Abstract
Purpose
The aim of this feasibility study was to evaluate the diagnostic accuracy of ultra-low-dose CT colonography using iterative reconstruction algorithms with reference to standard colonoscopy.
Materials and methods
Prior to this study, a phantom study was performed to investigate the optimal protocol for ultra-low-dose CT colonography. A total of 206 patients with average/high risk of colorectal cancer were recruited. After undergoing full bowel preparation, the patients were scanned in the prone and supine positions with the CT conditions set to 120 kV, standard deviation 45 to 50, and an adaptive iterative reconstruction algorithm applied. Two expert readers read the images independently. The main outcome measures were the per-patient and per-polyp accuracies for the detection of polyps ≥ 10 mm, with colonoscopy results as the reference standard.
Results
Two hundred patients (102 females, mean age 67.5 years) underwent both ultra-low-dose CT colonography and colonoscopy on the same day. The mean radiation exposure dose was 0.64 ± 0.34 mSv. On colonoscopy, 39 patients had 45 polyps ≥ 10 mm (non-polypoid morphology 7), including 4 cancers. Per-patient sensitivity, specificity, and accuracy of CT colonography for polyps ≥ 10 mm were 0.74, 0.96, and 0.92 for reader one, and 0.74, 0.99, and 0.94 for reader two, respectively. Per-polyp sensitivities for polyps ≥ 10 mm were 0.73 for reader one and 0.71 for reader two. On subgroup analysis by morphology, non-polypoid polyps ≥ 10 mm were not detected by both readers.
Conclusion
Extreme ultra-low-dose CT colonography had an insufficient diagnostic performance for the detection of polyps ≥ 10 mm, because it was unable to detect non-polypoid polyps. This study showed that the problem with ultra-low-dose CT colonography was the lack of detectability of small-size polyps, especially non-polypoid polyps. To use ultra-low-dose CT colonography clinically, it is necessary to resolve the problems identified by this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) colonography has been recognized as a less-invasive colonic imaging examination [1, 2], and it has been identified as an effective imaging technique for preoperative evaluation of colorectal cancer [3, 4]. Due to its high ability to identify colorectal polyps, CT colonography has been widely used for diagnosis of colorectal cancer, which is a major cause of morbidity and mortality in industrialized countries, and it has been well accepted by patients [5, 6].
Recently, increasing concerns have been voiced regarding the potential health risks of radiation exposure from CT [7, 8], and there has been a call for radiation dose reduction in CT examinations [9]. Although many of the previous studies were done with relatively low-dose conditions [5, 6], CT colonography unavoidably exposes patients to radiation, which remains a problem not completely resolved, particularly in the screening setting. Iterative reconstruction (IR) algorithms decrease the amount of quantum noise that is observed with a standard filtered back-projection reconstruction algorithm [10, 11]. Using this technique, radiation dose reduction is possible. In fact, there are studies that evaluated the image quality of hybrid types of IR algorithms with CT colonography examinations, and they reported that the radiation dose was reduced by approximately 50% while preserving the image quality, although some image noise and artifacts were still present [12,13,14].
On the other hand, reports detailing the value of various IR techniques for image noise reduction used in abdominal CT examinations have indicated that they did not always improve diagnostic accuracy [11, 15, 16]. There are some reports of ultra-low-dose CT colonography with IR [17,18,19], but in these studies, either only image quality assessment was done, or accuracy was lower than in the previous conventional CT colonography studies [1, 2, 5, 6]. Kang et al. reported that per-polyp sensitivity of sub-mSv CT colonography reconstructed with IR techniques for polyps ≥ 10 mm was 0.695 [17]. In contrast, Liu et al. reported that there was no difference in the diagnostic results of 125 cases between 120-kVp FBP-reconstructed colon CT and 100-kVp and 150-kVp CT colonography applying spectral filtration and advanced modeled iterative reconstruction [18]. According to the previous reports of the verification of CT colonography, per-patient sensitivity for polyps ≥ 10 mm was approximately 90% [1, 2, 5, 6]. Since the accuracy should not be impaired with a decrease in the radiation dose even for ultra-low-dose CT colonography, the accuracy is should be at least 90%. Since prospective comparisons are essential to evaluate the diagnostic potential of ultra-low-dose CT colonography for clinical use, the aim of this feasibility study was to evaluate the diagnostic accuracy of ultra-low-dose CT colonography with reference to standard colonoscopy.
Materials and methods
Study design and population
This prospective, single-center, feasibility study was designed to explore how we can reduce the radiation dose of ultra-low-dose CT colonography. The study protocol was implemented according to the Declaration of Helsinki and approved by our institutional review board and registered at the UMIN Clinical Trial Registry. The enrollment period of patients was from January 2014 to May 2015. Written, informed consent was obtained from all patients. Consecutive individuals who were 30 years of age or older and had abdominal symptoms such as melena or a recent positive immunochemical fecal occult blood test or had a plan to have follow-up surveillance due to a personal history of polyps were eligible for inclusion in the study.
Individuals who had medical conditions that could increase the risk of complications associated with bowel preparation and CT colonography were excluded. In addition, those who had colorectal polyps or cancers at any site already known at the time of enrollment and those who had a history of inflammatory bowel disease, Lynch syndrome, familial polyposis, colorectal surgery, hyperthyroidism, or iodine contrast-medium allergy were excluded.
Determination of the scanning protocol
To determine the scanning protocol with ultra-low-dose CT colonography, an experiment using a phantom (CT Colonography Phantom NCCS, Kyoto Kagaku, Kyoto, Japan) was carried out. The Adaptive Iterative Dose Reduction system using a three-dimensional processing algorithm (AIDR 3D; Canon Medical Systems Corp., Tochigi, Japan) was used for reconstruction [13]. The phantom was scanned, and the value of the standard deviation (SD) for the CT- Auto Exposure Control (AEC) and Dose–Length Product (DLP) was calculated according to the change of AIDR intensity under 4 conditions of combinations of rotation time and helical pitch factor (Supplementary Table 1). Tube voltage was fixed at 120 kV, and slice thickness was 0.5 mm. The SD value was changed from 10 to 60, and AIDR intensity was also evaluated. A cylindrical phantom that contained fake polyps set at the position of the rectum was scanned. Since the target was polyps ≥ 10 mm in this study, the diameter of six fake polyps was fixed at 10 mm, and their heights were changed from 7 to 0.5 mm (Fig. 1a). With the settings of conditions 1, 2, and 3, DLP (i.e., CT exposure dose) reached a plateau when SD was 40 or higher, and the exposure dose did not decrease even if SD was raised. With the setting of condition 4, the exposure dose was reduced even when SD was 40 or higher. In other words, under the setting of condition 4, the dose could be reduced according to the patient’s body shape; therefore, this condition was adopted. Polyps with a height of 2 mm or more can be visually recognized under all conditions, but for polyps with a height of 1 mm, recognition differed by readers from around SD 35–50; therefore, this can be considered a boundary area (Fig. 1b). When the SD value was changed in this boundary area and visually evaluated for problems such as artifacts on clinical images, there were no artifacts within the SD 40–50 as in the phantom experiment, and a smooth virtual endoscopic image was obtained. However, since there were cases with more obvious pelvic artifacts at SD 50 or higher, it was considered necessary to lower the SD value for the setting in the pelvis. Therefore, the Variable Helical Pitch Scan, which can scan with different SDs without stopping scanning, was adopted. The final AEC setting selected was SD 50 for the upper abdomen and SD 45 for the pelvis, taking into account artifacts (Table 1).
Examination of polyps in a phantom. a A total of 6 phantom polyps have a fixed diameter of 10 mm and height varying from 0.5 to 7 mm. b Virtual endoscopy image of six polyps in a phantom when SD is changed from 10 to 60. Even when the SD is 60, polyps with a height of up to 2 mm could be discerned in the phantom
Bowel preparation for CT colonography
To allow patients to undergo both ultra-low-dose CT colonography and colonoscopy on the same day, they underwent both a single, full-cathartic bowel preparation and a contrast-medium bowel preparation. On the morning of the examination day, each patient was given 1620 mL of PEG (Niflec; Ajinomoto Pharmaceuticals Co., Ltd, Tokyo, Japan), followed by 400 mL of PEG-C consisting of 380 mL of PEG plus 20 mL of sodium diatrizoate (Gastrografin; Bayer Yakuhin, Ltd., Osaka, Japan) for tagging of residual fluid. The accuracy of CT colonography using this bowel preparation has been verified in the previous reports [5].
Ultra-low-dose CT colonography technique and interpretation
After bowel preparation, medical staff confirmed that the patient’s stool changed from solid to watery, and the patient was placed in the left decubitus position for thin flexible rectal-tube insertion with a balloon. Spasmolytic agents and intravenous contrast medium were not used during CT colonography [20]. The colon was insufflated using an automated carbon dioxide insufflator (ENIMA CO2, Horii Pharmaceutical, Osaka, Japan). All CT colonography examinations at all sites were performed using an 80-row multi-detector CT (Aquilion PRIME, Canon Medical Systems) using an automatic x-, y-, and z-axis tube current modulation technique (Volume EC; Canon Medical Systems), which automatically uses the optimal tube current considering target noise (SD) with supine and prone positioning. Table 2 shows the scanning protocol: 120 kV, SD 45 to 50, an adaptive iterative reconstruction algorithm applied, and a section thickness of 0.5 mm.
CT colonography interpretation was performed by two experienced CT colonography readers who had participated in a 2-day lecture to review 100 examples of CT colonography studies confirmed with colonoscopy and scored higher than a predefined sensitivity threshold of 0.90 for neoplasms ≥ 10 mm. In addition, both readers had interpreted more than 300 clinical CT colonography examinations before starting this study. Both readers were unaware of the colonoscopy results. All interpretations were performed using a commercially available workstation (plug-in colon analysis application for AZE Virtual Place, version 320; Canon, Tokyo, Japan). Primary three-dimensional interpretation modes were available, and lesions were measured at the multiplanar reconstruction setting that showed the maximal diameter of the detected lesion. In the primary three-dimensional reading, readers observed by fly through from the rectum to the cecum and then flipped back from the cecum to the rectum to prevent oversight. Next, readers observed the inside of the residual liquid, which is the blind spot of the fly through in the two-dimensional image. These were performed for two positions each, and if lesions were found, comparative interpretation and matching were performed. In cases with copious amounts of solid feces in the colon, readers used a primary two-dimensional approach, with three-dimensional problem-solving. Computer-assisted diagnosis and electronic cleansing software were not used. For each polyp, the morphology was recorded according to the Paris classification [21].
Colonoscopy
An expert colonoscopist with 10 years of experience, a board-certified member of the Japan Gastroenterological Endoscopy Society who was blind to the results of CT colonography, performed the total colonoscopy immediately after CT colonography. Sedation, analgesics, and muscle relaxants were used if patients requested them or if the colonoscopist found them necessary on the basis of his clinical judgment. All polyps were recorded in terms of location and classified morphologically according to the Paris classification [21]. The size and height of all polyps were measured with forceps or in comparison with endoscopic measures. If possible, all polyps with a diameter ≥ 10 mm were removed endoscopically during the same procedure, and if the polyps were not removed, biopsy samples were obtained for pathological examination.
Histological review and polyp matching
A polyp found on CT colonography was matched to the corresponding polyp found on colonoscopy when it was located in the same or an adjacent colon segment and when its size differed less than 50% [1]. The reference standard for polyp diagnosis was the result of colonoscopy and the histological evaluation of the resected polyps.
Outcome measures
The primary endpoint of the study was per-patient performance of CT colonography in the detection of polyps, with colonoscopy results as the reference standard. The sensitivity, specificity, positive and negative predictive values, and accuracy of CT colonography for detecting polyps ≥ 10 mm were calculated with the data pooled from the two readers on a per-patient basis. The secondary endpoints were per-polyp sensitivity and positive predictive value analysis based on morphology and radiation exposure dose.
Statistical analysis
Diagnostic values are reported along with their 95% confidence intervals (CIs). The Clopper–Pearson method was used to assess the 95% CIs. All other quantitative variables are expressed as means and SD values or medians, and qualitative variables are expressed as numbers and percentages. The chi-squared test was used to assess the significance of differences among proportions. P < 0.05 was considered to indicate significance. These analyses were performed with Intercooled Stata 16.0 for Windows (Stata Corp., College Station, TX, USA).
Results
Patients’ demographic characteristics
A total of 206 patients at one institution were included in this study. Five patients withdrew their informed consent for the study. One patient was excluded because of an incomplete colonoscopy. The remaining 200 patients (98 men, 102 women; mean age, 67.5 years [range 31–89 years]) were analyzed (Fig. 2). Of these 200 patients, 22 (11%) were at average risk of colorectal cancer, 16 (8.0%) were at elevated risk, and 162 (81%) had recent positive immunochemical fecal occult blood tests. Colonoscopic examination showed that 73 (36.5%) patients had 137 polyps of ≥ 6 mm, 39 (19.5%) patients had 45 polyps ≥ 10 mm, and 4 (2.0%) patients had cancers (Table 2). Supplementary Table 2 shows the distribution of polyps ≥ 6 mm, which included 4 cancers, 127 tubular adenomas, 5 tubulovillous adenomas, and 1 sessile serrated adenoma/polyp. Pathologically proven hyperplastic polyps were excluded. No adverse events related to colonoscopy occurred.
Radiation exposure of ultra-low-dose CT colonography
The mean DLP was 21.3 ± 11.8 mGy·cm in the supine position and 21.3 ± 11 mGy·cm in the prone position (total, 42.6 ± 22.6 mGy·cm; range 16.3–175.8 mGy·cm). The mean effective radiation dose of CT colonography was 0.32 ± 0.18 mSv in the supine position and 0.32 ± 0.16 in the prone position (total, 0.64 ± 0.34 mSv; range 0.24–2.64 mSv) (converted using an effective dose-conversion coefficient of 0.015). No adverse events relating to the CT colonography preparation or examination occurred.
Per-patient assessment
Table 3 shows the per-patient performance of ultra-low-dose CT colonography.
The per-patient sensitivity, specificity, and positive and negative predictive values of detecting polyps ≥ 10 mm were 0.74 (29 of 39 patients), 0.96 (155 of 161 patients), 0.83 (29 of 35 patients), and 0.94 (155 of 165 patients), respectively, by reader one and 0.74 (29 of 39 patients), 0.99 (159 of 161 patients), 0.94 (29 of 31 patients), and 0.94 (159 of 169 patients), respectively, by reader two.
Per-polyp assessment
Twelve and 13 of 45 polyps ≥ 10 mm were missed at CT colonography by reader one and reader two, respectively. The sensitivity and positive predictive value of CT colonography for polyps ≥ 10 mm were 0.73 (33 of 45 polyps) and 0.79 (33 of 42 polyps), respectively, by reader one, and 0.71 (32 of 45 polyps) and 0.91 (32 of 35 polyps), respectively, by reader two. Those of CT colonography for polyps 6 to 9 mm were 0.50 (46 of 92 polyps) and 0.73 (46 of 63 polyps), respectively, by reader one, and 0.51 (47 of 92 polyps) and 0.85 (47 of 55 polyps), respectively, by reader two.
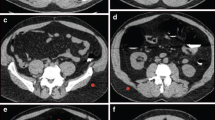
Per-polyp sensitivities based on morphology are shown in Table 4. Sensitivities for the detection of pedunculated, sessile, and non-polypoid polyps ≥ 10 mm by reader one were 0.83 (10 of 12 polyps), 0.88 (22 of 25 polyps), and 0.0 (none of seven polyps), respectively; values for reader two were 0.67 (8 of 12 polyps), 0.92 (23 of 25 polyps), and 0.0 (none of seven polyps), respectively. Sensitivities for non-polypoid polyps ≥ 10 mm were significantly lower than those for polypoid polyps (pedunculated or sessile) (P = 0.00: both readers). Of seven missed non-polypoid polyps, only one non-polypoid polyp could be found even on retrospective analysis. Of the remaining 6 lesions, however, no lesions were misidentified due to insufficient dilation or poor pretreatment. All four cancers were detected by CT colonography, but one of five tubulovillous adenomas was missed by both readers. Figure 3 shows a 20-mm polypoid polyp that was detected by CT colonography and was found to be a T1 cancer on pathologic examination.
Polypoid polyps of 20 mm were detected by CT colonography. a Virtual endoscopy image in a 70-year-old woman. A polypoid polyp with a flat component is seen in the rectum (arrowhead). b Multi-planar reconstruction image (arrow). c After indigo carmine dye spray is applied, the colonoscopy image shows a polypoid polyp with a flat component. The polyp was diagnosed as a 20-mm cancer. Endoscopic submucosal dissection was performed, and histologic examination shows a well-differentiated adenocarcinoma (T1). In addition, laparoscopic resection was performed
Discussion
For a long-term screening program in which exposure of asymptomatic individuals to radiation is unavoidable, radiation dose reduction is vital in CT colonography, along with reducing the burden of bowel preparation [22]. CT colonography examinations for screening purposes should be performed with the lowest possible dose while maintaining diagnostic capability, and one guideline according to the EU consensus [23] and the practical parameters in the United States [24] is to keep the average effective dose per test to about 3 to 5.7 mSv [25]. The U.S. Preventive Services Task Force and the National Comprehensive Cancer Network recommended CT colonography examinations every 5 years as a screening tool, and they also reported that radiation exposure remains a potential harm associated with CT colonography [26, 27]. In addition, a recent study reported that no effect of sub-mSv CT on human DNA was detected, but, in the same setting, DNA double-strand breaks and chromosome aberrations increased after standard-dose CT [28]. Accordingly, ultra-low-dose CT colonography can be one of the potential modalities for colon screening. In the present study, the radiation dose was successfully reduced as much as possible using the latest scanning technology. In the present study, the total average exposure dose was reduced to 0.64 mSv, even including scanning in both the supine and prone positions, which was lower than of the previous reports of ultra-low-dose CT colonography. In three previous studies of ultra-low-dose CT colonography, mean radiation doses were 0.90, 1.0, and 0.98 mSv. [17, 19, 29]. The present exposure dose was lower than that (almost 1.0 mSv) of typical plain abdominal radiographs [17]; thus, ultra-low-dose CT colonography may dispel the patients’ psychological resistance to radiation exposure, although it may not actually be a major health problem [25].
As for diagnostic accuracy, the present study showed that per-patient sensitivity of clinically significant polyps (≥ 10 mm) was 0.74 for both readers, and this result was almost equivalent to the previous studies of ultra-low-dose CT colonography [17, 19]. Detecting polypoid polyps ≥ 10 mm on ultra-low-dose CT colonography was possible with good diagnostic accuracy, although all seven non-polypoid polyps ≥ 10 mm were missed by two expert readers. These observations imply that ultra-low-dose CT colonography had limitations in detecting small polyps and non-polypoid polyps (Supplementary Fig. 1 a−c). Furthermore, the IRT-combined protocol determined by the phantom experiment simply removed the noise, but it did not function for the detection of non-polypoid polyps. Nevertheless, all cancers and most villous adenomas were detected by the two readers, suggesting that ultra-low-dose CT colonography could be clinically applicable, if the problems identified in this study are overcome. In addition, the present study also showed high specificity and high negative predictive value of ultra-low-dose CT colonography. When considering the use of CT colonography as a cancer screening examination, a high negative predictive value, as well as sensitivity, is important [30]. If the number of unnecessary colonoscopy examinations is substantially reduced, such triage examination is very useful. Because of this high negative predictive value, the present result suggests that, if 100 patients with a positive colorectal cancer screening result undergo CT colonography examination with a cut-off size of 10 mm, only 17 or 18 would need to proceed to colonoscopy examination. At the same time, however, it is important to notice that 6 false-negative patients were missed by the criteria.
Detection of non-polypoid polyps by CT colonography is generally difficult, because the subtle morphologic changes are not easily distinguished from normal mucosa [31,32,33]. Studies evaluating the accuracy of CT colonography for non-polypoid polyps showed varying results [31,32,33,34,35]. Togashi et al. [32] reported the detection rate of laterally spreading tumors. The detection rate was significantly higher for the polypoid type than for the non-polypoid type (0.71 vs 0.31). In addition, Utano et al. reported that per-lesion sensitivities for detection of polypoid and non-polypoid neoplasms ≥ 10 mm were 0.95 and 0.67, respectively [6]. On the other hand, Heresbach et al. [34] and Fidler et al. [35] reported no significant differences in accuracy on the basis of lesion morphology. Two multi-center studies with conventional CT colonography showed that sensitivity for non-polypoid polyps was similar (0.68 and 0.67) [5, 6]. In the present study, the sensitivity for polypoid polyps was almost equivalent to previous studies, but no non-polypoid polyps were detectable. Of the seven non-polypoid polyps in the present series, six could not be identified. This finding may suggest a limitation of detectability of this modality. Therefore, we consider that ultra-low-dose CT colonography had insufficient per-patient sensitivity for lesions ≥ 10 mm, and there was a significant difference (0.74 (29/39) vs. 0.93 (162/175), p = 0.001) compared with the results of a previous study using the same bowel preparation [5]. In addition, flat polyps with height of 2 mm were identifiable in the phantom study, but not at all in clinical settings. This indicates that extreme dose reduction in CT colonography is not yet acceptable. Per-lesion sensitivity did not reach 90% in any previous reports on ultra-low-dose CT colonography, suggesting that the exposure of 3.0–5.7 mSv, which is consistent with the EU consensus [23] and the practical parameters in the United States [24], is appropriate at present. However, six of the non-polypoid polyps detected in this study were low-grade adenomas, and one was a sessile serrated adenoma/polyp with mild atypia; therefore, their misidentification may not lead to grave outcomes in the short term. In recent years, the application of deep learning to CT colonography has also been attempted [36], and it has been reported that deep learning in computer-aided detection is a promising method for improving the performance of CT colonography [37]. It is expected in the future that improvements in scanner technology, iterative reconstruction techniques, and deep learning may solve the problems identified in the present study.
There are several limitations to this study. First, this was a single-center study in a tertiary care hospital in Japan; therefore, the lesion distribution may be different from that in western countries. Second, this study was limited by a relatively small number of patients with a high risk of colon cancer, and no power analysis was done in advance, because it was a feasibility study. To actually introduce ultra-low-dose CT colonography as a screening test, multi-center studies of a large number of patients with average risk would be necessary. Third, extracolonic lesions, the quality of bowel preparation, and the colon distention of CT colonography images were not evaluated, although intestinal dilation is vital for CT colonography to detect non-polypoid polyps. Fourth, we did not use an electronic cleansing software. The reason why we did not use it was because the capacity of electronic cleansing differs depending on each workstation, and also in the previous studies, it was not used [1, 2, 5, 6]. Furthermore, in ultra-low-dose CTCs as in this study, electronic cleansing would result in more noise than in CT images taken under normal conditions, and it cannot fully demonstrate its capability.
In conclusion, extreme ultra-low-dose CT colonography had an insufficient diagnostic performance for the detection of polyps ≥ 10 mm, because it was unable to detect small size and non-polypoid polyps. This study showed that the problem with ultra-low-dose CT colonography was the lack of detectability of small polyps, especially non-polypoid polyps. To use ultra-low-dose CT colonography clinically, it is necessary to resolve the problems identified in this study.
References
Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200.
Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–17.
Tsurumaru D, Nishimuta Y, Kai S, Oki E, Nishie A. Factors of incomplete colonoscopy for stenosing colorectal cancer: CT colonography features. Jpn J Radiol. 2020;38:973–8.
Tsurumaru D, Takatsu N, Kai S, Oki E, Ishigami K. Measurement of circumferential tumor extent of colorectal cancer on CT colonography: relation to clinicopathological features and patient prognosis after surgery. Jpn J Radiol. 2021;39:966–72.
Nagata K, Endo S, Honda T, et al. Accuracy of CT colonography for detection of polypoid and non-polypoid neoplasia by gastroenterologists and radiologists: a nationwide multi-center study in Japan. Am J Gastroenterol. 2017;112:163–71.
Utano K, Nagata K, Honda T, et al. Diagnostic performance and patient acceptance of reduced-laxative CT colonography for the detection of polypoid and non-polypoid neoplasms: a multi-center prospective trial. Radiology. 2017;282:399–407.
Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84.
Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201:1283–90.
Candela-Juan C, Montoro A, Ruiz-Martínez E, Villaescusa JI, Martí- BL. Current knowledge on tumour induction by computed tomography should be carefully used. Eur Radiol. 2014;24:649–56.
Flicek KT, Hara AK, Silva AC, Wu Q, Peter MB, Johnson CD. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: a pilot study. AJR Am J Roentgenol. 2010;195:126–31.
Yoon MA, Kim SH, Lee JM, et al. Adaptive statistical iterative reconstruction and Veo: assessment of image quality and diagnostic performance in CT colonography at various radiation doses. J Comput Assist Tomogr. 2012;36:596–601.
Shin CI, Kim SH, Lee ES, et al. Ultra-low peak voltage CT colonography: effect of iterative reconstruction algorithms on performance of radiologists who use anthropomorphic colonic phantoms. Radiology. 2014;273:759–71.
Nagata K, Fujiwara M, Kanazawa H, et al. Evaluation of dose reduction and image quality in CT colonography: comparison of low-dose CT with iterative reconstruction and routine-dose CT with filtered back projection. Eur Radiol. 2015;25:221–9.
Lubner MG, Pooler BD, Kitchin DR, et al. Sub-milli Sievert (sub-mSv) CT colonography: a prospective comparison of image quality and polyp conspicuity at reduced-dose versus standard-dose imaging. Eur Radiol. 2015;25:2089–102.
Song JS, Choi EJ, Kim EY, et al. Attenuation-based automatic kilovoltage selection and sinogram-affirmed iterative reconstruction: effects on radiation exposure and image quality of portal-phase liver CT. Korean J Radiol. 2015;16:69–79.
Chang W, Lee JM, Lee K, et al. Assessment of a model based, iterative reconstruction algorithm (MBIR) regarding image quality and dose reduction in liver computed tomography. Invest Radiol. 2013;48:598–606.
Kang HJ, Kim SH, Shin CI, et al. Sub-millisievert CT colonography: effect of knowledge-based iterative reconstruction on the detection of colonic polyps. Eur Radiol. 2018;28:5258–66.
Liu JJ, Xue HD, Liu W, et al. CT colonography with spectral filtration and advanced modeled iterative reconstruction in the third-generation dual-source CT: image quality, radiation dose and performance in clinical utility. Acad Radiol. 2021;28:e127–36.
Shin CI, Kim SH, Im JP, et al. One-mSv CT colonography: Effect of different iterative reconstruction algorithms on radiologists’ performance. Eur J Radiol. 2016;85:641–8.
Nagata K, Fujiwara M, Shimamoto T, Iida N, Mogi T, Mitsushima T. Colonic distension at CT colonography: randomized evaluation of both intravenous hyoscine butyl bromide and automated insufflation. AJR Am J Roentgenol. 2015;204:76–82.
No authors listed. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon—November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43.
Utano K, Takayanagi D, Nagata K, et al. A novel volume-reduced CT colonography regimen using hypertonic laxative (polyethylene glycol with ascorbic acid): randomized controlled trial. Eur Radiol. 2019;29:5236–46.
Neri E, Halligan S, Hellström M, et al. The second ESGAR consensus statement on CT colonography. Eur Radiol. 2013;23:720–9.
ACR–SAR–SCBT-MR practice parameter for the performance of computed tomography (CT) colonography in adults. Available via: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/CT-Colonog.pdf. Accessed 5 September 2021
Liedenbaum MH, Venema HW, Stoker J. Radiation dose in CT colonography–trends in time and differences between daily practice and screening protocols. Eur Radiol. 2008;18:2222–30.
Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564–75.
Provenzale D, Jasperson K, Ahnen DJ, et al. Colorectal cancer screening, version 1.2015. J Nat Compr Cancer Netw. 2015;13:959–68.
Sakane H, Ishida M, Shi L, et al. Biological effects of low-dose chest CT on chromosomal DNA. Radiology. 2020;295:439–45.
Cianci R, Delli Pizzi A, Esposito G, et al. Ultra-low dose CT colonography with automatic tube current modulation and sino2019gram-affirmed iterative reconstruction: effects on radiation exposure and image quality. J Appl Clin Med Phys. 2019;20:321–30.
Liedenbaum MH, van Rijn AF, de Vries AH, et al. Using CT colonography as a triage technique after a positive fecal occult blood test in colorectal cancer screening. Gut. 2009;58:1242–9.
Fidler JL, Johnson CD, MacCarty RL, et al. Detection of flat lesions in the colon with CT colonography. Abdom Imaging. 2002;27:292–300.
Togashi K, Utano K, Kijima S, et al. Laterally spreading tumors: limitations of computed tomography colonography. World J Gastroenterol. 2014;20:17552–7.
Utano K, Katsuki S, Matsuda T, et al. Colon capsule endoscopy versus CT colonography in patients with large non-polypoid tumours: a multicentre prospective comparative study (4CN Study). Digestion. 2020;101:615–23.
Heresbach D, Djabbari M, Riou F, et al. Accuracy of computed tomographic colonography in a nationwide multicentre trial, and its relation to radiologist expertise. Gut. 2011;60:658–65.
Fidler JL, Zhang Z, Herman BA, et al. CT colonography for the detection of non-polypoid adenomas: sensitivity assessed with restricted national CT colonography trial criteria. AJR Am J Roentgenol. 2014;203:W614–22.
Tachibana R, Näppi JJ, Ota J, et al. Deep learning electronic cleansing for single- and dual-energy CT colonography. Radiographics. 2018;38:2034–50.
Uemura T, Näppi JJ, Ryu Y, Watari C, Kamiya T, Yoshida H. A generative flow-based model for volumetric data augmentation in 3D deep learning for computed tomographic colonography. Int J Comput Assist Radiol Surg. 2021;16:81–9.
Acknowledgements
The authors would like to thank Rika Ishizuka of Aizu Medical Center for providing administrative support.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institution and with the Helsinki declaration. The study protocol was approved by the Institutional Review Board.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11604_2022_1266_MOESM1_ESM.tif
Supplementary file1 0-Is+IIa polyp, 14 mm in size, cecum, tubular adenoma. CT image at SD = 20. Flat component is recognizable around the ridge (arrow head). (TIF 4097 KB)
11604_2022_1266_MOESM2_ESM.tif
Supplementary file2 0-Is+IIa polyp, 14 mm in size, cecum, tubular adenoma. Almost no artifacts are seen, and the sessile part of the polyp is recognizable (arrow head). However, the surrounding flat component that could be seen at SD = 20 is difficult to recognize. It has been suggested that it is more difficult to depict non-polypoid components with ultra-low-dose CT colonography than with normal-dose CT colonography. (TIF 4096 KB)
11604_2022_1266_MOESM3_ESM.tif
Supplementary file3 0-Is+IIa polyp, 14 mm in size, cecum, tubular adenoma. Endoscopic image. Flat component is seen around the ridge (arrow head). (TIF 3757 KB)
About this article
Cite this article
Yasuda, T., Honda, T., Utano, K. et al. Diagnostic accuracy of ultra-low-dose CT colonography for the detection of colorectal polyps: a feasibility study. Jpn J Radiol 40, 831–839 (2022). https://doi.org/10.1007/s11604-022-01266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01266-1