Abstract
The purpose of this study was to perform an economic evaluation of hepatocyte-specific Gd-EOB-DTPA enhanced MRI (PV-MRI) compared to extracellular contrast-media-enhanced MRI (ECCM-MRI) and three-phase-MDCT as initial modalities in the work-up of patients with metachronous colorectal liver metastases. The economic evaluation was performed with a decision-tree model designed to estimate all aggregated costs depending on the initial investigation. Probabilities on the need for further imaging to come to a treatment decision were collected through interviews with 13 pairs of each a radiologist and a liver surgeon in Germany, Italy and Sweden. The rate of further imaging needed was 8.6% after initial PV-MRI, 18.5% after ECCM-MRI and 23.5% after MDCT. Considering the cost of all diagnostic work-up, intra-operative treatment changes and unnecessary surgery, a strategy starting with PV-MRI with 959 € was cost-saving compared to ECCM-MRI (1,123 €) and MDCT (1,044 €) in Sweden. In Italy and Germany, PV-MRI was cost-saving compared to ECCM-MRI and had total costs similar to MDCT. In conclusion, our results indicate that PV-MRI can lead to cost savings by improving pre-operative planning and decreasing intra-operative changes. The higher cost of imaging with PV-MRI is offset in such a scenario by lower costs for additional imaging and less intra-operative changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In a time of limited health-care resources it is important to compare not only the sensitivity and the specificity of new diagnostic techniques, but also the impact of the procedure on decision-making, clinical outcomes and costs. Evaluation of sensitivity and specificity alone does not provide the information required to decide whether or not a new technology should be implemented [1]. In particular, the cost of a single diagnostic investigation should not be viewed in isolation. The need and cost of further imaging to come to a treatment decision should also be taken into consideration, as well as the effects on the pre-operative planning and the frequency of changes in operative plans during surgery.
The liver is a common site of metastases from colorectal carcinoma [2, 3]. High precision in detecting, localizing and characterizing liver lesions is important, because the treatment decision may have consequences for the prognosis of the patient with regard to both life expectancy and quality of life. Since surgical resection of colorectal liver metastases (CLM) is the only potentially curative treatment, an important goal of the diagnostic work-up is to identify those patients who would benefit from surgery [2]. More accurate imaging may also have economic consequences, as pre-operative planning is improved and unnecessary surgery can be avoided [4].
Gd-EOB-DTPA (gadoxetic acid disodium; Primovist®, Eovist® in the US, EOB-Primovist® in Japan) is a gadolinium-based paramagnetic diagnostic contrast agent used in the detection, localization and characterization of focal liver lesions with magnetic resonance imaging (MRI) [5, 6]. Gd-EOB-DTPA is taken up by hepatocytes in healthy liver tissue in an amount of about 50% of injected dose, and because malignant primary and secondary tumours usually do not contain functioning hepatocytes as a result of the contrast effect the lesions will appear as dark areas against healthy liver parenchyma. Clinical trials have shown that Gd-EOB-DTPA increases the accuracy of lesion detection and characterization compared with spiral computed tomography (CT) [5–10]. The increase in detected lesions as compared to biphasic spiral CT in a general population has been given with up to 10% by a recent publication by Hammerstingl et al. [9].
The objective of this study was to perform an economic evaluation of Gd-EOB-DTPA-enhanced MRI as a pre-operative diagnostic tool in patients with CLM. Of particular interest in the health–economic model was the need for further diagnostic imaging to come to a treatment decision, and the costs for modified surgical procedures and unnecessary surgery with Gd-EOB-DTPA-enhanced MRI (PV-MRI) compared to three-phase multidetector CT (MDCT) and extracellular contrast media-enhanced MRI (ECCM-MRI).
Methods
Model for diagnostic evaluation of metachronous colorectal liver metastases
A decision-tree model was developed to compare different diagnostic options for the evaluation of CLM in patients with a history of colorectal cancer and known or suspected metachronous liver metastases. The model takes as its starting point cases where further imaging is needed after follow-up staging with monophasic CT, i.e. when it is not clear whether or not the patient is eligible for potentially curative resection. The model compares three alternative imaging modalities: (1) MDCT (2) ECCM-MRI (3) PV-MRI.
Expert panel and interviews
The decision model was developed and validated at three expert panel meetings in 2006 and 2007, with physicians invited from Italy, Germany, Sweden, Ireland and Japan. During the first meeting, the CLM decision-tree structure was developed, during the second meeting the decision-tree model was validated, and the third meeting was used in order to clarify any unresolved issue. The probabilities for different clinical courses in the complete decision-tree were established through in-depth interviews with 13 pairs of physicians (each with one radiologist and one liver surgeon): five pairs in Germany, five in Italy and three in Sweden. The 26 physicians participating in the interviews were recruited from university hospitals and generally had long experience as radiologists and liver surgeons.
During each of the 13 interviews the decision-tree was displayed on three posters, with one arm of the decision-tree represented on each poster, i.e. one poster for MDCT, one poster for ECCM-MRI and one poster for PV-MRI as the initial imaging strategy. Probabilities associated with each arm of the decision-tree were calculated by taking the average of the probabilities estimated in the physician interviews. The results were presented at the third expert panel meeting, where any areas of uncertainty were discussed and resolved.
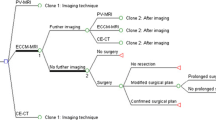
The structure of the decision-tree model is shown in Fig. 1. The model has been simplified by using clones for elements that appear repeatedly in the tree (clones are copies of a certain decision-tree structure, but may differ from each other in terms of probabilities and outcomes). Some branches in the tree were not relevant for all diagnostic options. Technically, these issues were handled by setting the probability of unrealistic diagnostic options to zero in the decision-tree.
Cost estimations
Costs were estimated from a health-care payer perspective, and included only direct medical costs. Costs for single CT and MRI procedures were found in price lists published by the German Hospital Federation for Germany [11], the Lombardy Regional Tariffs for Italy [12], and regional hospital price lists for Sweden [13] (see Table 1). The prices for contrast agents in Germany were obtained from current sales prices to hospitals according to the pharmaceutical database Ifap index [14], in Italy from current sales prices to hospitals according to Italian National Drug Agency product list [15] and in Sweden from the national Swedish pharmaceutical reference book [16].
The costs for liver surgery were based on the time in the operating theatre (anaesthesia time), time in intensive care and the number of hospital days. In this way we could estimate the costs for different types of surgical resection of CLM (high risk or low risk; modified or confirmed surgical plan, or no resection) that are needed for the decision-tree model. Low-risk resection was defined as a standard (or minor) resection involving at most four liver segments with 40% or more of functional liver parenchyma remaining after surgery. High-risk resection was defined as an extended (or major) resection involving more than four liver segments with less than 40% functional liver parenchyma remaining after surgery.
We asked the surgeons participating in the interviews for the typical time required for each type of surgery (high risk, low risk, with confirmed or modified plan). We also asked about the typical number of days in intensive and in standard inpatient care after each type of intervention. The costs for standard and intensive hospital care in Germany were estimated based on data from health insurance and DRG costs, respectively [17, 18], in Italy from other published sources [19, 20] and in Sweden from hospital price lists (see Table 1). Both the DRG costs and the costs from hospital price lists represent the amount the hospitals are paid for performing these services. The cost per minute in the operating theatre was not directly available in any official price lists in Germany and Italy, but was possible to calculate based on DRG and per diem costs for liver surgery in combination with data on the length of stay for relevant DRGs [18, 21]. In Sweden, the cost per minute of anaesthesia was directly available in hospital price lists [13], as were the costs for standard and intensive hospital care [13, 22].
Results
The possible outcomes after initial imaging and the distribution of patients based on average values from the interviews are shown in Figs. 2 and 3, respectively. According to these estimates, the proportions of patients in the “resectable high-risk”, “unresectable” and “no malignant lesions found” categories were higher with PV-MRI in comparison to both MDCT and ECCM-MRI. The need for further diagnostic tests was considerably lower with PV-MRI than with the other diagnostic modalities.
In patients considered to be eligible for hepatic resection and scheduled for low risk resection, the proportions of “confirmed surgical plans” were estimated to be higher and the proportions of “modified surgical plans” lower following initial imaging with PV-MRI than with MDCT or ECCM-MRI (Figs. 4 and 5). The rate of intra-operatively detected unresectable patients was also lower following PV-MRI compared with alternative imaging modalities. The estimations for MDCT and ECCM-MRI were very similar, and an average for these two modalities was used in the model.
There were two options in the model if a high-risk resection was indicated after initial imaging: either the patient proceeds directly to surgery, or confirmatory diagnostic tests with PV-MRI could be performed (Fig. 6). The mean proportion of patients estimated to be in each branch, according to the initial imaging investigation, is shown in Table 2. If PV-MRI was used initially, however, only the direct surgery branch would be used and not a second PV-MRI. It was estimated that about 40% of patients would require confirmatory imaging with PV-MRI when MDCT- or ECCM-enhanced MRI were used as the initial imaging investigation.
In case further diagnostic testing was required after initial imaging, for both initial MDCT and ECCM-MRI, PV-MRI was the preferred imaging test, with 94.6% going to PV-MRI after MDCT, and 74.5% going to PV-MRI after ECCM-MRI. For both initial MDCT and ECCM-MRI, a number of patients with “no malignant lesions” could be identified at confirmatory imaging, whereas it was estimated that these could be detected at an earlier stage with PV-MRI. The experts estimated that patients would rarely proceed directly to laparotomy after initial MDCT, while this option would be pursued in 10% of patients after initial ECCM-MRI and in 50% after initial imaging with PV-MRI. The mean proportions are shown in Table 3.
The estimated time required for surgery and hospitalization is presented in Table 4 for low-risk and high-risk surgery, and for different intra-operative scenarios, i.e. confirmed surgical plan, modified surgical plan and patients in whom unresectability was determined with IOUS and palpation only during surgery. In Fig. 7 the costs of surgery and hospitalization based on the time estimations are shown.
The results showed that the total costs for the whole imaging strategy, i.e. including all modalities needed to come to a therapy decision, were lowest using MDCT as initial imaging investigation in Germany (375 €) (Table 5). This was primarily due to a lower cost for the initial MDCT procedure (223 €). The imaging strategies starting with ECCM-MRI and PV-MRI as initial imaging modalities had about equal total cost, with 498 € for ECCM-MRI and 502 € for PV-MRI (Table 5). It is worth noting that the proportion of further imaging as percentage of the total cost of imaging was by far the lowest for the PV-MRI-imaging strategy with 2.0% (1.8 + 0.2%), and the highest for the MDCT imaging strategy with 40.5% (39.2 + 1.3%).The results for Italy and for Sweden were similar, but the general cost level for imaging was a little lower in Italy and a little higher in Sweden compared to the results in Germany (Fig. 8).
In Germany and Italy, the PV-MRI imaging strategy had a higher total cost of imaging than either MDCT or ECCM-MRI, but when taking the cost of modified or unnecessary surgical procedures into account, the total cost was lower for the PV-MRI imaging strategy than for the ECCM-MRI and the cost difference compared to MDCT was also substantially reduced. In Sweden, the savings due to modified or unnecessary surgical procedures were so large that overall the PV-MRI imaging strategy was less expensive than MDCT. In Germany and Italy the total cost with PV-MRI as initial imaging was lower than for ECCM-MRI and comparable to MDCT. The reasons behind these savings are explained by the distribution of confirmed and modified resections in Fig. 9. The rate of confirmed surgical plan was around 5–10% higher for PV-MRI than for MDCT and ECCM-MRI as initial imaging modalities. The rate of no resection was lower with PV-MRI, because a larger proportion of unresectable cases were identified already prior to surgery (Fig. 3).
Discussion
The model was based on mean values estimated from a limited number of interviews with medical expert pairs of each a radiologist and a liver surgeon. This means that there is some uncertainty concerning the generalizability of the results, and that the results may also be sensitive to selection bias. Most of the physicians worked at large university hospitals and were very experienced, which puts them in a good position to make an estimate of the diagnostic accuracy of PV-MRI in clinical practice compared to MDCT and ECCM-MRI.
An almost inevitable aspect of comparative investigations, no matter whether it is a clinical trial or a health–economic model, is to impose some restrictions on the number of clinical options available to clinicians. In this model, more options could have been included, e.g. contrast-enhanced ultrasound or positron emission tomography (PET) combined as PET-CT, but increasing the number of diagnostic options would have made the health–economic model difficult to work with, as an increased number of options will multiply the number of alternative diagnostic pathways. Already with the current model, it was often challenging for the expert pairs to assign probabilities to all branches of the decision-tree during the 3-h interviews. We therefore limited the number of options in the model to the most relevant comparators for PV-MRI.
In order to avoid restricting the choice of diagnostic options too much after initial imaging, PV-MRI can be used for further imaging in the MDCT and ECCM-MRI arms of the model. However, this means that the analysis may not fully capture the potential benefits of PV-MRI over MDCT and ECCM-MRI, as PV-MRI is to some extent compared with itself. As a result, the differences in outcomes between the three imaging strategies will tend to be smaller than if the comparison had been based on only one type of imaging investigation in each arm. On the other hand, the purpose of the economic evaluation was to compare PV-MRI to ECCM-MRI and MDCT as initial imaging strategy, rather than directly comparing the different diagnostic modalities with each other.
This was mainly a cost study, but cost minimization is not the primary objective in medical care. It is therefore worth noting that we have not taken into account the clinical benefits for patients due to higher diagnostic accuracy. Since better diagnostic precision leads to more patients correctly allocated to high-risk vs. low-risk surgery, it is possible that also patient outcomes would be affected by the choice of diagnostic strategy. As no long-term clinical data are available for testing this hypothesis, this was beyond the scope of the present study.
Relatively few comparable studies on health–economic aspects of contrast agents in liver disease seem to have been published [4, 23–26]. These have shown that contrast-enhanced MRI has the potential to improve medical management and save health-care costs compared with contrast-enhanced CT, mainly due to the avoidance of unnecessary surgical procedures. A retrospective economic evaluation of Mangafodipir trisodium (Teslascan®) in pre-operative assessment of focal liver disease and tumours was performed based on medical chart review [25]. The findings from the study indicated that Teslascan® MRI was more sensitive than contrast-enhanced CT in the pre-operative prediction of the resectability of hepatic lesions, which leads to cost savings, as many cases of unnecessary surgery could be avoided.
In a recent study by Annemans et al. [4], the health–economic impact of ferucarbotran-enhanced MRI in the diagnosis of hepatic colorectal cancer metastases was investigated. A decision-tree comparing diagnosis based on contrast-enhanced spiral CT with ferucarbotran-enhanced MRI was constructed. The analysis showed that in patients receiving additional ferucarbotran-enhanced MRI investigation, the total expected management cost amounted to 16,634 €, compared to 18,416 € for spiral CT-based decision-making. Despite an initial extra cost of 338 € for ferucarbotran-enhanced MRI, a significant net saving of 1,443 € was obtained compared to spiral CT, mainly because of the avoidance of unnecessary surgery.
Economic evaluation of diagnostic agents is a field that is still very much in development [1, 27]. This is a challenge that clinical trials involving diagnostics tend to focus on technical aspects such as sensitivity and specificity, while the effect on subsequent diagnostic or treatment decisions is generally not known. By studying the effects on subsequent diagnostic work-up and surgical decisions, it would be possible to investigate whether using a more accurate and state-of-the-art initial diagnostic modality also leads to cost savings that offset part or all of the higher acquisition cost. In further research in the diagnostic field, it could therefore be recommended to routinely include economic aspects of the diagnostic work-up process when clinical trials are planned. This would increase the amount of data gathered in clinical trials, but in comparison with the large amount of data already collected, the increases in data collection needed for this purpose would be relatively small.
There are limitations in this study. As mentioned before, a model is never able to depict the clinical reality with all its possible choices and potential outcomes. With the help of the expert panel consisting of radiologists working in diagnostics, interventional radiologists and liver surgeons, we tried to identify the most important options and pathways for patients with metachronous liver metastases, being aware that options such as local thermo-ablation or neo-adjuvant chemotherapy have not been taken into account. The input data collected in the structured interviews do not have the same grade of evidence as data from a clinical trial. However, in the absence of comparative health–economic clinical-trial data, we think that the present data are of relevance for the radiological community as a first step towards properly designed clinical trials addressing the health–economic issues. In this respect the results of a recent publication of a multicentre trial, showing that PV-MRI changed the surgical strategy in 16.8% of patients (22/131) compared to biphasic spiral CT [9], are coherent with our collected interview data.
In conclusion, the results of the interviews and the model indicate that an imaging strategy with initial PV-MRI in patients with suspected CLM has the potential to avoid repeated diagnostic procedures by providing more accurate information at an earlier stage in the diagnostic work-up. Compared to doing MDCT initially, there was a moderate additional cost of imaging when PV-MRI was used as initial imaging. However, the total diagnostic cost was lower for the imaging strategy with initial PV-MRI compared to using ECCM-MRI as initial diagnostic modality in Sweden and only a little higher than the cost for ECCM-MRI in Germany and Italy. The results also indicate that PV-MRI improves pre-operative planning and decreases intra-operative changes in planning. This leads to cost savings through shorter operative time, benefits for patients by shorter anaesthesia time, and higher rate of avoidance of unnecessary surgical procedures. If the cost savings due to better pre-operative planning are taken into account, PV-MRI as initial imaging investigation has costs similar to MDCT, and is cost-saving compared to ECCM-MRI. In addition, better diagnostic precision may lead to more patients being allocated to the correct type of treatment. Without clinical data, however, this advantage is difficult to translate into cost savings or improved outcomes for patients in terms of gains in life expectancy or quality of life. These aspects would be important to incorporate in further research.
References
Hunink MG, Krestin GP (2002) Study design for concurrent development, assessment, and implementation of new diagnostic imaging technology. Radiol 222:604–614
Fusai G, Davidson BR (2003) Management of colorectal liver metastases. Colorectal Dis 5:2–23
Ruers T, Bleichrodt RP (2002) Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer 38:1023–1033
Annemans L, Lencioni R, Warie H et al (2008) Health economic evaluation of ferucarbotran-enhanced MRI in the diagnosis of liver metastases in colorectal cancer patients. Int J Colorectal Dis 23:77–83
Huppertz A, Balzer T, Blakeborough A et al (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230:266–275
Huppertz A, Haraida S, Kraus A et al (2005) Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology 234:468–478
Halavaara J, Breuer J, Ayuso C et al (2006) Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT—a multicenter trial. J Comput Assist Tomogr 30:345–354
Bluemke DA, Sahani D, Amendola M et al (2005) Efficacy and safety of MR imaging with liver-specific contrast agent: US multicenter phase-III study. Radiology 237:89–98
Hammerstingl R, Huppertz A, Breuer J et al (2008) Diagnostic efficacy of gadoxetic acid (Primovist®)-enhanced MRI and spiral CT for a therapeutic strategy: Comparison to intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 18:457–467
Schima W, Kulinna C, Langenberger H et al (2005) Liver metastases of colorectal cancer: US, CT or MR? Cancer Imaging 5(Spec no A):S149–S156
Deutsche Krankenhausgesellschaft DKG-NT Band I /BG-T (2007)Verlag W. Kohlhammer, Stuttgart
Lombardy Regional Tariffs (2007) Regione Lombardia, Milano, 2007. Available from: http://www.sanita.regione.lombardia.it/. Accessed 2007-05-16
Price List SRVN Malmö/Lund (2007) Lund: Södra Regionvårdsnämnden
Ifap Index–Die Arzneimitteldatenbank (2007) IfAp Service - Institut für Ärzte und Apotheker GmbH, Neu Golm. Available from: http://www.ifap.de. Accessed 2007-07-01
National Drug Agency product list (2007) (Classe H in commercio. Determinazione AIFA 9-2-2007) Prezzi Rimborso e Mercato. L’Agenzia Italiana del farmaco (AIFA), Rome. Available from http://www.agenziafarmaco.it/aifa/servlet/section.ktml?target=&area_tematica=PREZ_RIMB_MER§ion_code=AIFA_PREZ_RIMB_MER&entity_id=111.82243.1172659031689. Accessed 2007-05-16
FASS–Pharmaceutical Specialties in Sweden (2007) Läkemedelsindustriföreningen, Stockholm
Report of the private health insurances in Germany 2002/2003 (2003) (Die private Krankenversicherung 2002/2003. Zahlenbericht) Verband der privaten Krankenversicherung, Köln
G-DRG. German diagnosis related groups (2007) Institut für das Entgeltsystem im Krankenhaus gGmbH [Institute for the Renumeration System in Hospitals (InEK)] Siegburg. Available from: http://www.g-drg.de/. Accessed 2007-07-05
Bambini che non guariranno: una rete di assistenza pediatrica per curarli a casa. Sole 24 ore Sanità (2007) Gruppo Il Sole 24 ORE SpA, Milano. Available from http://www.saluteeuropa.it/news/2007/03/0316001.htm. Accessed 2007-05-16
Lucioni C, Mazzi S, Neeser K (2004) Analisi di costo-efficacia della terapia combinata con pioglitazone nel trattamento del diabete mellito di tipo 2 in Italia. Pharmacoeconomics Italian Research Articles 6:81–93
National DRG Tariffs (2006) Tariffario Unico Convenzionale 2006. La Conferenza Stato-Regioni, Rome
Price List Linköping University Hospital (2007) Linköping: Sydöstra Sjukvårdsregionen
Helmberger T, Annemans L, Warie H et al (2004) Health economic evaluation of liver MRI in colorectal cancer patients. Eur Radiol 14(Suppl 1):C13
Helmberger T, Gregor M, Holzknecht N et al (2000) Effects of biphasic spiral CT, conventional and iron oxide-enhanced MRI on therapy and therapy costs in patients with focal liver lesions. Rofo 172:251–259
Mann GN, Marx HF, Lai LL et al (2001) Clinical and cost effectiveness of a new hepatocellular MRI contrast agent, Mangafodipir trisodium, in the preoperative assessment of liver resectability. Ann Surg Oncol 8:573–579
Schultz JF, Bell JD, Goldstein RM et al (1999) Hepatic tumor imaging using iron oxide MRI: comparison with computed tomography, clinical impact, and cost analysis. Ann Surg Oncol 6:691–698
Hunink MG, Kuntz KM, Fleischmann KE et al (1999) Noninvasive imaging for the diagnosis of coronary artery disease: focusing the development of new diagnostic technology. Ann Intern Med 131:673–680
Conflict of interest:
This work was funded by Bayer Schering Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zech, C.J., Grazioli, L., Jonas, E. et al. Health-economic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast media-enhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. Eur Radiol 19 (Suppl 3), 753–763 (2009). https://doi.org/10.1007/s00330-009-1432-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1432-4