD (7400 € versus 6710 €; P = 0.053), and this was due to the significantly longer operating time of TP with respect to PD (380 versus 335 min; P < 0.001). On the contrary, the hospital stay costs were lower for TP than for PD (4560 € versus 5320 €; P = 0.049) even if the length of hospital stay was the same for the two procedures (16 days). It is evident that complications after PD, especially POPF, resulted in a higher cost of treatment (antibiotic therapy, somatostatin analogs, parenteral and enteral nutrition, etc.), and the hospital stay can be less predictable (longer range with respect to TP) than those of patients who underwent TP. Finally, the total cost for 1 year of therapy for pancreatogenic diabetes resulted significantly higher in TP than in PD (247 € versus 14 €; P < 0.001). In these cases, preoperative diabetes, and its relative higher cost, was significantly more frequent in patients who underwent TP than in those who underwent PD (Table 2; 35.6 versus 16.8 %; P = 0.002). In addition, approximately half of the patients who underwent PD developed diabetes and approximately ¼ required insulin therapy.
Finally, in a search of the English literature regarding TP and PD, a real cost-effectiveness analysis for TP compared with PD is lacking. This type of analysis, reporting not only the total cost of the procedures but also the effectiveness of the treatment, would be very useful in giving a proper and complete picture of the two procedures. Thus, considering the costs and the quality of life of the patients in the two groups, the cost-effectiveness analysis was in favor of patients who underwent TP with respect to those who underwent PD. In fact, the higher cost for the treatment of pancreatogenic diabetes of the patients who underwent TP was clearly compensated by a lower cost regarding hospital stay, even if complications occurred with respect to PD. The maximum raw cost of the hospital stay was much higher for PD patients than for TP patients (92,013 € versus 37,647 €). Thus, the cost of PD was very difficult to estimate preoperatively while the cost of TP was more predictable. Similarly, even if the mean QoL of the two procedures was similar, the range of values was greater in PD (−0.393–1) than in TP (0.345–1). Therefore, TP was more frequently cost-effective with respect to PD because it was more often less expensive and resulted in a better health status.
This study has some limitations in relation to the small sample size, to the heterogeneity of the two groups of patients and to the fact that it was a single center study with a non-randomized design.
In conclusion, despite the limitations, the present study yielded some new information regarding TP by its comparison with PD. In fact, it seems reasonable to suggest that total pancreatectomy can be considered not only as safe and feasible (similar postoperative outcomes) as PD but also efficacious (similar overall survival, quality of life, and impact of diabetes) and acceptable in terms of cost-effectiveness with costs and quality of life more frequently predictable than PD. Thus, for a good indication, total pancreatectomy is justified because mortality and morbidity are equals to pancreaticoduodenectomy and quality of life thereafter is acceptable. Finally, despite the high incidence of postoperative pancreatogenic diabetes in patients who underwent total pancreatectomy, it is easily manageable with acceptable disadvantages and costs. Regarding the extension of the indications for TP (frail, high risk patients, vascular reconstruction), further prospective, comparative studies involving large cohorts of patients are necessary.
References
Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RA, Edil BH and Pawlik TM. Total Pancreatectomy for Pancreatic Adenocarcinoma. Evaluation of Morbidity and Long-Term Survival. Ann Surg 2009;250:282–287.
Casadei R, Monari F, Buscemi S, Laterza M, Ricci C, Rega D, D’Ambra M, Pezzilli R, Calculli L, Santini D, Minni F. Total pancreatectomy: indications, operative technique, and results. A single centre experience and review of literature. Updates Surg, 2010;62:41–46.
Nikfarjam M, Low N, Weinberg L, Chia PH, He H and Christophi C. Total pancreatectomy for the treatment of pancreatic neoplasms. ANZ J Surg, 2014;84:823–826.
Hartwig W, Gluth A, Hinz U, Bergmann F, Spronk PER, Hackert T, Werner J, and Buchler MW. Total Pancreatectomy for Primary Pancreatic Neoplasms. Renaissance of an Unpopular Operation. Ann Surg 2014;00:1–10
Johnston WC, Hoen HM, Cassera MA, Newell PH, Hammill CW, Hansen PD, Wolf RF. Total pancreatectomy for pancreatic ductal adenocarcinoma: review of the National Cancer Data Base. HPB (Oxford). 2016;18:21-28)
Casadei R, Ricci C, Monari F, Laterza M, Rega D, D’Ambra M, Pezzilli R, Buscemi S, Minni F. Clinical Outcome of Patients Who Underwent Total Pancreatectomy. Pancreas. 2010;39:546-547
Roberts KJ, Blanco G, Webber J, Marudanayagam R,. Sutcliffe RP, Muiesan P, Bramhall SR, Isaac J, Mirza DF. How severe is diabetes after total pancreatectomy? A case-matched analysis. HPB 2014;16:814–821.
Watanabe Y, Ohtsuka T, Matsunaga T, Kimura H, Tamura K, Ideno N, Aso T, Miyasaka Y, Ueda J, Takahata S, Igarashi H, Inoguchi T, Ito T, Tanaka M. Long-Term Outcomes After Total Pancreatectomy: Special Reference to Survivors’ Living Conditions and Quality of Life. World J Surg, 2015;39:1231–1239.
Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, Muiesan P, Mirza D. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB 2015;17:416–421).
Alexakis N, Sutton R, Neoptolemos JP. Surgical treatment of pancreatic fistula. Dig Surg 2004;21:262–274.
Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg 2004;21:54–59.
Melloul E, Raptis DA, Clavien PA, Lesurtel M. Poor level of agreement on the management of postoperative pancreatic fistula: results of an international survey. HPB 2013;15:307–314.
McMillan MT, Vollmer CM. Predictive factors for pancreatic fistula following pancreatectomy. Langenbecks Arch Surg 2014;399:811–824.
Roberts KJ, Sutcliffe RP, Marudanayagam R, Hodson J, Isaac J, Muiesan P et al. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy. A UK Multicenter Study. Ann Surg 2014;00:1–7
Casadei R, Ricci C, Taffurelli G, D’Ambra M, Pacilio CA, Ingaldi C, Minni F. Are there preoperative factors related to a “soft pancreas” and are they predictive of pancreatic fistulas after pancreatic resection? Surg Today. 2015;45:708-714.
McMillan MT, Vollmer CM, Asbun HJ, Ball CG, Bassi C, Beane JD, et al. The Characterization and Prediction of ISGPF Grade C Fistulas Following Pancreatoduodenectomy. J Gastrointest Surg 2016;20:262–276
Self-Reported Population Health. An International Perspective based on EQ-5D. Eds. Szende A, Janssen B, Cabases J. London: Springer; 2014.
Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyzes. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1339-1341.
Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106 8.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-213
Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13.
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy haemorrhage (PPH)-An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25.
Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health Econ. 2010;19(3):316-333.
Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, Breisch-Girbig D, Ceyhan GO, Buchler MW. Is There Still a Role for Total Pancreatectomy? Ann Surg 2007;246: 966–975.
Bhayani NH, Miller JL, Ortenzi G,. Kaifi JT, Kimchi ET, Staveley-O’Carroll KF, Gusani NJ. Perioperative outcomes of pancreaticoduodenectomy compared to total pancreatectomy for neoplasia. J Gastrointest Surg. 2014;18:549-554.
Epelboym I, Winner M, DiNorcia J, Lee MK, Lee JA, Schrope B, Chabot JA, Allendorf JD. Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J Surg Res. 2014;187:189-196.
Satoi S, Murakami Y, Motoi F, Uemura K, Kawai M, Kurata M, Sho M, Matsumoto I, Yanagimoto H, Yamamoto T, Mizuma M, Unno M, Hashimoto Y, Hirono S, Yamaue H, Honda G, Nagai M, Nakajima Y, Shinzeki M, Fukumoto T, Kwon AH. Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg. 2015;19:6-14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Authorship
All the authors have participated sufficiently in the work according the guidelines of the International Committee of Medical Journal Editors (ICMJE).
Rights and permissions
About this article
Cite this article
Casadei, R., Ricci, C., Taffurelli, G. et al. Is total pancreatectomy as feasible, safe, efficacious, and cost-effective as pancreaticoduodenectomy? A single center, prospective, observational study. J Gastrointest Surg 20, 1595–1607 (2016). https://doi.org/10.1007/s11605-016-3201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3201-4