Abstract
Purpose
The Working Group of the Gynecological Tumor Committee of the Japanese Radiation Oncology Study Group (JROSG) published recommendations for contouring high-risk clinical target volumes (HR-CTV) on CT for 3-dimentional image-guided brachytherapy for cervical cancer. The purpose of this study was to compare HR-CTV delineated on CT, referencing recommendations from JROSG, with HR-CTV delineated on MRI, referencing consensus guidelines from the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC ESTRO).
Material and methods
Fourteen patients were evaluated. After the insertion of the CT/MR applicator into the patient, CT and MRI were performed. HR-CTVMR was delineated on MRI for clinical examination and HR-CTVCT was delineated on CT retrospectively referencing the MRI just before brachytherapy within a week. The volume and dosimetry of HR-CTV based on a 6 Gy dose of the Manchester system were evaluated.
Results
The median volumes of HR-CTVMR and HR-CTVCT were 24.4 ml (range, 13.6–50.4 ml) and 29.9 ml (range, 13.2–49.2 ml), respectively. Median D90 of HR-CTVMR and HR-CTVCT were 6.7 Gy (range, 5.8–10.1 Gy) and 6.8 Gy (range, 5.1–10.4 Gy), respectively.
Conclusion
Somewhat difference could be seen between HR-CTVMR and HR-CTVCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brachytherapy is an essential component of curative treatment for patients with locally advanced uterine cervix carcinoma [1].
The Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC ESTRO) proposed the concept of 3-dimensional (3D) image-guided brachytherapy (IGBT) for cervical cancer [2,3,4]. GEC ESTRO developed a common language for magnetic resonance image (MRI) -guided IGBT, such as delineation of the high-risk clinical target volume (HR-CTV).[2] Since then, the use of IGBT has been spreading, particularly in Europe, North America and Asia [5,6,7,8].
GEC ESTRO consensus guidelines were originally designed for MRI-guided IGBT. However, in many institutions, computed tomography (CT) is commonly used for IGBT because of increased accessibility of CT compared with MRI [5, 7, 9,10,11,12,13].
Viswanathan et al. published contouring guidelines for CT-based HR-CTV [14, 15]. After comparing CT-based HR-CTV (HR-CTVCT) with MRI-based HR-CTV (HR-CTVMR), they concluded that the width of HR-CTVCT are greater than that of HR-CTVMR, particularly in cases with parametrial extension.
To reduce these differences in HR-CTV between CT and MRI, the Working Group of the Gynecological Tumor Committee of the Japanese Radiation Oncology Study Group (JROSG) published recommendations for contouring the HR-CTV for CT-based IGBT, referencing MRI just before brachytherapy [16]. The recommendations of JROSG were made on the basis of the GEC-ESTRO consensus guidelines of MRI-based HR-CTV. JROSG emphasized the necessity of MRI just before brachytherapy for CT-based HR-CTV to identify the extent of residual tumors [17]. However, no study has confirmed this JROSG recommendation.
In our institution, MRI-based IGBT is performed and CT images are obtained at the same time. Therefore, we compared delineation of HR-CTV contoured on CT referencing JROSG recommendations with delineation of HR-CTV contoured on MRI referencing GEC-ESTRO consensus guidelines.
Materials and methods
Patient enrollment
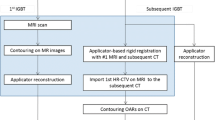
This was a retrospective analysis of patients with histologically proven carcinoma of the uterine cervix treated by external beam irradiation and IGBT. JROSG recommended that T2-weighted MR images, acquired at the time of diagnosis and just before brachytherapy without an applicator, should be routinely used as a reference. To confirm JROSG delineation, all patients were scanned by MRI at least three times: at diagnosis, within a week before brachytherapy (just before BT), and at brachytherapy applicator in situ (IGBT). As MRI-based brachytherapy was introduced in 2014, we included patients from 2014 in this study. Of 90 patients treated between July 2014 and December 2016, we included patients who underwent MRI at diagnosis, just before BT and at IGBT completely (Fig. 1). There were 14 patients who underwent all these examinations. Our institutional review board approved this study.
Treatment protocol
Patients initially underwent external beam radiotherapy (EBRT) with or without chemotherapy. Patients underwent brachytherapy three or four times after 30–40 Gy of EBRT. EBRT continued to a median dose of 50 Gy using 4 cm wide midline block.
CT/MR applicators (Fletcher CT/MR Applicator, Tandem/ovoid applicator, Elekta AB, Stockholm, Sweden) were used for brachytherapy. Applicators were placed under sedation using low-dose propofol and ketamine [18].
CT and MRI at brachytherapy
After the insertion of applicators, in-room CT was performed. The CT images were acquired on an Alexion CT scanner (Canon Medical Systems, Tokyo, Japan) in 2 mm slice intervals without intravenous contrast.
Following CT, patients were transferred to an MRI room. The MR images were acquired by a GE Signa 1.5 T MRI scanner (GE Medical System, Milwaukee, USA) with an eight-channel phased-array cardiac coil. MR sequences complied with GEC-ESTRO recommendations [4]. MRI using a 3D isotropic sequence (variable refocusing flip angle-fast spin echo: VRFA) were acquired in the sagittal plane with a 2000 ms repetition time (TR), a 101 ms echo time (TE), a 80 echo train length, a 256 mm field of view, and a 1.4 × 1.4 × 1.4 mm voxel size were reconstructed into axial images [19]. For reducing bowel motion, scopolamine butylbromide or glucagon was administered.
The CT images were acquired at every brachytherapy, whereas the MR images were acquired at the first brachytherapy. CT and MR images of the first brachytherapy were used for this study.
Contouring of HR-CTV
HR-CTV was delineated on MRI(HR-CTVMR) and on CT(HR-CTVCT) for each patient. HR-CTVMR was delineated by each radiation oncologist based on GEC-ESTRO consensus guidelines in clinical practice. HR-CTVMR was delineated using Oncentra brachy version4.3 (Elekta AB, Stockholm, Sweden).
HR-CTVCT was delineated by Y.I. radiation oncologist (with 2 years of experience of 3D-IGBT) retrospectively following JROSG recommendations. These recommendations defined craniocaudal, lateral and anteroposterior boundaries on CT images using MR images acquired at diagnosis and just before BT. In general, transverse tumor diameter was carefully evaluated on just before BT MR axial image in cases with paremetrial invasion. For cases with uterine corpus invasion, tumor length toward uterine fundus was measured on just before BT MR sagittal images. Findings of pelvic examination were documented on medical records. Pelvic examination findings of tumor size and vaginal, parametrial, rectal, and bladder invasion judged by gynecologists and radiation oncologists at diagnosis and just before brachytherapy were schematically illustrated on a specific chart, and carefully referenced at the time of delineation of HR-CTVCT. Delineation of HR-CTVCT was done without any prior MRI information with an applicator in situ. Each HR-CTVCT was reviewed by the other radiation oncologist (4 years of experience of 3D-IGBT). HR-CTVCT were delineated using MIM Maestro.version6.4.4 (MIM Software, Cleveland, USA).
Evaluation of HR-CTV
The values for height (SI), width (LR), thickness (AP), and volume were generated for the HR-CTVMR and HR-CTVCT contours. LR is maximum lateral length of HR-CTV. Lateral lengths were measured on each slice of the images. The highest value was selected as the maximum length. SI is superior-inferior length. The number of slices from the top of HR-CTV to bottom was counted. SI is calculated from the number of slices and slice thickness. AP is the maximum anterior–posterior length that was measured using a similar technique that was used for LR (Fig. 2). Each length of HR-CTVMR with that of HR-CTVCT was compared using a paired sample t-test. The analyses were done with the JMP computer software (version 13).
Volumes displayed on MIM Maestro were also recorded as HR-CTV. The volume ratio of HR-CTVMR to HR-CTVCT of each patient was determined. To evaluate of conformity of HR-CTVMR and HR-CTVCT, the Dice coefficient was used [20]. MR images and HR-CTVMR were co-registered on CT images by manual fusion, adjusting the tandem and ovoid of both images to evaluate Dice coefficient.
A Volume of HR-CTVMRB Volume of HR-CTVCT
To examine the impact of variations in HR-CTV delineation on dosimetric parameters, virtual Manchester plans were applied to each contour sets on Oncentra Brachy (Elekta, Stockholm, Sweden) and dose-volume histogram (DVH) parameters were determined. The minimum dose to 90% (D90) of the HR-CTV was determined for each HR-CTVMR and HR-CTVCT. D90 of the HR-CTV were plotted with respect to volumes of HR-CTV. With every case, the arrow starts from HR-CTVCT and ends to HR-CTVMR (Fig. 6).
Results
Patients characteristics
The median age was 49 (range, 28–86). FIGO 2008 stages were IB1 in 1, IB2 in 5, IIA1 in 2, IIB in 2, IIIB in 3, and IVA in 1. Parametrial invasion was observed in six patients.
Lengths and volumes
The median lengths of HR-CTVMR and HR-CTVCT are shown in Table 1. The LR of HR-CTVMR and HR-CTVCT were 41.7 mm (range, 32.6–49.9 mm) and 42.25 mm (range, 30.0–59.5 mm), the SI were 38 mm (range, 27–65 mm) and 42 mm (range, 30–63 mm), the AP of HR-CTVMR and HR-CTVCT were 36.4 mm (range, 29.5–50.7 mm) and 40 mm (range, 30.7–48.1 mm), respectively (Fig. 3a–c). In five of six patients with parametrial invasion, the LR of HR-CTVCT were longer than LR of HR-CTVMR (Fig. 3d).
The median volume of HR-CTVMR and HR-CTVCT was 24.4 ml (range, 13.6–50.4 ml) and 29.9 ml (range, 13.2–49.2 ml), respectively (p = 0.012 paired t-test) (Fig. 4). The median volume ratio of HR-CTVMR to HR-CTVCT was 0.82 (range, 0.54–1.25). HR-CTVMR was significantly lower than HR-CTVCT. Median Dice coefficients of volume between HR-CTVMR and HR-CTVCT was 74.1% (range, 61.2–82.1%). The case with the lowest Dice coefficient was stage IIIB with poor abdominal fat. The CT intensity of the patient’s parametrium was as high as the uterus because of parametrial invasion. It was difficult to recognize the border between the uterus and bowels. In some cases of uterine myoma, it was difficult to recognize the border between the uterus and myoma as well.
Dosimetry
Median D90 of HR-CTVMR and HR-CTVCT were 6.7 Gy (range, 5.8–10.1 Gy) and 6.8 Gy (range, 5.1–10.4 Gy), respectively. In three cases, there was a divergence of more than 1 Gy between HR-CTVMR and HR-CTVCT (Fig. 5). The D90 of HR-CTVCT were lower than that of HR-CTVMR in these three cases, and the volume of HR-CTVCT were higher than that of HR-CTVMR. The delineation of HR-CTVCT of these three cases was difficult because of poor abdominal fat in two cases, and uterine myoma in the other case. Optimizing dosimetry for these structures, patients treated based on HR-CTVCT can receive higher doses than on MRI.
Dosimetry of HR-CTV when 6 Gy is administered for point A. Dosimetry is evaluated as D90 of HR-CTV. Each arrow corresponds to a patient. Starting point of arrow indicates volume and dosimetry of HR-CTVCT, and ending point indicates HR-CTVMR. Dotted arrows indicate that the volume of HR-CTVMR was higher than HR-CTVCT
Parameters of the six cases with parametrial invasion are shown in Table 2. In five of these cases, D90 of HR-CTVMR were higher than that of HR-CTVCT. LR and volume of HR-CTVCT were slightly higher than those of HR-CTVMR. When the volume of HR-CTVMR was lower than that of HR-CTV CT, D90 of HR-CTVMR was higher than that of HR-CTVCT.
Discussion
We evaluated HR-CTV delineations of 14 patients who were scanned both by CT and MRI. Lateral lengths and volumes of HR-CTVMR were slightly lower than those of HR-CTVCT especially in patients with parametrial invasion.
Parametrial invasions were recognized as having the same density as the uterus on CT, while the border of the uterus and parametrial invasions could be recognized in MRI. Consistent with previous reports, HR-CTVCT were slightly wider than HR-CTVMR.[14, 15].
For delineation, MRI was more useful than CT especially for patients with parametrial invasion, uterine myoma and/or minimal abdominal fat. The border between extra uterine organs, such as small intestine and uterus, is often difficult to be recognized on CT (Fig. 6). Recognizing the border between uterus and intestine is very important because intestines are organs at risk. In other reports, CT with intravenous contrast or contrast in the rectum was recommended to recognize the intestine [21]. However, the administration of exogenous contrast agents is not always feasible due to possible nephrotoxicity or radiation enteritis.
For contouring HR-CTV on CT, just before BT MRI is useful for identifying myoma and the border between bowels and uterus. However, the uterus is extended and moved by the insertion of the applicator. Thus, using just before BT MRI as a reference image for contouring HR-CTV on CT with the applicator in situ can pose challenges. Though deformable image registration is an emerging technology, it has difficulties accounting for the anatomical differences caused by an applicator in situ.[22]
Limitations
One limitation of this study is the small study sample from a single institution. Possible inter-physician and inter-institution differences were not assessed. In this study, HR-CTVMR were delineated by radiation oncologists at first IGBT. Conversely, HR-CTVCT were delineated by one radiation oncologist retrospectively. To ensure objectivity and quality of HR-CTVCT, HR-CTVCT were reviewed by the other radiation oncologist.
Selection bias is another limitation. In this study, selected patients underwent CT and MRI at IGBT and just before BT MRI. In our institution, almost all patients undergo CT and MRI at the first IGBT. However, the number of patients who undergo MRI to evaluate tumor response and uterine deformation just before brachytherapy is still limited.
Conclusion
We compared the delineations of HR-CTVMR with HR-CTVCT. Somewhat difference could be seen between HR-CTVMR and HR-CTVCT. The volume of HR-CTVMR was slightly lower than that of HR-CTVCT. For delineation on CT, referencing the just before BT MRI is especially useful in cases with parametrial invasion, uterine myoma, and minimal abdominal fat. However, it is important to consider that the uterus can deform and change direction after insertion of applicators.
Even if the same treatment strategy for cervical cancer is chosen, the dose between CT and MRI CTV may be different, especially in patients with locally advanced tumors.
References
Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie-Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. 2018;127:404–16.
Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Bradandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (I): concepts and terms in 3D image-based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–45.
Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Bradandere M, Dimopoulos J, et al. GEC-ESTRO Working Group: Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77.
Dimopoulos JC, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image-based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103:113–22.
Tan LT. Implementation of image-guided brachytherapy for cervix cancer in the UK: progress update. Clin Oncol. 2011;23:681–4.
Pavamani S, D’Souza DP, Portelance L, Craighead PS, Pearce AG, Traptow LL, et al. Image-guided brachytherapy for cervical cancer: a Canadian Brachytherapy Group survey. Brachytherapy. 2011;10:345–51.
van Dyk S, Byram D, Bernshaw. Use of 3D imaging and awareness of GEC–ESTRO recommendations for cervix cancer brachytherapy throughout Australia and New Zealand. J Med Imaging Radiat Oncol. 2010; 54:383–7.
Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104–9.
Kusada T, Toita T, Ariga T, Maemoto H, Hashimoto S, Shiina H, et al. Computed tomography–based image-guided brachytherapy for cervical cancer: correlations between dose–volume parameters and clinical outcomes. J Radiat Res. 2018;59:67–766.
Dimopoulos JC, Lang S, Kirisits C, Fidarova EF, Berger D, Georg P, et al. Dose–volume histogram parameters and local tumor control in magnetic resonance image–guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:56–63.
Grover S, Harkenrider MM, Cho LP, Erickson B, Small C, Small W, et al. Image guided cervical brachytherapy: 2014 survey of the american brachytherapy society. Int J Radiat Oncol Biol Phys. 2016;94:598–604.
Phan T, Mula-Hussain L, Pavamani S, Peace A, D’Souza D, Patil NG, et al. The changing landscape of brachytherapy for cervical cancer: a Canadian practice survey. Curr Oncol. 2015;22:356–60.
Toita T, Ohno T, Ikushima H, Nishimura T, Uno T, Ogawa K, et al. National survey of intracavitary brachytherapy for intact uterine cervical cancer in Japan. J Radiat Res. 2018;569:469–76.
Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–8.
Viswanathan AN, Erickson B, Gaffney DK, Bosch W, members of the CT/MR Atlas Committee Comparison and Consensus Guidelines for Delineation of Clinical Target Volume for CT- and MR-Based Brachytherapy in Locally Advanced Cervical Cancer. Int J Radiat Oncol Biol Phys. 2014;90:320–8.
Ohno T, Wakatsuki M, Toita T, Kaneyasu Y, Yoshida K, Kato S, et al. Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res. 2017;3:341–50.
Hegazy N, Pötter R, Kirisits C, Berger D, Federico M, Sturdza A, et al. High-risk clinical target volume delineation in CT-guided cervical cancer brachytherapy: impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol. 2013;52:1345–52.
Nemoto WM, Nozaki-Taguchi N, Togasaki G, Kanazawa A, Kurokawa M, Harada R, et al. New approach to relieving pain and distress during high-dose-rate intracavitary irradiation for cervical cancer. Brachytherapy. 2015;14:642–7.
Nemoto WM, Iwai Y, Togasaki G, Kurokawa M, Harada R, Kobayashi H, et al. Preliminary results of a new workflow for MRI/CT-based image-guided brachytherapy in cervical carcinoma. Jpn J Radiol. 2017;35:760–5.
Dice LR. Measures of the amount of ecologic association between species. Ecology.1945;26 (3):297–302.
Erickson B, Albano K, Gillin M. CT-guided interstitial implantation of gynecologic malignancies. Int J Radiat Oncol Biol. 1996;36:699–709.
Tait LM, Hoffman D, Benedict S, Valicenti R, Mayadev JS. The use of MRI deformable image registration for CT-based brachytherapy in locally advanced cervical cancer. Brachytherapy. 2016;15:333–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study was approved by the local ethics review board and subjects’ informed consent were obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iwai, Y., Nemoto, M.W., Horikoshi, T. et al. Comparison of CT-based and MRI-based high-risk clinical target volumes in image guided-brachytherapy for cervical cancer, referencing recommendations from the Japanese Radiation Oncology Study Group (JROSG) and consensus statement guidelines from the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC ESTRO). Jpn J Radiol 38, 899–905 (2020). https://doi.org/10.1007/s11604-020-00980-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-00980-y