Abstract
Background
Accurate detection of colorectal liver metastasis is paramount in the role of management. This study aims to compare magnetic resonance imaging (MRI) with gadoxetate disodium (a hepatocyte-specific agent—Eovist®) to triple-phase enhanced computed tomography in detecting colorectal liver metastases.
Methods
A retrospective chart analysis of 30 patients from 2011 to 2013 with colorectal liver metastases was performed. Patients with more than 6 weeks or two cycles of chemotherapy between the two imaging modalities were excluded. The number of lesions identified on triple-phase enhanced computed tomography vs. MRI with Eovist® was compared.
Results
Of the 30 patients that met the inclusion criteria, 12 (40 %) patients had more lesions identified on MRI with Eovist® compared to triple-phase enhanced computed tomography. Eighteen (60 %) had no change in the number of lesions identified. When MRI with Eovist® detected more lesions, the mean number of additional lesions detected was 1.5. Eovist® MRI changed the surgical management in 36.7 % of patients.
Conclusion
MRI with Eovist® is superior to enhanced computed tomography in identifying colorectal liver metastases. The increased number of lesion identified on MRI with Eovist® can profoundly change the surgeon’s management. It should be considered the “imaging modality of choice” in preoperative imaging for liver metastases in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most common cancer in the USA, with annual incidence of one million cases across the world.1 Approximately 50 % of patients with colorectal cancer present with liver metastases at the time of diagnosis or as a result of recurrent disease.1 Liver resection remains the gold standard for those patients that are deemed possible surgical candidates; this offers the best chance of a cure.1 Imaging plays a key role in patient evaluation and in preoperative selection, for those patients that may be candidates for liver surgery. The modalities currently available for preoperative assessment include ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), 18-fluorodeoxyglucose positron emission tomography (FDG PET), and integrated PET-CT.1 CT scan of the chest, abdomen and pelvis is most commonly used for staging colorectal cancer, especially for assessment of liver metastases. Enhanced CT scan has a 69 to 71 % sensitivity and 86 to 91 % specificity, and MRI has a 81 to 86 % sensitivity and 93 % specificity for detecting liver metastasis.2 However, with the recent introduction of liver parenchyma-specific contrast agents, assessment of liver metastases with MRI is being recommended more commonly.3 Mangafodipir trisodium (MT) was the first liver-specific contrast agent to enhance the T1-weighted images used in the detection, localization, and characterization of liver lesions.4 However, this agent had limited assessment of vascular structures due to its inability to be administered as a bolus due to hypersensitivity reactions. Agent was taken off the market in the year 2005 due to concerns of toxicity related to neurological symptoms and liver toxicity.3 Gadobenate dimeglumine (Gd-BOPTA) was the second liver-specific contrast agent approved in 2004. Gd-BOPTA acts as both an extracellular agent as well as a hepatobiliary contrast agent.3 Three percent to 5 % is taken into the hepatocytes and excreted into the biliary system.3 While it is approved in Europe, it is used off label in the USA.3 The newly approved contrast agent gadoxetate disodium Gd-EOB-DPTA (Eovist®, Bayer HealthCare Pharmaceuticals) is a gadolinium-based formulation, which has both extracellular and hepatocyte-specific properties.4 Approximately, 50 % of the Eovist® is renally excreted and the other 50 % is actively transported into the hepatocytes then excreted into the biliary system.4 Initial studies using gadolinium-based contrast agents showed advantages of MRI over CT, but with some mixed results.3 The aim of this study was to compare MRI with Eovist® to CT in detecting colorectal liver metastases and to determine if this can further change surgical management.
Methods
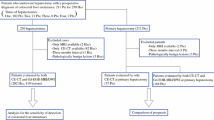
All patients referred to HPB service with the diagnosis of colorectal cancer and liver metastases (CRLM) between 2011 and 2013 were included in this retrospective study. Data on demographic and clinical characteristics including age, gender, primary tumor stage, CT scan and MRI imaging findings of liver metastases, initial treatment, and change in the treatment plan was abstracted after obtaining institutional review board approval. Colorectal liver metastases characteristics for number of lesions and size differences were compared between CT scan and MRI. Inclusion criteria were as follows:
-
1.
Patients with CRLM
-
2.
Patients who has good triple phase CT (TPCT) and MRI with Eovist® at our institution
-
(a)
A triple-phase CT scan consists of a non-contrast phase, arterial phase, portal venous phase, and delayed phase. A 40-slice CT scanner was used. The slice thickness in the non-contrast phase is 5 mm, arterial phase is 3 mm with contrast timing 15–30 s, portal venous phase 5 mm with contrast timing 70–90 s, and the delayed phase 5 mm with contrast timing 3 min. This is considered a high-quality triple-phase CT scan at our institution and comparable to our MRI with Eovist. The quality of our institutional CT scans followed the protocol of the American College of Radiology CT Accreditation program. Radiologist at our institution have also concurred that the protocol mentioned above is a high-quality CT scan that is comparable to MRI
-
(b)
Pre-contrast MRI followed by the administration of Eovist with MRI performed immediately (the “dynamic” phase) and at 10 to 20 min following EOVIST administration (the “hepatocyte” phase)
-
(a)
-
3.
Patients who had less than 6 weeks or two cycles of chemotherapy, between the two imaging modalities
Five different radiologists had reviewed both CT scan and MRI. Usually, the same radiologist reviewed both studies; therefore, they were not blinded to previous studies. One radiologist did read 50 % of the cases. This does lead to some bias; however, one surgeon looked at all studies and with any discrepancy, we have the same radiologist or second radiologist review both CT and MRI again.
Sensitivity for liver metastases <1 and >1 cm was calculated separately for both CT scan and MRI by looking at the pathological findings. Further change in the treatment plan based on MRI findings was also calculated.
Results
A total of 30 patients (15 females and 15 males) met the criteria. The mean age of the patients was 62 SD ± 10. The total number of lesions identified by CT scan was 47 whereas it was 64 by MRI with Eovist®. Twelve (40 %) patients had more lesions identified on MRI with Eovist® whereas 18 (60 %) patients had no change in the number of lesions identified. The average number of lesions identified by CT scan was 1.56 lesions compared to 2.13 lesions on MRI with Eovist® (Table 1). When evaluated by the size of the lesions, for lesions <1 cm, CT scan identified only two lesions compared to 15 lesions detected by MRI with Eovist®. For lesions >1 cm, CT scan identified a total of 45 lesions compared to 49 lesions by MRI with Eovist® (Table 2). The average number of lesions <1 cm detected by CT scan was 0.07 lesions compared to 0.5 lesions by MRI with Eovist®, with a 614.2 % increased detection rate of lesions <1 cm by MRI with Eovist®. Similarly, the average number of lesions >1 cm detected by CT scan was 1.5 lesions compared to 1.63 lesions by MRI with Eovist®, with an 8.7 % increased detection rate of lesions >1 cm by MRI with Eovist®.
The sensitivity of enhanced CT scan was 75.8 % with 95 % CI (61.1 %; 90.5 %), and the sensitivity for MRI with Eovist® was 100 % in this study based on pathological review. There was change in treatment plan for 36.7 % (N = 11) of patients based on by MRI with Eovist® findings.
Discussion
The accurate detection of metastatic disease especially liver metastases at initial presentation or during the course of treatment of colorectal cancer remains crucial to patient management. Our study showed that 12 (40 %) patients had more lesions identified on MRI with Eovist® compared to TPCT. Eighteen (60 %) had no change in the number of lesions identified, and Eovist® MRI changed the surgical management in 36.7 % of patients with CRLM. When looking at the size of the lesions, MRI with Eovist® had a sixfold increase in detecting lesions <1 cm.
Surgical resection offers the highest survival benefit; hence, early identification of liver metastases provides the opportunity for simultaneous resection of primary tumor with the metastatectomy.5 Both CT and MRI imaging have benefited from rapid technological advances, particularly MRI imaging from the advent of newer liver-specific contrast agents.6 Eovist® is a gadolinium-based formulation, which has both extracellular and hepatocyte-specific properties.4 Approximately 50 % of the Eovist® is renally excreted, and the other 50 % is actively transported into the hepatocytes and then excreted into the biliary system.4 The liver will appear bright on T1-weighted MRI whereas non-hepatocytes (e.g., malignant metastases) will not take up the agent and appear dark.4 Eovist® is also characterized by a high level of relaxivity (the ability of magnetic compounds to increase the relaxation rates of the surrounding water proton spins), which determines how bright the contrast agent will appear on a T1-weighted MRI. This elevated relaxivity allows for smaller dosing as compared to other gadolinium-based agents.4 Enhancement of the normal liver parenchyma on T1-weighted sequence peaks at approximately 20 min after injection and persists for up to 2 h, providing a wide window of opportunity to image during the hepatocyte phase.4
Liver lesions with minimal or no hepatocyte function do not show accumulation of Eovist®. These lesions include cysts, hemangiomas, metastases, and the majority of poorly differentiated hepatocellular carcinomas.7 In these cases, liver lesions will be more easily detected secondarily to increased contrast between the lesions and normal enhanced liver.3 In contrast, hepatic lesions with functioning hepatocytes as for focal nodular hyperplasia and a small percentage of well-differentiated hepatocellular carcinoma take up Eovist® on hepatic phase.7
Multiple groups have evaluated Eovist® (Gd-EOB-DTPA)-enhanced MRI for detecting liver metastases comparing them to unenhanced MRI and CT scan. The European EOB Study Group (Huppertz et al. 2004) looked at 302 lesions in 130 patients with biopsy or intraoperative ultrasound-proven focal lesions. Eighty-one of these patients had metastases from colorectal tumor primary. T1 an T2 phase MRIs, pre- and post-Gd-EOB-DPTA, were performed. Results differed in 21 of the 129 patients between the pre- and post-contrast MRI. Nineteen of which resulted in a significant (p < 0.001) difference in correct detection with Gd-EOB-DTPA. It was also showed that a 7 % increase in correct lesion characterization with Gd-EOB-DTPA compared to precontrast MRI.8
In 2006, Halavaara et al. showed superiority of Gd-EOB-DTPA MRI compared to CT. They looked at both benign and malignant lesions. They found increased lesion identification (95 vs. 89 %), sensitivity (95 vs 92 %), and specificity (94 vs. 90 %) with MRI especially when lesions less than 1 cm were considered.9 This contrasted our study which selectively looked at CRLM with specific inclusions and exclusion criteria. This was further supported by studies by Ichikawa et al. (2010) who demonstrated superiority of gadoxetic-acid-enhanced MRI over unenhanced MRI and triphasic contrast-enhanced CT for detection of lesions <2 cm related to hepatocellular carcinoma and chronic liver disease.10 Similar study by Hammerstingl et al. (2008) looked at 302 lesions and showed that the frequency of correctly detected lesions was significantly higher (10.44 %) on Gd-EOB-DTPA-enhanced MRI compare to biphasic helical CT scan.11 However, this was looking at identifying all types of lesions in the liver with no definite inclusion/exclusion criteria for timing intervals and treatment modalities between the two scans.
Two studies have compared Gd-EOB-GTPA-enhanced MRI with PET/CT. Donati et al. (2010) looked at 85 liver lesions in 29 patients. Forty-five of these were metastases from colorectal primary. When looking at these lesions as a whole, there was a significant difference in lesion detection between PET/CT and Gd-EOB-GTPA MRI (64 and 85 %, p = 0.002), respectively. Also, there was a significant difference in lesions less than 1 cm in diameter that were detected (29 and 71 % p = 0.013).12 Seo et al. (2011) compared Gd-EOB-DTPA MRI to PET/CT for the detection of liver metastases, specifically from colorectal cancer. The study retrospectively looked at 135 metastases from 68 patients. They found 25 more lesions less than 1 cm with Gd-EOB-DTPA MRI compared to PET/CT.13 More recently Chen et al. performed a met-analysis of 1900 lesions from 13 studies showing the sensitivity of Gd-EOB-DTPA-enhanced MRI for detection of liver metastases to be 93 % and specificity of 95 % for all types of lesions.14
The issue of imaging patient following neoadjuvant chemotherapy can be difficult due to the fact that these patients develop hepatic steatosis. This was evaluated by Berger-Kulemann et al. (2012); 68 metastases were evaluated with triphasic CT scan and Gd-EOB-DTPA-enhanced MRI. For lesions less than 1 cm, CT scan detected only 41.9 % while Gd-EOB-DTPA-enhanced MRI detected 93 % of the metastases (p < 0.001). All patients underwent surgical resection after evaluation.15
While there have been multiple studies looking at the sensitivity and specificities of Gd-EOB-DTPA-enhanced MRI compared to other imaging modalities as stated above. However, CT scans do remain a diagnostic tool for focal liver lesions at many institutions; we aimed to compare the diagnostic performance between the two. Our current study was quite unique that contrasted the studies mentioned above. We sought to identify the number of lesions on an Eovist®-enhanced MRI compared to enhanced triple-phase CT scan looking at only lesions related to CRLM. There were clear inclusion and exclusion criteria for each of these patients that were not seen in the previous studies mentioned above. A total of 30 patients were assessed; MRI with Eovist® identified more liver lesions in 40 % of patients, with a (614.2 %) sixfold increased detection rate of lesions <1 cm and 8.7 % increased detection rate of lesions >1 cm; and 36.7 % (N = 11) of patients had change in treatment plan based on by MRI with Eovist® findings. These changes in treatment plans for the 11 patients further need extended resection or needed ablation that was not previously planned based on CT scan. We related this to the pathological specimens (Table 3) to correlate the sensitivity of Eovist®-enhanced MRI and also determined the change in treatment plan that occurred with the MRI with Eovist®. Six patients with lesions seen on Eovist MRI and not CT were detected by pathology. Five patients had lesions seen on MRI that could not be clearly identified on pathology. All patients were shown to have the areas of concern adequately resected on post-operative follow-up imaging studies (Table 3). This data would suggest that Eovist MRI may be overly sensitive. The authors would argue that this is in fact what is needed in order to prevent unnecessary liver resection. The limitations to this study include the small sample size as well as failure to obtain final histopathological confirmation for all the lesions identified on MRI with Eovist®. Most of these patients however received neoadjuvant chemotherapy in which some of these smaller lesions had a pathological response and not identified post-operatively.
While MRI with Eovist® does show promise in superiority compared to enhanced CT as from the previous studies and our current study. Zech et al. looked at the economic considerations and performed a cost analysis comparing MRI with Eovist®, extracellular enhanced MRI, and three-phase MDCT as the initial evaluation of patients with metachronous colorectal liver metastases in Germany, Italy, and Sweden. It demonstrated that MRI with Eovist® required fewer additional imaging studies (8.6 %) than extracellular enhanced MRI (18.5 %) and CT (23.5 %).16 Although MRI with Eovist® has an initial higher cost than the other two modalities, it was in fact cost-effective when reimaging was factored in. This cost analysis can further be evaluated if we look at the unnecessary surgical procedures that can be saved with Eovist®-enhanced MRI as was identified in the study by Hammerstingl et al.11
Conclusion
In conclusion, the ideal imaging study for evaluating liver metastases would be one that provides diagnostic information which is highly sensitive and specific with low rate of false positive as well as cost-effective. The studies described above have shown that gadoxetic acid disodium-enhanced MRI offers the highest sensitivity and specificity and is safe and cost-effective. These are important aspects of preoperative planning for resection of metastatic hepatic lesions. Further, accurately mapping the location of metastatic lesions in the liver is crucial in a surgeon’s perspective that can dramatically alter surgical approach and allows for better counseling of the patient. As further studies are conducted on evaluation of Eovist®-enhanced MRI showing its superiority to other imaging modalities in the detection, localizations, characterizations, and management may become the standard of care for patients with colorectal liver metastases.
References
Mohammad WM, Balaa FK. Surgical management of colorectal liver metastases. Colon Rectal Surg 2009; 22(4): 225–232.
Choi J. Imaging of hepatic metastases. Cancer Control 2006;13(1): 6–12.
Lafaro KJ, Roumanis P, Demirjian AN, Lall C, Imagawa DK. Gd-EOB-DTPA-Enhanced MRI for detection of liver metastases from colorectal cancer. International Journal of Hepatology 2013; 2013:572307.
Leyendecker JR. Gadoxetate disodium for contrast MRI of the liver. Gastroenterology Hepatol, 2009; 5(10): 698.
Bakalakos EA, Kim JA, Young DC, Martin EW. Determinants of survival following hepatic resection or metastatic colorectal cancer. World J Surg 1998;22(4):399–404.
Sica GT, Ji H, Ros PR. CT and MR Imaging of hepatic metastases. American Journal of Roentgenology 2000; 174(3): 691–698.
Do RK, Rusinek H, Taouli B. Dynamic contrast-enhanced MRI of the liver: current status and future directions. MRI Clinics of North America 2009; 17(2): 339–349.
Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, Laniado M, Manfredi RM, Mathieu DG, Mueller D, Reimer P, Robinson PJ, Strotzer M, Taupitz M, Vogl TJ. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 2004; 230(1): 266–275.
Halavaara J, Breuer J, Ayuso C, Balzer T, Bellin MF, Blomgvist L, Carter R, Grazioli L, Hammerstingl R, Huppertz A, Jung G, Krause D, Laghi A, Leen E, Lupatelli L, Marsili L, Martin J, Pretorius ES, Reinhold C, Stiskal M, Stolpen AH. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasicCT—a multicenter trial. Journal of Computer Assisted Tomography. 2006; 30(3): 345–354.
Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K, Hirohashi S, Nishie A, Saito Y, Onaya H, Kuwatsuru R, Morimoto A, Ueda K, Kurauchi M, Breuer J. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Investigative Radiology 2010; 45(3): 133–141.
Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, Fusté LC, Heinz-Peer G, Judmaier W, Laniado M, Manfredi RM, Mathieu DG, Müller D, Mortelè K, Reimer P, Reiser MF, Robinson PJ, Shamsi K, Strotzer M, Taupitz M, Tombach B, Valeri G, van Beers BE, Vogl TJ. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. European Radiology 2008; 18(3): 457–467.
Donati OF, Hany TF, Reiner CS, von Schulthess GK, Marincek B, Seifert B, Weishaupt D. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA enhanced MRI. Journal of Nuclear Medicine 2010; 51(5): 692–699.
Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Investigative Radiology 2011; 46(9): 548–555.
Chen L, Zhang J, Zhang L, Bao J, Liu C, Xia Y, Huang X, Wang J. Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One 2012; 7(11):e48681.
Berger-Kulemann V, Schima W, Baroud S, Koelblinger C, Kaczirek K, Gruenberger T, Schindl M, Maresch J, Weber M, BaSsalamah A. Gadoxetic acid-enhanced 3.0T MR imaging versus multidetector-row CT in the detection of colorectal metastases in fatty liver using intraoperative ultrasound and histopathology as a standard of reference. European Journal of Surgical Oncology 2012; 38(8): 670–676.
Zech CJ, Grazioli L, Jonas E, Ekman M, Niebecker R, Gschwend S, Breuer J, Jonsson L, Kienbaum S. Health-economic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast media-enhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. European Radiology 2009; 19(3): S753–S763.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, S., Cheek, S., Osman, H. et al. MRI with Gadoxetate Disodium for Colorectal Liver Metastasis: Is It the New “Imaging Modality of Choice”?. J Gastrointest Surg 18, 2130–2135 (2014). https://doi.org/10.1007/s11605-014-2676-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2676-0