Abstract
Purpose
We propose a method of image-guided brachytherapy (IGBT) that combines MRI-based target volume delineation for the first fraction with CT datasets of subsequent fractions, using an automatic, applicator-based co-registration, and report our preliminary experience.
Materials and methods
The MRI of the first fraction was used for the first brachytherapy planning. For each subsequent brachytherapy fraction, after the same applicator insertion, a new CT scan with the applicator in place was obtained. The MR image set was registered to the subsequent brachytherapy treatment planning CT using the applicator for rigid body registration. To demonstrate the registration quality, we used here the Dice index as a measurement of tandem delineation overlap between CT and MRI.
Results
The median Dice index was 0.879 (range 0.610–0.932), which indicated that the contours on CT and MRI fitted well. With this combination method, the median D90 of HR CTV and the calculated D2 cm3 of the bladder, rectum, and sigmoid in each fraction were 7.2 (4.0–10.4), 5.9 (2.3–7.7), 4.0 (1.9–6.7), and 3.8 (0.6–7.2) Gy, respectively.
Conclusion
Our described method of MRI-guided IGBT offers a practical option for the benefits of target delineation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracavitary brachytherapy is an essential component of the curative treatment of cervical cancer [1]. Appropriate applicator placement and optimal dose distribution must be achieved to obtain good local control and survival with acceptable morbidity rates. Originally validated with orthogonal X-rays to verify applicator position and standard source loading [2], in recent years there has been a transition toward three-dimensional (3D) volumetric planning and optimization [1, 3, 4].
Although use of magnetic resonance imaging (MRI) has demonstrated superiority in target volume delineation [3, 5, 6], it is not routinely offered in brachytherapy centers in Japan because of the lack of MRI accessibility [7]. MRI scanners are large and expensive, and have strict magnetic shielding requirements. On the other hand, computed tomography (CT) scanners are ubiquitous within radiation oncology departments, and staff are well accustomed to their use. As a result, CT-based image-guided brachytherapy (IGBT) remains the dominant modality for the treatment of cervical cancer in Japan, in the United States, and also in Europe [7,8,9,10].
In order to exploit the full potential of CT and MRI, Watanabe et al. have previously reported that patients in our center are routinely treated with IGBT using a CT/MRI approach with an MRI only at the time of the first treatment, because of limited access to MRI for brachytherapy planning [11, 12]. We propose a method of IGBT that combines the MRI-based target volume delineation for the first fraction with CT datasets of subsequent fractions, using an automatic, applicator-based co-registration, and report our preliminary experience.
Materials and methods
Image-guided brachytherapy
First brachytherapy fraction
Patients were sedated with propofol and ketamine [13] in the brachytherapy suite and placed in the dorsal lithotomy position, then a Foley catheter was inserted. After examination, CT/MR applicators (Fletcher CT/MR Applicator, Tandem/ovoid applicator, Nucletron, Veenendaal, The Netherlands) were placed and vaginal packing was performed in the standard fashion [1]. CT images were then acquired using an in-room Alexion CT scanner (Toshiba Medical Systems, Japan).
Following the CT, patients were transferred to an MRI scanner, which took less than 5 min for one way. Patients underwent MR imaging in a GE Signa 1.5 T MRI scanner (GE Medical System, Milwaukee, USA) with an eight-channel phased-array cardiac coil. To reduce bowel motion, an intravenous antispasmodic drug (N-butylscopolan or glucagon chlorhydrate) was administered. Normal saline solution (100 ml) was added to the bladder in order to achieve reproducible bladder filling during image acquisition and treatment delivery. The MR sequences included in this study are widely available in current clinical practice and comply with GEC-ESTRO recommendations [15]. Imaging was completed in only 15 min. The details of the imaging parameters of the above sequences are described in Table 1. MRI using a 3D isotropic sequence (variable refocusing flip angle-fast spin echo: VRFA) were acquired in the sagittal plane with a 2000 ms repetition time (TR), a 101 ms echo time (TE), a 256 mm field of view, and a 1.4 × 1.4 × 1.4 mm voxel size, and were reconstructed into axial images. T2-weighted MRI fast spin echo (FSE) images were obtained in the axial (two-dimensional) plane with a slice thickness of 3 mm. Periodically Rotated Overlapping ParallEL Lines with Enhanced Reconstruction (PROPELLER) (two-dimensional) images were obtained in the sagittal plane with a slice thickness of 4 mm. Patients were then returned to the brachytherapy treatment room.
Planning in the first brachytherapy fractions
The MRI was used for the first brachytherapy planning. From the 3D data set, axial, para-axial, and para-coronal images were reconstructed. A contouring procedure was performed on axial images resampled from the 3D MRI. The gross tumor volume at brachytherapy (GTVB), HR CTV, and organs at risks (OARs) were then identified on the MR images, incorporating the principles defined by the GEC ESTRO [3, 4]. Based on the MRI with the applicator in place, brachytherapy treatment planning was performed using Oncentra Brachy 4.3 (Nucletron).
IGBT was performed with a prescribed dose of 6 Gy at point A using the Manchester method and followed by manual dose optimization to ensure coverage of the HR CTV and to minimize doses to the OARs if necessary. Brachytherapy dose prescription and tolerance dose limits to OARs were based on established guidelines [1, 3, 4, 14]. Plans were optimized to have HR-CTV D90% (the minimum dose received by 90% of the HR CTV) of the prescribed 6 Gy, while dose constraints for OARs were determined as follows: D2 cm3 (the minimum dose to the highest irradiated 2 cm3 volume) bladder < 6.6 Gy per fraction, D2 cm3 rectum < 6 Gy per fraction.
Doses to the HR CTV and OARs were recorded for each fraction, and cumulative doses were tracked on a spreadsheet. After treatment delivery using a 192Ir remote after-loading system (RALS, MicroSelectron HDR, Nucletron), the packing and applicators were removed from the patient.
Subsequent brachytherapy fractions
For each subsequent HDR brachytherapy fraction, after the same applicator insertion and bladder filling with 100 ml normal saline, a new CT scan with the applicator in place was obtained. Acquisition of MRI with the applicator in place was omitted.
Image registration and planning in subsequent brachytherapy fractions
The first IGBT MRI with structure set (HR CTV) and the subsequent CT images were transferred to MIM Maestro ver.6.4.4 (MIM Software Inc., Cleveland, OH, USA). The MR image set was registered to the subsequent brachytherapy treatment planning CT, using the applicator for rigid body registration. We then imported the HR CTV delineation of the first fraction to the subsequent CT images and made adjustments manually if necessary. We delineated the rectum and bladder on subsequent CT images. A well-trained radiation oncologist reviewed the image registration and contours on the CT. These CT images and structure sets were transferred to Oncentra Brachy, and were used for the subsequent brachytherapy planning. The brachytherapy dose was then manually optimized to deliver the full dose to the HR CTV while keeping the critical structure doses within reported tolerance levels, in the same way as the first fraction. The MRI/CT-based image-guided brachytherapy workflow is shown in Fig. 1.
To demonstrate the registration quality, we used here the Dice index as a measurement of tandem delineation overlap between CT and MRI. Contouring the volumes, A as CT tandem and B as MRI tandem, it is defined as:
Dice index \( = \frac{{2\left| {A\mathop \cap \nolimits B} \right|}}{\left| A \right| + \left| B \right|} \).
Radiotherapy technique
The total pelvic sidewall dose from external beam radiation therapy was 50 Gy in 25 fractions. The initial 20–40 Gy was delivered to the whole pelvis with a 4-field box followed by pelvic irradiation with a 3–4-cm width of central shield (CS) being used, reducing OAR exposure. After the central shield was inserted, IGBT was performed in 3–4 fractions, once a week.
Patients’ study
From August 2014 to May 2015, 21 consecutive patients with cervical carcinoma were included in this study. The Fédération Internationale de Gynécologie et d’Obstetrique (FIGO) stage distribution was as follows: IB = 9, IIA = 3, IIB = 5, and IIIB = 4. The median age was 58 years (range 38–72 years). This study was conducted according to the Helsinki Declaration. Informed consent was obtained from all patients before enrollment.
This study was approved by the ethics committee of the Institutional Review Board.
Results
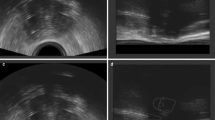
Figure 2a shows a reconstructed sagittal CT image of the second IGBT with the same MRI-compatible applicator. Without MRI, it was almost impossible to delineate GTVB on the CT image. Figure 2b demonstrates a sagittal T2-weighted MR image of the first IGBT. The residual gross tumor was contoured in pink and HR CTV was contoured in blue. We transferred both images to the first MIM Maestro. The MR image set was registered to the second brachytherapy treatment planning CT, using the applicator for rigid body registration. Figure 2c shows the fusion display of the co-registered MRI and CT from the second fraction of brachytherapy. Note that the co-registration was based on the applicator. The quality of the fusion of the CT and MR images was considered to be good, and the fused image set provided excellent resolution. The median Dice index was 0.879 (range 0.610–0.932), which indicated that the contours on CT and MRI fitted well. We then imported the HR CTV delineation of the first fraction to the subsequent CT images and made adjustments manually if necessary (Fig. 2d). Imported HR CTV-based MRI (pink) was protruded into the vaginal packing. Taking into account tumor shrinkage, HR CTV after adjustment was shown as a yellow line.
a A reconstructed sagittal CT image of the second IGBT. b A sagittal T2-weighted MR image of the first IGBT. The residual gross tumor was contoured in pink. HR CTV was contoured in blue. c MR and CT images were co-registered based on the applicator. d We imported the HR CTV delineation of the first fraction to the subsequent CT images and made adjustments manually if necessary. HR CTV after adjustment was contoured in yellow
We delineated the rectum and bladder on subsequent CT images. A well-trained radiation oncologist reviewed the image registration and contours on the CT. With this combination method, the median D90 of HR CTV and the calculated D2 cm3 of the bladder, rectum, and sigmoid in each fraction were 7.2 (4.0–10.4), 5.9 (2.3–7.7), 4.0 (1.9–6.7), and 3.8 (0.6–7.2) Gy, respectively, which were acceptable (Table 2).
Discussion
We have sought to take advantage of the combination of the high tissue resolution of MRI and the high versatility of CT, because insufficient accessibility of MRI makes MRI-IGBT difficult in clinical settings in Japan. Our method of MRI-guided IGBT offered a practical option for the benefits of MRI-based target delineation. We now routinely use this workflow for cervical cancer patients without medical contraindication to MRI.
We have found this approach to be feasible in our institution. First, HR-CTV contouring for the subsequent fractions on CT required only minor adjustments with this combination of MRI and CT, eliminating uncertainty in the simple CT-based target delineation. Second, there are several advantages to this technique over MRI-based IGBT. As CT scan times are faster than MRI scan times, this approach can reduce the total sedation (or anesthesia) time and the time required with the applicator in place in subsequent brachytherapy fraction.
Wakatsuki et al. [16] and Kang et al. [17] used MRI acquired before the IGBT treatment, without the applicator in situ, to improve contouring of the HR CTV on subsequent CT images at the time of brachytherapy. In these cases, the combination use can modify/deviate the position of the uterus. It seems difficult to register correctly a pre-implant MRI image to CT images with the applicator in place. Although the brachytherapy plan could be improved to a more realistic target structure than that identified by contouring on CT alone, the combination of CT and MRI with the applicator in situ is more practical and accurate.
One of the limitations of this study was that it used the combination of MRI and CT, without comparing MRI-based planning. Nesvacil [18] argued that for larger tumors and complex applications, as well as situations with unfavorable OAR topography, MRI-based adaptive BT planning remains the superior method.
The dose delivered to HR CTV will generally be higher than that calculated from the first fraction due to tumor shrinkage, since the target contour can be obtained from the first MRI-based plan and transferred to subsequent CT plans. In comparison with HR CTV, OARs can be delineated accurately on CT. The dose optimization can take into account the organ topography at the time of the subsequent brachytherapy treatment. In the absence of MRI for all fractions, the use of CT makes it possible to adapt the dose distribution to the OAR. This combination of MRI and CT can be based on dedicated registration procedures that can help to reduce the uncertainties in delineating the appropriate dimensions of the CTV on CT images.
Conclusion
Our described method of MRI/CT-based IGBT offers a practical option for easily delineating HR CTV on CT images under the assistance of applicator-based co-registration of MRI.
References
Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix part I: general principles. Brachytherapy. 2012;11:33–6.
Tod M, Meredith WJ. Treatment of cancer of the cervix uteri, a revised Manchester method. Br J Radiol. 1953;26:252–7.
Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC-ESTRO working group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–45.
Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy––3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77.
Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–8.
Viswanathan AN, Erickson B, Gaffney DK, Beriwal S, Bhatia SK, Lee Burnett O, et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2014;90:320–8.
Ohno T, Toita T, Tsujino K, Uchida N, Hatano K, Nishimura T, et al. A questionnaire-based survey on 3D image-guided brachytherapy for cervical cancer in Japan: advances and obstacles. J Radiat Res. 2015;56(6):897–903.
Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104–9.
Pavamani S, D’Souza DP, Portelance L, Craighead PS, Pearce AG, Traptow LL, et al. Image-guided brachytherapy for cervical cancer: a Canadian Brachytherapy Group survey. Brachytherapy. 2011;10:345–51.
van Dyk S, Byram D, Bernshaw D. Use of 3D imaging and awareness of GEC-ESTRO recommendations for cervix cancer brachytherapy throughout Australia and New Zealand. J Med Imaging Radiat Oncol. 2010;54:383–7.
Watanabe M, Iwai Y, Togasaki G, Kanazawa A, Kurokawa M, Harada R, et al. Preliminary results of MRI/CT based image guided brachytherapy in cervical carcinoma. Brachytherapy. 2016;15(Suppl):11.
Nemoto MW, Ikeda Y, Ii N, Toita T, Togasaki G, Kanazawa A, et al. Multi-Institutional Comparative Study of MRI technique in cervical cancer image-based brachytherapy (IGBT): 3D MRI with high sampling efficiency versus conventional 2D multiplanar MRI. Int J Radiat Oncol Biol Phys. 2016;96(Suppl):E304–5.
Watanabe Nemoto M, Nozaki-Taguchi N, Togasaki G, Kanazawa A, Kurokawa M, Harada R, et al. New approach to relieving pain and distress during high-dose-rate intracavitary irradiation for cervical cancer. Brachytherapy. 2015;14:642–7.
Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix Part II: High-dose-rate brachytherapy. Brachytherapy. 2012;11:47–52.
Dimopoulos JC, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, et al. Recommendations from gynaecological (GYN) GEC-ESTRO working group (IV): basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103:113–22.
Wakatsuki M, Ohno T, Yoshida D, Noda S, Saitoh J, Shibuya K, et al. Intracavitary combined with CT-guided interstitial brachytherapy for locally advanced uterine cervical cancer: introduction of the technique and a case presentation. J Radiat Res. 2011;52:54–8.
Kang H-C, Shin KH, Park S-Y, Kim J-Y. 3D CT-based high-dose-rate brachytherapy for cervical cancer: clinical impact on late rectal bleeding and local control. Radiother Oncol. 2010;97:507–13.
Nesvacil N, Pötter R, Sturdza A, Hegazy N, Federico M, Kirisits C. Adaptive image guided brachytherapy for cervical cancer: a combined MRI-/CT-planning technique with MRI only at first fraction. Radiother Oncol. 2013;107:75–81.
Acknowledgements
This work was supported by Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (B) 16K19808.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Nemoto, M.W., Iwai, Y., Togasaki, G. et al. Preliminary results of a new workflow for MRI/CT-based image-guided brachytherapy in cervical carcinoma. Jpn J Radiol 35, 760–765 (2017). https://doi.org/10.1007/s11604-017-0690-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0690-3