Abstract
The use of adequate fluid therapy during cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) remains controversial. The aim of the study was to assess whether the use of fluid therapy protocol combined with goal-directed therapy (GDT) is associated with a significant change in morbidity, length of hospital stay, and mortality compared to standard fluid therapy. Patients American Society of Anesthesiologists (ASA) II–III undergoing CRS and HIPEC were randomized into two groups. The GDT group (N = 38) received fluid therapy according to a protocol guided by monitored hemodynamic parameters. The control group (N = 42) received standard fluid therapy. We evaluated incidence of major complications, total length of hospital stay, total amount of fluids administered, and mortality rate. The incidence of major abdominal complications was 10.5 % in GDT group and 38.1 % in the control group (P = 0.005). The median duration of hospitalization was 19 days in GDT group and 29 days in the control group (P < 0.0001). The mortality rate was zero in GDT group vs 9.5 % in the control group (P = 0.12). GDT group received a significantly (P < 0.0001) lower amount of fluid (5812 ± 1244 ml) than the control group (8269 ± 1452 ml), with a significantly (P < 0.0001) lower volume of crystalloids (3884 ± 1003 vs 68,528 ± 1413 ml). In CRS and HIPEC, the use of a GDT improves outcome in terms of incidence of major abdominal and systemic postoperative complications and length of hospital stay, compared to standard fluid therapy protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is indicated in primary and secondary peritoneum neoplasm. It consists of almost complete removal of the peritoneal surface, multiple visceral resections, a variable number of intestinal anastomosis, followed by perfusion of chemotherapy inside the abdominal cavity, for 90 min at 42 °C.1 , 2 The wide extent of surgical resection and physicochemical trauma of the HIPEC alters capillary permeability, resulting in tissue damage and facilitating abdominal and systemic complications with postoperative morbidity and mortality ranging from 22 to 41 % and from 2 to 5 % respectively, with a significant increase in hospitalization time.3 , 4 Proper management of perioperative fluid therapy during CRS and HIPEC remains controversial. Permissive infusion regimen was proposed in the past5 , 6 to counteract fluid, blood, and protein losses; however, it excessively exposes the patient to the risk of fluid overload, tissue edema, and severe abdominal complications. On the other hand, the use of restrictive infusional regimens may expose the patient to hemodynamic instability, determining tissue hypoperfusion, organ damage, and worsen the nephrotoxic chemotherapy drug effect. Clinical evidence suggests that goal-directed fluid therapy (GDFT) is associated with a significant reduction of intestinal and systemic complications and improvement of prognosis in major abdominal surgery.7 – 12 The aim of our study was to assess whether the use of a protocol of fluid therapy combined with GDT may be associated with a significant change in outcome, compared to standard fluid therapy protocol. The primary endpoint was the incidence of major abdominal complications (anastomotic dehiscence, enteric fistulae, intestinal perforation, abdominal abscesses); secondary endpoints consisted in the incidence of systemic complications, duration of hospital stay, and mortality.
Materials and Methods
The study was approved by the ethics committee of the Regina Elena National Cancer Institute, Rome, Italy, with registration number 89/10; the procedures followed were in accordance with the Declaration of Helsinki 1975, revised Hong Kong 1989. After having obtained the written informed consent, all consecutive ASA II–III patients with peritoneal carcinomatosis, candidates for peritonectomy and HIPEC, who met the inclusion criteria, were enrolled in the period between June 2010 and September 2012. Patients under the age of 18 and patients with hemodynamically significant aortic regurgitation and heart rhythm disorders were excluded from the study. This is a single-center, prospective, randomized study. An operator who is not directly involved in the study randomly divided patients into two treatment groups. This process of randomization was carried out according to specific dedicated software, developed in-house, by GW Basic programmer, which generated an assignment code verified immediately before inducing anesthesia. A blinded observer recorded the outcomes. One group received fluid therapy according to therapy targeted at optimizing monitored hemodynamic parameters (GDT group); the other group received fluid therapy (control group) in accordance with a standardized protocol. In the GDT group, the target was identified in maintaining the minimum cardiac index (CI) threshold value, assessed using the FloTrac/Vigileo system, and according to a specific treatment protocol. In all patients, the same operating team performed the surgical procedure, and general anesthesia was carried out in accordance with a standard protocol.
The cytoreductive technique consisted of a total peritonectomy (parietal and visceral), omentectomy, and any multiple intestinal resections associated with hysteroannessiectomy, splenectomy, and caustic of nodules of carcinomatosis on the hepatic capsule and on the bowel loops.
The aim of the surgical resection was to achieve cc0/cc1, which is a non-visible macroscopic residual tumor or microscopic residual tumor up to 2.5 mm.13
When this was not feasible, patients were not eligible to undergo the phase of perfusion and were therefore not included in the study. One week before surgery, patients underwent staging with videolaparoscopy to verify whether surgery was feasible: Those candidates who were not eligible for HIPEC were those who presented an extension of carcinomatosis thus preventing feasibility of surgical resection cc0/cc1. The cytoreductive phase was followed by perfusion chemotherapy, carried out within the abdominal cavity for 90 min at 42 °C. Patients in both groups were premedicated with midazolam 0.01 mg/kg IV, and general anesthesia was induced with fentanyl 2–5 mcg/kg, propofol 1.5 to 2 mg/kg, cisatracurium 0.07 mg/kg IV. After tracheal intubation, anesthesia was maintained with a mixture of sevoflurane/O2/air; the sevoflurane was adjusted to maintain an end-tidal sevoflurane of 1.4–2.5 vol%, with positive pressure ventilation in volumetric 37 mmHg and a tidal volume of 8–10 ml/kg.
Boluses of fentanyl were administered according to anesthetic requirements and hemodynamic changes. A continuous infusion of cisatracurium 0.06–0.12 mg/kg/h was maintained.
All patients were managed according to standard monitoring of care which involves the continuous evaluation of electrocardiograms, heart rate (HR), continuous arterial blood pressure (ABP) measurement, pulse oximetry (SpO2), body temperature, hourly dieresis, and inspired and expired gas.
In the control group, the fluid therapy regime was mainly restrictive, according to basal infusion of crystalloid variable from 4 to 10 ml/kg/hour.7 , 8 Mean arterial pressure (MAP) was maintained at values between 65 and 95 mmHg. It was possible to administer boluses of colloids (hydroxyethyl starch (HES) 130/0.4) of 250 ml in 15 min and infuse inotropic agents (dopamine) if CVP was ≤15 mmHg or if diuresis was ≤1 ml/kg/h or if MAP was ≤70 % of preinduction.
In the GDT group, the FloTrac/Vigileo system (Edwards Lifesciences, Irvine, CA, USA, software version 1.14) was applied in all patients in order to continuously monitor CI, indexed stroke volume (SVI), and stroke volume variation (SVV). The CI was maintained at values greater than or equal to 2.5 l/min/m2. Fluid therapy protocol was mainly restrictive involving basal infusion of crystalloids at 4 ml/kg/h and boluses of colloids (HES 130/0.4) of CI < 2.5 l/min/m2, SVI < 35 ml/m2, and SVV > 15 %. In the case of CI < 2.5 l/min/m2 and SVI < 35 ml/m2 with SVV < 15 %, an infusion with dopamine was initiated (Fig. 1).
In both groups, patients were transfused with concentrated red cells for Hb values <8 mg/dl (9 mg/dl in patients with congestive heart failure or coronary heart disease). In both groups during the HIPEC (duration 90 min), fresh frozen plasma (FFP) was administered (1 U/15 min) for a total of six units, in accordance with the standardized technique applied at our institute. Diuresis was maintained at values equal to or greater than 120 ml/15 min; the administration of diuretics (furosemide) was free up to a maximum of 250 mg.
At the end of the operation, patients were extubated in the operating theater and transported to the intensive care unit (ICU), where depending on the their clinical conditions whether stabile and normal, returned to their original hospital ward. In the postoperative period, the same standardized regimen of fluid therapy was applied to both groups: Infusion solutions through total parenteral nutrition (TPN) up to 3000 ml/day was administered and gradually converted into enteral nutrition (EN) after 5–7 days, starting 10 ml/h to achieve the dose of 60 ml/h, until mouth feeding resumed.
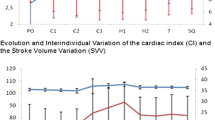
The main hemodynamic parameters were recorded in all patients at different times (T0: induced anesthesia; T1: 30 min from the beginning of HIPEC, T2: 30 min after the end of surgery) (ABP, HR, SpO2, hourly diuresis). In the GDT group, the CI, SVI, and SVV were measured. At the end of surgery, readings in both groups were taken of the total amount of fluids administered, their breakdown (crystalloid/colloid), the total number of colloid boluses administered, and the use of diuretics and inotropic agents.
In both groups, we evaluated the incidence of major abdominal and systemic complications that occurred within 30 days, as well as the total duration of hospital stay and mortality up to 30 days. The incidence of postoperative complications was rated by anesthesiologists who were not involved in the intraoperative management of patients. The major abdominal complications were anastomotic leakage, enteric fistulas, perforation, and abdominal abscesses (confirmed by computerized tomography); systemic complications were divided into cardiac (electrocardiographic signs or laboratory data of myocardial infarction, angina, or arrhythmia), hepatic (persistent alteration in hepatic function tests including bilirubin, prothrombin time, ammonia concentration, aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase), respiratory (X-ray findings of airspace or interstitial opacity, lobar consolidation, or pleural effusions; severe respiratory failure requiring respiratory support), and renal (oliguria with urine output < 0.5 ml/kg/h for more than 4 h, creatinine increase > 30 % of preoperative values, dialysis). Furthermore, the incidence of readmission to the ICU was also assessed.
Statistical Analysis
The study was designed as a two-arm parallel prospective, randomized trial. The primary endpoint of the study was the occurrence rate of abdominal complications (dehiscence of anastomosis, intestinal perforation, abdominal abscesses). The number of patients required for the study was determined by assuming that in the standard arm, the rate of abdominal complications was equal to 40 %4 and assuming that GDT could reduce this by 30 % in absolute value, setting the significance level to 5 % and the power to 80 %, a sample size of 80 patients was identified. The analysis was carried out on the population “per protocol”. The Fisher’s exact test was used to assess the primary endpoint and measure the association between categorical variables. Once the assumption of normality with the Kolmogorov-Smirnov test was verified, the continuous variables were compared using the Student’s t test. The Kaplan-Meier method was used to estimate the duration of hospitalization, and the difference between the two curves was evaluated by the log-rank test. All P values were two-sided. IBM SPSS Statistics version 20.0 was used to implement the statistical analyses.
Results
The flowchart of the patients who participated in the study is shown in Fig. 2. Eighty patients in total were randomized: 42 patients were assigned to the control group and 38 to the GDT group. The groups were similar in age, sex, weight, comorbidity, type and duration of surgery, ASA classification, and cancer type (Table 1). The intraoperative hemodynamic data recorded is shown in Table 2. The incidence of major abdominal complications in the experimental group was 10.5 % (95 % CI: 4.1–24.1) and significantly lower than that of the control group, which was equal to 38.1 % (P = 0.005, 95 % CI: 25.0–53.2), with an absolute risk reduction of 27.6 % (95 % CI: 8.7–43.9). The median duration of hospitalization was 29 days (95 % CI: 25–33) in the control group and 19 days (95 % CI: 17–21) in the GDT group (P < 0.0001, log-rank test) (Fig. 2). The mortality was 4 out of 42 patients (9.5 %, 95 % CI: 3.8–22.1), in the control group (3 patients developed multiple organ dysfunction syndrome (MODS) resulting from abdominal abscess; 1 patient had MODS resulting from anastomotic leakage), and no deaths occurred in the GDT group (P = 0.12). The number of patients who developed at least one complication in the postoperative period was significantly lower (P < 0.0001) in the GDT group (10 in 38 patients; 26.3 %, 95 % CI: 15.0–42.0) compared to the control group (39 in 42; 92.9 %, 95 % CI: 81.0–97.5). The incidence of specific systemic complications in the two groups is shown in Table 3. The incidence of readmission to the ICU was 11.9 % (95 % CI: 81.0–97.5) in the control group and 0 % in the GDT group (P = 0.05). The total amount of fluids administered intraoperatively was significantly higher in the control group compared to the GDT group (8269 ± 1452 vs 5812 ± 1244 ml; P < 0.0001). A larger volume of crystalloids was administered intraoperatively in the control group than in the GDT group (6852 ± 1413 vs 3884 ± 1003 ml; P < 0.0001). On the other hand, a slightly higher amount of colloids was used in the GDT group compared to the control group (1927 ± 318 vs 1417 ± 279 ml; P < 0.0001) (Table 4). Lactate levels at the end of surgery were significantly higher in the control group compared to the GDT (1.94 ± 0.77 vs 2.66 ± 1.25 mmol/l; P = 0.003). In the GDT group, 30 (78.9 %) patients were receiving intraoperative furosemide, whereas there were 0 in the control group (P < 0.0001). There were no significant differences in urine output, blood loss, and number of patients receiving red blood cells or inotropes (Table 3).
Patient flow throughout the study. HIPEC hypertermic intraperitoneal chemotherapy. *Deterioration of the patient’s clinical condition 1Supervening change in heart rate (sustained sinus tachycardia) which has required use of cardioactive drugs not covered by the protocol 2Supervening change in heart rate (prolonged bradycardia) which has required use of cardioactive drugs not covered by the protocol
Discussion
In our study, GDT was confirmed as an effective method for reducing the length of hospital stay and postoperative complications in patients undergoing CRS and HIPEC compared to conventional fluid therapy treatment, as reported in previous experiences in traditional surgery.8 – 11 Moreover, the mortality rate decreased and is not considered statistically significant. In CRS with HIPEC extensive surgical trauma, important exposure of the viscera and long duration of the procedure may grow to a considerable size and predispose the patient to a state of hypovolemia and hemodynamic instability during the subsequent phase of the HIPEC.12 Furthermore, the administration of potential nephrotoxic chemotherapeutic drugs imposes the need to adopt adequate strategies of renal protection, avoiding dehydration. However, CRS and HIPEC procedure determines important and sometimes conflicting pathophysiological dynamics; the limit between hypovolemia and hypervolemia is very subtle and may change during surgery. Tissues, in fact, are due to prolonged mechanical, thermal, and chemical damage associated with the procedure and are consequently particularly prone to developing interstitial edema, even in hypovolemic conditions.
Under these circumstances, proper management of balancing fluid plays a critical role through maintaining optimal blood volume during the different phases in order to ensure an adequate supply of oxygen to the tissues,14 while avoiding states of over hydration.
In our study, the CI was used as a target for the GDT. Although the oxygen delivery index (DO2I) is, according to many authors, the reference target, its determination requires a calculation obtained through repeated blood gas sampling and cannot be easily applied during surgery. The FloTrac/Vigileo system allows the continuous determination of cardiac output by analyzing the wave morphology of the arterial pulse, and its easy use is particularly indicated in the operating room. The continuous detection of CI can be considered a valid surrogate of the DO2I, and its use as a hemodynamic target constitutes a valid alternative, in combination with occasional appropriate sample of hemoglobin values and arterial saturation.
A CI of 2.5 l/min/m2, the minimum threshold value to ensure an adequate supply of oxygen to the tissues, is a conceptually different approach from what was originally proposed by Shoemaker and subsequently developed by many other authors who have used the predetermined over limit hemodynamic values of CI and DO2I as a therapeutic target.15
A GDT based on the pursuit of predetermined over limit hemodynamic values can in some circumstances be ineffective, if not harmful. It is therefore necessary to customize the GDT by identifying reference hemodynamic parameters based on the hemodynamic ability of the patient and the operating context.16 A GDT based on achieving over limit DO2I, CI, or SVI values in CRS and HIPEC operations necessarily involves the infusion of large quantities of liquids or the administration of high doses of inotropes, thus exposing patients to the risk of an overload in fluids or abnormal rhythms that are difficult to sustain.
Our findings show a reduction in mortality and impressive absolute reductions in complications and hospital length of stay in intervention group. The GDT group was managed by personalized treatment protocols adapted to the type of surgery; the hemodynamic variations were recorded in real time, allowing action to be taken before the resulting tissue alterations. In control group, although there were predefined protocols for fluid administration, management protocol did include clinical criteria of suspicion of persistent hypovolemia based on evidence. This management may lead to delay or inaccuracies in the decisions made regarding fluid therapy in the control group.
Moreover, in our study, dopamine was administered mainly in the GDT group. There is considerable evidence to demonstrate the benefits of increasing oxygen delivery in high-risk surgical patients during the perioperative period.17 To achieve adequate tissue, oxygenation boluses of fluid may not be sufficient. The use of inotropes and vasopressors has been shown to be vital when integrating GDT protocols.15 In particular, dopamine, a positive inotropic agent and splanchnic and renal vasodilator, may play a crucial role in achieving tissue oxygenation.9 This could have contributed to a better outcome than the control group. This is also confirmed by the significant increase in the level of lactate at the end of surgery in the control group, since the concentration of lactate is the expression of a critical decrease in tissue oxygenation and is inversely proportional to the values of DO2.18
However, our clinical trial is a small single-center trial, and although the two groups were randomized and the differences between the two groups are not statistically significant, it remains difficult for small samples to conclude that the result is related to the anesthetic procedure rather than the imbalance between the groups.
In the control group, a higher volume of crystalloids was used compared to the GDT group. Hypovolemia may be associated with prolonged periods of tissue hypoperfusion; on the other hand, the effect on the increased blood volume by crystalloids is limited in time and results in interstitial edema at the level of intestinal anastomoses.19 Similar to our results, Brandstrup, Lobo, and Nisanevich in three separate studies concerning perioperative fluid therapy in patients undergoing major abdominal surgery have reported a lower incidence of postoperative complications in patients where a system of restrictive fluid therapy was used.20 – 22 Nevertheless, in recent years, the question “liberal” vs “restrictive” fluid therapy seems to have turned into a “balanced” vs “imbalanced”,23 with some recent studies that do not show a reduction in risk, in terms of complications and length of stay, of the restrictive fluid therapy in major abdominal surgery.24 , 25 Therefore, during chemotherapic perfusion, the crucial point seems to be avoiding over hydration and optimizing fluid balance by targeting cardiac output.26 , 27
In this particular context, maintaining a balance in fluid with flexible and individualized volume replacement seems to be the more rational approach. It is evident that the patterns of fixed volume do not give due consideration to individuals and very important differences, especially changes in blood volume that are very frequent during CRS and HIPEC.28 , 29 The fluid therapy protocol we adopted involved the basal infusion of crystalloids with the boluses of colloids for CI < 2.5 l/min/m2 and SVV > 15 % explained by the need to avoid transient and unrecognized peripheral hypoperfusion.
In the study, SVV was used as an index for preload and to identify a state of relative hypovolemia. Several authors have demonstrated the utility of dynamic filling indices for determining the responsiveness to the volemic load.30 , 31 In our experience, a value of SVV > 15 % has been identified as a threshold value for administering a bolus of colloids. This limit is slightly higher than other values identified in previous studies. The SVV values that identify the responsiveness to the volemic load vary in different cases from 9.6 to 12 % depending on the efficacy parameters considered and the type of surgery. In the course of peritonectomy and HIPEC, surgical, thermal, and chemical stress prepares for tissue edema as well as small fluid overloads. We therefore felt that the identification of a SVV threshold for the colloid bolus infusion that was slightly higher than that identified in similar experiences was appropriate, which protects the patient from the risk of relative hypervolemia. Maintaining adequate CI values, even under conditions of moderate water restriction, can be obtained alternatively by administering moderate doses of inotrope.
Our experience agrees with the results of other studies that correlate GDT using colloids with a lower incidence of postoperative complications (especially bowel) in high-risk patients undergoing major abdominal surgery.31 – 33 In fact during peritonectomy and HIPEC, it is particularly important to maintain a good oncotic pressure due to the massive loss of protein due to the surgical procedure. We must consider that colloids allow a volume replacement of about 90 %, while the increase of intravascular volume of crystalloids is less of 20 %. This can contribute to interstitial edema with damage to the renal tubular cells, so the renal damage may be greater for hypovolemia, tissue hypoxia, or cellular edema rather than to the administration of colloids. At present, it is difficult to establish whether a beneficial effect of colloid exists (systemic effects of the improvement in the patient’s hemodynamic profile, effects on the regional intestinal flow, or a combination of both), especially considering the controversial events related to the regulation of the use of HES in Europe. However, after a precautionary ban on the use, the European Community concluded the risk-benefit ratio favorable for HES in the treatment of acute hypovolemia, giving specific contraindications. Limitations in our study were the sample size and the lack of blinding for operating team.
Conclusion
In patients undergoing peritonectomy with HIPEC, the use of a restrictive fluid therapy regimen combined with a GDT aimed at maintaining the minimum threshold of CI greater than or equal to 2.5 l/min/m2 improves outcome in terms of the incidence of major abdominal and systemic postoperative complications and length of stay compared to a standard fluid therapy protocol; the incidence of mortality is decreased and is not statistically significant.
References
Sugarbaker PH: Peritonectomy procedures. Ann Surg 1995; 221:29-42.
Esquivel J, Sticca R, Sugarbaker P et al.; Society of Surgical Oncology Annual Meeting: Cytoreductive surgery and hypertermic intraperitoneal chemiotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Onc 2007;14:128-133
Gusani NJ, Cho SW, Colovos C, et al: Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high volume tertiary cancer center. Ann Surg Oncol 2008; 15: 754-763
Baratti D, Kusamura S, Laterza B, Balestra MR, Deraco M: Early and long term postoperative management following cytoreductive surgery and hypertermic intraperitoneal chemotherapy. World J Gastrointest Oncol 2010;2:36-43.
Esquivel J, Angulo F, Bland R, Stephens A D, Sugarbaker PH: Hemodynamic and cardiac function parameters during heated intraperitoneal chemotherapy using the open coliseum technique. Ann Surg Onc 2000; 74:296-300.
Kanakoudis F, Petrou A, Michaloudis D, Chortaria G, Konstantinidou A: Anaesthesia for intra-peritoneal perfusion of hypertermic chemotherapy. Anaesthesia 1996; 51:1033-1036.
Raspe C, Piso O, Wiesenack C, Buchera M: Anesthetic management in patients undergoing hyperthermic chemotherapy. Curr Op Anesthsiol 2012; 25:348-55
Donati A, Loggi S, Preiser JC, et al Goal directed intraoperative therapy reduces morbidity and length of hospital stay in high risk surgical patients. Chest 2007; 132:1817-1824.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennet ED: Early goal directed therapy after major surgery reduces complication and hospital stay. A randomized controlled trial. Crit Care 2005; 9: R687-R693.
Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Michard F: Goal-directed fluid management based on pulse pressure variation monitoring during high risk surgery: a pilot randomized trial. Crit Care 2007; 11:R100.
Giglio MT, Marucci M, Testini M, Brienza N: Goal-directed haemodynamic therapy and gastrointestinal complication in major surgery: a meta-analysis of randomized controlled trial. Br J Anaesth 2009; 103:637-646.
Shime N, Lee M, Hatanaka T: Cardiovascular changes during continuous hypertermic peritoneal perfusion. Anesth Analg 1994; 78:938-942.
Sugarbaker PH: Peritonectomy procedures. Surg Oncol Clin N Am 2003;12:703–727.
Lees N, Hamilton M, Rhodes A: Clinical review: Goal directed therapy in high risk surgery patients. Crit Care 2009; 13:231.
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lees TS: Prospective trial of supranormal values of survivor as therapeutic goals in high risk surgical patients. Chest 1988, 94:1176-1186.
Mackenzie SJ: Should perioperative management target oxygen delivery? Br J Anaesth 2003; 91:615-618.
Lugo G, Arizpe D, Domínguez G, Ramírez M, Tamariz O Relationship between oxygen consumption and oxygen delivery during anesthesia in high-risk surgical patients. Crit Care Med. 1993 Jan; 21(1):64-9.
EP Rivers, M Katranji, KA Jaehne, S Brown et al. Early intervention in sever sepsis and septic shock: a review of the evidence one decade later. Minerva Anestesiol. 2012 Jun;78:712-24
Jafari MD, Halabi WJ, Stamos MJ, Nguyen VQ. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg. 2014 Feb 1;149:170-5.
Brandstrupp B, Tønnesen H, Beier-Holgersen R, et al. Effect of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens. Ann Surg 2003; 238: 641-648.
Lobo S, Ronchi L, Oliveira N, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high risk surgery. Crit Care 2011; 15:R226.
Nisavenich V, Felsenstein I, Almogy G, Weissman C, et al.: Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005; 103:25-32.
Boland MR, Noorani A, Varty K, Coffey JC, et al. Perioperative Fluid Restriction in Major Abdominal Surgery: Systematic Review and Meta-analysis of Randomized, Clinical Trials World J Surg 2013; 37:1193–1202
Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010; 69:488–498
Abraham-Nordling M, Hjern F, Pollack J, Prytz M, et al. Randomized clinical trial of fluid restriction in colorectal surgery. Br J Surg 2012; 99:186–191
Nygren J, Thacker J, Carli F, Fearon KC et al. Guidelines for Perioperative Care in Elective Rectal/Pelvic Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations World J Surg 2013;37: 285–305
Lassen K, Marielle M, Coolsen E, Slim K, et al. Guidelines for Perioperative Care for Pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS) Society Recommendations World J Surg 2013;37; 240–25
Schmidt C, Moritz S, Rath S, et al: Perioperative management of patients with cytoreductive surgery for peritoneal carcinomatosis. J Surg Oncol 2009; 100: 297-301
Hadian M, Pinsky MR: Functional hemodynamic monitoring. Curr Opin Crit Care 2007;13:318-323
Benes J, Chytra I, Altmann P, et al: Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010; 14:R118
Chowdhury AH, Lobo DN: Fluids and gastrointestinal function. Curr Opin Clin Nutr Metab Care 2011;14:469-476.
Hiltebrand L, Kimberger O, Arnberger M, Brandt S, Kurz A, Sigurdsson GH: Crystalloids versus colloids for goal directed therapy in major surgery. Crit Care 2009;13: R40.
Kimberger O: Goal directed colloid administration improves the microcirculation of healthy and perianastomotic colon. Anesthesiology 2009; 110:496-450
Acknowledgments
Departmental funding only.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colantonio, L., Claroni, C., Fabrizi, L. et al. A Randomized Trial of Goal Directed vs Standard Fluid Therapy in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. J Gastrointest Surg 19, 722–729 (2015). https://doi.org/10.1007/s11605-015-2743-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2743-1