Abstract
Purpose
To assess the feasibility of combining Auto-kVp selection technique, higher preset ASIR-V and noise index (NI) to realize individualized sub-mSv CT colonography (CTC) for accurate colorectal tumor detection and localization.
Methods
Ninety patients with suspected colorectal cancer (CRC) were prospectively enrolled to undergo standard dose CTC (SDCTC) in the prone and ultra-low dose CTC (ULDCTC) in the supine position. SDCTC used 120 kVp, preset ASIR-V of 30%, SmartmA for a NI of 13; ULDCTC used Auto-kVp selection technique with 80 or 100 kVp, preset ASIR-V of 60%, SmartmA for a NI of 13 for 80 kVp, and NI of 15 for 100 kVp. The effective dose (ED), image quality [signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) of colorectal neoplasms] between the two protocols were compared and the accuracies of tumor locations were evaluated for CTC in comparison with the surgery results.
Results
The mean ED of the ULDCTC-80 kVp subgroup was 0.70 mSv, 71.43% lower than the 2.45 mSv for the 120 kVp group, while that of the ULDCTC-100 kVp subgroup was 0.98 mSv, 73.00% lower than the 3.63 mSv for the 120 kVp group (P < 0.001). The tumor SNR and CNR of the ULDCTC were higher than those of SDCTC (P < 0.05), while there was no difference in the subjective image quality between them with good inter-observer agreement (Kappa: 0.805–0.923). Both SDCTC and ULDCTC groups had high detection rate of colorectal tumors, along with good consistency in determining tumor location compared with surgery reports (Kappa: 0.718–0.989).
Conclusion
The combination of Auto-kVp selection, higher preset ASIR-V and NI achieves individualized sub-mSv CTC with good performance in detecting and locating CRC with surgery and consistent results between SDCTC and ULDCTC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) has become the third leading cause of death in patients diagnosed with malignant neoplasms in the digestive system [1]. Since its primary cause is inability to diagnose at an early stage, early detection is essential to CRC prevention [2]. Optical colonoscopy (OC), serving as the gold standard, plays an important role in the reliable diagnosis of CRC [3]. However, the invasive nature of the procedure and high requirement of the physician’s experience narrow the application of OC in some circumstances [4, 5]. Nowadays, CT Colonography (CTC), also known as virtual colonoscopy, becomes a compensation for incomplete colonoscopy (IC), because of its ability to provide the entire colon evaluation, especially for the proximal colon obstructed by occlusive CRC [6]. Furthermore, an increasing number of organizations, including American College of Gastroenterology, the European Society of Gastrointestinal Endoscopy and European Society of Gastrointestinal and Abdominal Radiology, have involved CTC as a routine screening test for CRC, which highlighted the increasing clinical value of CTC [7, 8]. However, the routine CTC requires two scans in prone and supine positions, causing the risk of radiation exposure a non-negligible issue. Since the potential harm from radiation has raised great awareness, exploring effective dose reduction approaches has always been a hot topic in the field of medical imaging.
With the continuous development of CT technology, various tools have been introduced to decrease radiation dose without sacrificing image quality. One of them is the automated kilovoltage peak selection (Auto-kVp), been known as Auto-prescription technique, provides the patient with the most appropriate tube voltage based on the x-ray attenuation features derived from patient’s body habitus [9]. Taking advantage of Auto-kVp can realize individualized low-dose imaging, which has been studied in the thoracoabdominal aorta and portal vein [10, 11]. Another dose reduction approach, been frequently neglected, is adjusting the preset strength of adaptive statistical iterative reconstruction algorithm-V (preset ASIR-V). As the weight of preset ASIR-V increases, the radiation dose required to achieve the same noise level for a given patient decreases exponentially [12]. Furthermore, increasing noise index (NI) can also decrease radiation dose. The increasing image noise caused by the higher NI can be dealt with applying high strength of post-set ASIR-V, which has great denoising capacity to compensate for the image degradation [13, 14]. To the best of our knowledge, combining the above-mentioned dose-reduction techniques in CRC patients has not been paid much attention.
Therefore, this study aims to assess the feasibility of combining Auto-prescription technique, higher preset ASIR-V and higher NI to realize individualized sub-mSv CTC, as well as evaluating its diagnostic performance by the detection rate of CRC under ultra-low dose protocol and the accuracy of defining tumor location in comparison with the results of surgery.
Materials and methods
This prospective study was approved by the Institutional Review Board of our Hospital. Written informed consent for study participation was obtained from all involved patients.
Study participants
During the period of December 2023 to April 2024, a total of 110 consecutive patients who were scheduled to undergo CTC examination with clinically suspected CRC in our hospital were prospectively enrolled in this study. The exclusion criteria were as follows: patients with poor bowel preparation, unable to tolerate gas injection, metal artifacts from lumbar implants or total hip replacement, and patients who were recommended to use 120 kVp tube voltage by Auto prescription technique. Among the 110 patients, 11 patients who had no pathological report, 5 patients who had maximum tumor diameter less than 10 mm, and another 4 patients who underwent chemotherapy for treatment were further excluded from analysis. Thus, 90 patients finally made up of our study population (Fig. 1).
Sub-millisievert CT colonography
Prior to the CTC, a clear liquid diet was restricted to all patients three days before the examination. A standard bowel preparation was conducted on all patients with one sachet of soluble polyethylene glycol electrolyte powder (Hygecon, Jiangxi Hygecon Pharmecutical) dissolved in one litre of warm water as laxative, and without fecal tagging. In addition, patients were instructed to consume 30 millilitres (mls) of Simethicone dissolved in 100 mls of warm water to alleviate possible abdominal bloating. On the day of CTC examination, all patients had fasted overnight and lied in the left lateral decubitus position on the CT examination table. A senior radiologist assisted by a dedicated nurse manually inflated CO2 into the colon via a flexible rectal tube, until the patient experienced abdominal discomfort. The CTC scanning started with patients in prone position with care taken to avoid abdominal compression by putting a pillow under the chest. After the patients rotated into the supine position, the colon distension was checked based on the supine scout image. If the residual air was inadequate, further inflation would be performed before the second scan to ensure the CTC image quality. To avoid repeated bowel preparation, the CTC examinations were conducted on the same day with colonoscopy.
All CTC examinations were performed with a 256-row spectral CT scanner (Revolution CT, GE HealthCare, Milwaukee, WI, USA) using the following parameters: detector width, 80 mm; pitch, 0.992; rotation time, 0.5 s; slice thickness, 5 mm; tube voltage, 120 kVp in the prone position [standard dose CTC (SDCTC)] and Auto-prescription kVp selection in the supine position [ultra-low dose CTC (ULDCTC)] with subgroups of 80 kVp or 100 kVp; tube current, Smart mA mode (range, 20–450 mA); preset ASIR-V, 30% for the SDCTC and 60% for the ULDCTC. Radiation doses were controlled by using noise index (NI) setting: 13 for the 120 kVp group and 80 kVp subgroup and 15 for the 100 kVp subgroup. All raw data were reconstructed using a STND kernel with 30% and 80% post-set ASIR-V for the SDCTC and the ULDCTC, respectively. The reconstruction thickness and increment were both 0.625 mm.
Radiation dose metrics
The volume CT dose index (CTDIvol) and dose-length product (DLP) were recorded for comparison. Moreover, the scan length was obtained from the starting and ending points for comparison between the prone and supine positions. Furthermore, the effective dose (ED) was calculated from DLP using 0.015 mSv/ (mGy·cm) as the conversion factor in abdomen, recommended by the European guidelines on quality criteria [15].
Quantitative evaluation
All axial images were transferred to an AW 4.7 (GE HealthCare, Milwaukee, WI, USA) workstation for the following reformatted images: two-dimensional (2D) Multi-planar Reformation (MPR), three-dimensional (3D) endoluminal view from CT Virtual Colonoscopy (CTVC) and 3D Raysum. On the axial images, the circular regions of interest (ROIs) were drawn on the homogenous area of the colorectal tumor and intraluminal air to measure the CT value and the standard deviation (SD) values, in order to calculate the signal-to-noise ratio (SNR) and the contrast-to-noise ratio (CNR) of tumors. The formulas were as follows: SNR=CT tumor/SD tumor, CNR=(CT tumor-CT intraluminal air)/SD intraluminal air. The tumor ROIs were depicted on the slice of maximum diameter, and were manually placed on the prone and supine images, keeping the ROI size accounting for 70-80% of the tumor. The measurements on three consecutive slices were recorded and averaged to avoid measuring bias, and the ROI sizes ranged from 50 to 100 mm2.
Qualitative evaluation
Two abdominal radiologists (one junior radiologist with 5-year experience, and one senior radiologist with 8-year experience in abdominal imaging), who were blinded to the group division, independently performed subjective image quality assessment on the workstation. The 2D MPR images and 3D CTVC images were graded using a five-point scale. The images scored greater than or equal to 3 points were considered diagnostically acceptable. The marking criteria were demonstrated as follows:
5 points: clear image, virtually no image noise, excellent confidence to detect colorectal lesions;
4 points: relatively clear image, low image noise, good confidence to detect colorectal lesions;
3 points: fair image, moderate image noise, fair confidence to detect colorectal lesions;
2 points: slightly blurry image, large image noise, low confidence to detect colorectal lesions;
1 point: blurry image, heavy image noise, uninterpretable.
Different criteria were applied to 3D images:
5 points: smooth endoluminal wall, clear lesion morphology;
4 points: relatively smooth endoluminal wall, distinguishable lesion morphology;
3 points: irregular endoluminal wall, relatively distinguishable lesion morphology;
2 points: rough endoluminal wall, barely distinguishable lesion morphology;
1 point: highly rough endoluminal wall, indistinguishable lesion morphology.
Tumor detection and location
The colonic and rectal tumor locations were recorded by the same radiologists mentioned above while assessing the image quality. The colon and rectum were divided into nine segments [16]: ileocecal junction, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and rectum. If divergent opinions appeared, a consensus reading was performed to determine a final conclusion. An intergroup comparison was made between the tumor locations reported by the two radiologists and by a surgeon, using the operation result as the reference standard.
Statistical analysis
Data analysis was performed with SPSS 26.0 statistical software. For the normally distributed continuous variables, expressed as mean ± SD, the Paired sample t test was used, whereas for the non-normally distributed variables, expressed as median (Interquartile range, IQR), Wilcoxon sign rank test was used. The comparison of subjective scores between the control group and Auto-kVp groups used Wilcoxon sign rank test. The Kappa test was used to compare the inter-reader consistency and tumor location consistency between CTC and surgery: Kappa value ≥ 0.75, good consistency, 0.75 > Kappa value > 0.4, moderate consistency, Kappa value ≤ 0.4, poor consistency. P < 0.05 represented a statistically significant difference.
Results
Patient demographics
After applying the inclusion and exclusion criteria, a total of 90 patients (44 males and 46 females; age range, 37–85 years; mean age 65.80 ± 8.90 years) were finally included in our study, with an average body mass index (BMI) of 23.26 ± 2.96 kg/m2 (range, 16.67–30.11 kg/m2). The detailed patient demographics and histopathological information of ULDCTC-80 kVp and ULDCTC-100 kVp were demonstrated in Table 1.
Radiation dose
The scan lengths (in mm) of the prone and supine images, respectively representing the SDCTC (120 kVp) and ULDCTC (80 or 100 kVp), had statistically insignificant difference (427.00 ± 36.61 vs. 420.92 ± 30.85, 441.65 ± 40.22 vs. 434.38 ± 46.36, both P>0.05). The mean CTDIvol and DLP with ULDCTC-80 kVp decreased by 70.46% and 71.43%, respectively compared with the same patient of 120 kVp (both P < 0.001, Table 2). The average ED for ULDCTC-80 kVp was 0.70 mSv, compared to 2.45 mSv for 120 kVp, resulting in a dose reduction of 71.43% (P < 0.001). All cases (50/50) had an ED less than 1 mSv, which all met the ultra-low dose requirement. When it comes to the ULDCTC-100 kVp subgroup, the average CTDIvol, DLP, and ED were 1.29 mGy, 65.19 mGy·cm, and 0.98 mSv, compared to the 4.63 mGy, 241.78 mGy·cm, and 3.63 mSv for 120 kVp, resulting in a dose reduction of 72.14%, 73.04%, and 73.00%, respectively (all P < 0.001, Table 2). 40/40 cases had an ED less than 1.10 mSv, which was comparable to the dose of ULDCTC-80 kVp group.
Quantitative image analysis
The CT values of tumors with ULDCTC-80 kVp were significantly higher than those with 120 kVp, and the mean image noise of ULDCTC-80 kVp was significantly lower than that of 120 kVp (both P < 0.001), because the higher postset ASIR-V weight of ULDCTC-80 kVp significantly reduced image noise. Hence, the tumour SNR and CNR of ULDCTC-80 kVp were higher than those of 120 kVp (P < 0.001, Fig. 2). In addition, the same results could be found in the comparison between ULDCTC-100 kVp and 120 kVp. The image standard deviations of tumors were significantly lower and their CT values, SNR and CNR values were significantly higher with ULDCTC-100 kVp than with 120 kVp (all P < 0.001).
Qualitative image analysis
No significant difference was observed in the subjective scores between the ULDCTC and SDCTC, no matter in 2D or 3D images (all P>0.05). A good consistency of subjective image quality existed among the two reviewers (Kappa value 0.805~0.923, P<0.001). In both groups, no images were found to be non-diagnostic (Fig. 3). Representative examples are presented in Figs. 4 and 5.
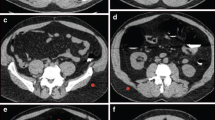
A 70-year-old female with a 3.5-cm tubular adenocarcinoma in the distal rectum (BMI 22.89 kg/m2). The tumor is clearly depicted (red arrow) on the (a) 2D MPR reconstructed by 30% post-set ASIR-V, (b) 3D CTVC, and (c) 3D Raysum image, with an ED of 2.41 mSv. In the supine position, the tumor is well visualized (red arrow) on the (d) 2D MPR reconstructed by 80% post-set ASIR-V, (e) 3D CTVC, and (f) 3D Raysum images, with an ED of 0.70 mSv. After reducing the radiation dose by 70.95%, the image qualities of the supine images were still comparable to those of prone images
A 67-year-old male with a 4.0-cm tubular adenocarcinoma in the sigmoid colon (BMI 27.55 kg/m2). The tumor is clearly depicted (red arrow) on the (a) 2D MPR reconstructed by 30% post-set ASIR-V, (b) 3D CTVC, and (c) 3D Raysum image, with an ED of 3.96 mSv. In the supine position, the tumor is well visualized (red arrow) on the (d) 2D MPR, (e) 3D CTVC, and (f) 3D Raysum images, with an ED of 0.93 mSv. After reducing the radiation dose by 76.52%, the image qualities of the supine images were still comparable to those of prone images
Diagnostic performance of SDCTC and ULDCTC
The locations of colorectal tumors reported in CTC and surgery are summarized in Table 3. A total of 91 colorectal tumors were reported in surgery, including 82 CRC with one patient had two cancers (one in the ascending colon, and one in the rectum), 8 premalignant tubulovillous adenomas with high-grade dysplasia, and 1 benign tubular adenoma. Compared with surgical results, SDCTC and ULDCTC both detected 90 out of 91 tumors (98.9%), including the patient who had two tumors in the colon. The percentage of colorectal tumors detected in ULDCTC-80 kVp group were 100%, whereas only one tumor was missed in ULDCTC-100 kVp group (97.5%), which was confirmed to be a benign tubular adenoma in ileocecal junction by pathology. The minimal tumor diameters reported by surgery were 1.6 cm and 1.7 cm in ULDCTC-80 kVp and ULDCTC-100 kVp subgroups, respectively, which were two premalignant tubular adenomas been successfully detected on the ULDCTC as well. Both the SDCTC and ULDCTC had good consistency in determining tumor location with surgery reports (Kappa value 0.718~0.989, P<0.05). In addition, 16/90 (17.7%) patients had incomplete colonoscopy due to occlusive CRC, leaving 87/810 (10.7%) colon segments inaccessible to colonoscopy. In our study, ULDCTC was successfully achieved in surgical candidates.
Discussion
Our study realized individualized ultra-low dose CTC (ULDCTC) without degrading image quality and tumor diagnosis. The air injected during CTC examination, serving as a negative contrast, not only increased the contrast between the intestinal tract and lesions, but also provided a great potential for a dramatic dose reduction, making sub-mSv CTC technically feasible [17]. Our results demonstrated that for the CTC patients given 80 kVp, the ULDCTC could be achieved by combining 60% preset ASIR-V with a NI of 13, whereas for the subgroup of 100 kVp, the target could be achieved by combining 60% preset ASIR-V with a NI of 15.
In our study, few patients were recommended 120 kVp by the Auto-prescription technique, might be due to the relatively small body habitus of the Asian population compared with patients in the western countries. Therefore, those patients given 120 kVp were excluded in order to ensure the ULDCTC image quality. Even though a few studies indeed realized a sub-mSv CTC under 120 kVp, their limitations were also obvious. For example, although a previous study by Yasuda et al. [18] achieved an even lower average ED of 0.64 mSv, its maximum ED reached up to 2.64 mSv, implying that a significant proportion of patients in their study received the radiation dosage higher than 1 mSv. Another case in point was that Cianci et al. [19] decreased tube current of CTC from a a quality reference of 55 milliamper second (mAs) to an ultra-low level of 25 mAs, resulting in a 63.2% effective dose reduction. However, 14 out of 82 patients (17.1%) in Cianci’s study received a median ED of 1.31 mSv, demonstrating that the ED in patients with large body sizes failed to reach an ultra-low level. On the contrary, the outperforming advantage of our study is that not only the mean effective dose (ED) reached the standard of ultra-low dose, but the ED for each patient in both 80 kVp and 100 kVp groups was lower than 1 mSv. At this point, our study realized an actual sub-mSv CTC imaging, with a personalized radiation dose below 1 mSv, which was superior to other previous research [17,18,19]. Furthermore, the body mass indices of our enrolled patients ranged from 16.67 to 30.11 kg/m2, which covered a relatively large population with different body sizes, indicating that our ULDCTC protocol had a great general applicability.
In the context of individualized low-dose imaging, Li et al. [20] manually selected tube voltage based on the CTC patients’ BMI, resulting in a 63.6%, 44.6% and 32.1% ED reduction in patients scanned by 70 kVp, 80 kVp, and 100 kVp, respectively. Another study by Wei et al. [21] applied tailored tube voltage of 80 kVp and 110 kVp for patients BMI ≤ 21 kg/m2 and > 21 kg/m2, respectively, to obtain a low-dose CT-guided hook wire localization for pulmonary nodules. However, the resulting average ED for patients whose BMI were more than 21 kg/m2 was 2.33 mSv, indicating that the sub-mSv imaging was only achieved among the population with small body sizes, limited the clinical application value of this protocol. Even if BMI was the most frequent index for group divisions, the potential selection bias was unavoidable because of the subjective decision made during kVp selection. By contrast, our study utilized Auto prescription technique, which automatically gave tube voltage recommendation on the basis of anteroposterior (AP) and lateral scout images, so that the individual tube voltage could be given objectively in actual, avoiding manual grouping bias [22]. In addition, the above-mentioned studies only achieved personalized CT imaging, but their average EDs were higher than 1 mSv.
Moreover, ASIR-V in the CT systems of GE Healthcare can be divided into preset and post-set ASIR-V, which both can be adjusted within the range from 0 to 100%. The function of preset ASIR-V reduces radiation dose by lowering the tube current, with the weight of 30% being conventional in our department, while post-set ASIR-V improves image quality by reducing image noise. Preset ASIR-V reduces tube current (milliampere, mA) using automatic tube current modulation without affecting the CT value [23]. According to a research by Zhu et al. [24], the image quality of non-contrast CT abdomen could be maintained when the post-set ASIR-V strength was equal to or higher than that of the preset ASIR-V. Thus, in the ULDCTC group, the preset and post-set ASIR-V weights were increased to 60% and 80%, respectively. NI is slightly different from preset ASIR-V, which is used to set the desired noise level, thereby indirectly controlling the radiation dose. In other words, integrating preset ASIR-V and NI allows for the precise adjustments of radiation dose. In the clinical practice, however, the dose reduction and parameter adjustment of our study are well-founded. According to Zhao et al’s study [12], when the weight of preset ASIR-V was higher than 70%, the image noise significantly increased, strongly affecting the subjective scores of image quality. Therefore, our study only increased the weight of preset ASIR-V to 60%, pursuing the balance between dose reduction and image quality. Additionally, since a One-Size-Fits-All method to scan all patients was technically inappropriate, different noise indices were used in our study for the Auto-kVp group. According to Zhao et al.’s study [25], NI was increased to 15 for patients whose BMI ≥ 24 kg/m2 in hepatic CTA. Although NI could be increased even larger to achieve a considerable dose reduction [26], the image quality would be heavily deteriorated, such as affecting the delineation of colorectal tumor and the margin sharpness, which led to low subjective scores. Therefore, our study only increased NI to 15 for patients scanned by 100 kVp, not only to match the individual ED to a sub-mSv level, but keeping the overall image quality at a diagnostic acceptable level as well.
An interesting point appeared in our study was that prone and supine CTC images reported the same result in defining colonic tumor locations. In other words, our results indicated that the supine ULDCTC images had comparable performance with the prone SDCTC images. Furthermore, our study divided colon and rectum into nine segments, which was more detailed than four or seven-segment division in prior studies, fulfilling the preoperative requirement of precise tumor localization [6, 27]. In addition, 16/90 (17.7%) patients had incomplete colonoscopy due to occlusive CRC, leaving 87/810 (10.7%) colon segments inaccessible to colonoscopy, thereby affecting preoperative evaluation and the outcome of radical surgery for CRC. However, since our SDCTC and ULDCTC can provide images of the same quality, clinicians were still able to reference these images to perform surgical treatment on these patients.
Despite of the good results, our study has several limitations that should be acknowledged. Firstly, this was a study from a single center with a relatively small study population. Therefore, multi-center studies with larger number of participants should be conducted for further investigation. Regarding the bowel preparation, on the one hand, it was relatively suboptimal due to no fecal tagging. On the other hand, the dosage of laxatives was the same for all patients, irrespective of whether the patients had occlusive CRC. However, this may be due to the different standards of bowel preparation in different nations. To ensure the image quality, patients with poor bowel distention and poor air inflation were excluded in our study. Furthermore, for image reconstruction, we selected the same post-set ASIR-V weight for both ULDCTC-80 kVp and ULDCTC-100 kVp images, even though they used different NI. The reason is that the more essential focus of our study was to explore how to realize ULDCTC rather than to explore the effect of iterative reconstruction algorithm on the image quality. Thus, a more specific comparison of utilizing variable strengths of post-set ASIR-V should be considered in the future ULDCTC research. In addition, our study was unable to apply deep learning reconstruction algorithms (DL) to the ULDCTC images, due to the software upgrade issue. Hence, comparing the denoising effect of DL with post-set ASIR-V in ULDCTC is a potential aspect to be explored.
Conclusion
In conclusion, the combination of automatic tube voltage selection, 60% preset ASIR-V and noise index at 13 or 15 can achieve ULDCTC, with the mean effective dose less than 1 mSv in the patient population within a wide range of BMI values. The diagnostic performance was revealed by the high detection rate and good consistency of ULDCTC in locating colorectal cancer with surgical results. Therefore, the modified sub-mSv CTC protocol has the potential to be recommended in clinical practice.
Data availability
Data is provided within the manuscript.
References
Valletta R, Faccioli N, Bonatti M et al (2022) Role of CT colonography in differentiating sigmoid cancer from chronic diverticular disease. Jpn J Radiol 40(1):48–55. https://doi.org/10.1007/s11604-021-01176-8
Zhang Y, Wang Y, Zhang B, Li P, Zhao Y (2023) Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. Biomed Pharmacother 163:114786. https://doi.org/10.1016/j.biopha.2023.114786
Latos W, Aebisher D, Latos M et al (2022) Colonoscopy: Preparation and Potential Complications. Diagnostics (Basel) 12(3):747. https://doi.org/10.3390/diagnostics12030747
Chervenkov L, Sirakov N, Georgiev A et al (2023) High Concordance of CT Colonography and Colonoscopy Allows for the Distinguishing and Diagnosing of Intestinal Diseases. Life (Basel) 13(9):1906. https://doi.org/10.3390/life13091906
Wesp P, Grosu S, Graser A et al (2022) Deep learning in CT colonography: differentiating premalignant from benign colorectal polyps. Eur Radiol 32(7):4749–4759. https://doi.org/10.1007/s00330-021-08532-2
Horvat N, Raj A, Ward JM, Smith JJ, Markowitz AJ, Gollub MJ (2018) Clinical Value of CT Colonography Versus Preoperative Colonoscopy in the Surgical Management of Occlusive Colorectal Cancer. AJR Am J Roentgenol 210(2):333–340. https://doi.org/10.2214/AJR.17.18144
de Kanter C, Dhaliwal S, Hawks M (2022) Colorectal Cancer Screening: Updated Guidelines From the American College of Gastroenterology. Am Fam Physician 105(3):327–329.
Spada C, Hassan C, Bellini D et al (2021) Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline-Update 2020. Eur Radiol 31(5):2967–2982. https://doi.org/10.1007/s00330-020-07413-4
Papadakis AE, Damilakis J (2019) Automatic Tube Current Modulation and Tube Voltage Selection in Pediatric Computed Tomography: A Phantom Study on Radiation Dose and Image Quality. Invest Radiol 54(5):265–272. https://doi.org/10.1097/RLI.0000000000000537
Euler A, Taslimi T, Eberhard M et al (2021) Computed Tomography Angiography of the Aorta-Optimization of Automatic Tube Voltage Selection Settings to Reduce Radiation Dose or Contrast Medium in a Prospective Randomized Trial. Invest Radiol 56(5):283–291. https://doi.org/10.1097/RLI.0000000000000740
Choi MH, Lee YJ, Jung SE (2020) A lesson from automatic tube voltage selection: feasibility of 100 kVp in portal venous phase abdominal CT. Radiat Prot Dosimetry 188(4):424–431. https://doi.org/10.1093/rpd/ncz302
Zhao Y, Li D, Liu Z, Geng X, Zhang T, Xu Y (2021) Comparison of image quality and radiation dose using different pre-ASiR-V and post-ASiR-V levels in coronary computed tomography angiography. J Xray Sci Technol 29(1):125–134. https://doi.org/10.3233/XST-200754
Liu JJ, Xue HD, Liu W et al (2021) CT colonography with spectral filtration and advanced modeled iterative reconstruction in the third-generation dual-source CT: image quality, radiation dose and performance in clinical utility. Acad Radiol 28(5):e127-e136. https://doi.org/10.1016/j.acra.2020.03.040
Afadzi M, Lysvik EK, Andersen HK, Martinsen ACT (2019) Ultra-low dose chest computed tomography: Effect of iterative reconstruction levels on image quality. Eur J Radiol 114:62–68. https://doi.org/10.1016/j.ejrad.2019.02.021
Israel GM, Cicchiello L, Brink J, Huda W (2010) Patient size and radiation exposure in thoracic, pelvic, and abdominal CT examinations performed with automatic exposure control. AJR Am J Roentgenol 195(6):1342–6. https://doi.org/10.2214/AJR.09.3331
Manigrasso M, Milone M, Musella M et al (2022) Preoperative Localization in Colonic Surgery (PLoCoS Study): a multicentric experience on behalf of the Italian Society of Colorectal Surgery (SICCR). Updates Surg 74(1):137–144. https://doi.org/10.1007/s13304-021-01180-7
Liu JJ, Xue HD, Liu W et al (2021) CT colonography with spectral filtration and advanced modeled iterative reconstruction in the third-generation dual-source CT: image quality, radiation dose and performance in clinical utility. Acad Radiol 28(5):e127-e136. https://doi.org/10.1016/j.acra.2020.03.040
Yasuda T, Honda T, Utano K et al (2022) Diagnostic accuracy of ultra-low-dose CT colonography for the detection of colorectal polyps: a feasibility study. Jpn J Radiol 40(8):831–839. https://doi.org/10.1007/s11604-022-01266-1
Cianci R, Delli Pizzi A, Esposito G et al (2019) Ultra-low dose CT colonography with automatic tube current modulation and sinogram-affirmed iterative reconstruction: Effects on radiation exposure and image quality. J Appl Clin Med Phys 20(1):321–330. https://doi.org/10.1002/acm2.12510
Li B, Wang X, Fan Y et al (2023) Evaluation of BMI-based tube voltage selection in CT colonography: A prospective comparison of low kV versus routine 120 kV protocol. J Appl Clin Med Phys 24(5):e13955. https://doi.org/10.1002/acm2.13955
Wei W, Wang SG, Zhang JY et al (2023) Implementation of Individualized Low-Dose Computed Tomography-Guided Hook Wire Localization of Pulmonary Nodules: Feasibility and Safety in the Clinical Setting. Diagnostics (Basel) 13(20):3235. https://doi.org/10.3390/diagnostics13203235
He W, Chen X, Hu R, Sun W, Tan W (2022) Influence of Contrast Agent Injection Scheme Customized by Dual-Source CT Based on Automatic Tube Voltage Technology on Image Quality and Radiation Dose of Coronary Artery Imaging. Front Surg 9:862697. https://doi.org/10.3389/fsurg.2022.862697
Zhao Y, Li D, Liu Z, Geng X, Zhang T, Xu Y (2021) Comparison of image quality and radiation dose using different pre-ASiR-V and post-ASiR-V levels in coronary computed tomography angiography. J Xray Sci Technol 29(1):125–134. https://doi.org/10.3233/XST-200754
Zhu Z, Zhao Y, Zhao X et al (2021) Impact of preset and postset adaptive statistical iterative reconstruction-V on image quality in nonenhanced abdominal-pelvic CT on wide-detector revolution CT. Quant Imaging Med Surg 11(1):264–275. https://doi.org/10.21037/qims-19-945
Zhao S, Liu ZC, Zhao YX, Zhang TL, Zuo ZW (2023) A feasibility study of different GSI noise indexes and concentrations of contrast medium in hepatic CT angiography of overweight patients: image quality, radiation dose, and iodine intake. Jpn J Radiol 41(6):669–679. https://doi.org/10.1007/s11604-022-01384-w
Cao L, Liu X, Li J et al (2021) A study of using a deep learning image reconstruction to improve the image quality of extremely low-dose contrast-enhanced abdominal CT for patients with hepatic lesions. Br J Radiol 94(1118):20201086. https://doi.org/10.1259/bjr.20201086
Taguchi N, Oda S, Imuta M et al (2018) Model-based Iterative Reconstruction in Low-radiation-dose Computed Tomography Colonography: Preoperative Assessment in Patients with Colorectal Cancer. Acad Radiol 25(4):415–422. https://doi.org/10.1016/j.acra.2017.10.008
Author information
Authors and Affiliations
Contributions
J.Z wrote the main manuscript text. M.H. and Q.C. were responsible for methodology and visualization. S.W. and J.L. reviewed and edited the manuscript. Y.L. was responsible for the conceptualization and supervision of the study. Y.Z. was responsible for the validation and supervision. W.W. was responsible for data curation, software and manuscript reviewing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Hu, M., Cheng, Q. et al. Achieving sub-millisievert CT colonography for accurate colorectal tumor detection using smart examination protocols: a prospective self-controlled study. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04557-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04557-5