Abstract
Purpose
To apply for detecting low-concentration gadolinium contrast agent (Gd), such as those in the perilymph and cerebrospinal fluid (CSF) at 4 h after intravenous administrations (IV), we tried to clarify the degree of increased signal intensity (SI) of low-concentration Gd by longer repetition time (TR) in heavily T2-weighed 3D-FLAIR.
Materials and methods
A phantom was designed using Gd diluted with saline at 0.250–0.008 mM and obtained images by varying the TR from 9000 to 16,000. We measured the SI of five slices for each concentration and compared the mean SI (SImean) values. Normalized SI (SInorm) values, defined as the SImean value of each TR divided by the SImean value of 9000-ms TR for each concentration were compared.
Results
Longer TR increased the SImean values in all solutions. In the 0.250 mM solution, the SImean value of 16,000-ms TR was 1.07-times greater than that of 9000-ms TR. In contrast, the SImean value of 16,000-ms TR was 1.67-times greater than that of 9000-ms TR in 0.008 mM solution.
Conclusion
SI increased with longer TR and lower Gd concentration. Thus, longer TR was useful in detecting low-concentration Gd, such as those in the perilymph and CSF at 4 h after IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavily T2-weighted three-dimensional fluid-attenuated inversion recovery (hT2w-3D-FLAIR) is useful for detecting low concentrations of gadolinium contrast agent (Gd) in the evaluation of endolymphatic hydrops in patients with Meniere’s disease [1]. Intravenously administered Gd enters the perilymph, but not the endolymph, which allows separate visualization of the perilymph and endolymph. hT2w-3D-FLAIR is far more sensitive to subtle T1 differences in the fluid because of the extremely long echo time, optimal inversion time (TI), and constant flip angle mode. However, due to the low signal-to-noise ratio (SNR), it takes approximately 15 min of scan time to obtain the hT2w-3D-FLAIR image. Long scan time results in patient motion, which reduces the throughput of magnetic resonance (MR) examination. Furthermore, this sequence has been reported to be useful not only for detecting endolymphatic hydrops but also for evaluating Gd leakage into the cranial nerve endings, anterior chamber aqueous humor, CSF, and perivascular space [2,3,4]. The increased SI of hT2w-3D-FLAIR to low Gd concentrations in the fluid may also be useful for elucidating the glymphatic system, a recently discovered macroscopic waste clearance system in the brain [5,6,7,8,9]. Therefore, it is necessary to further improve the image quality and to shorten the practical scan time in this sequence. A previous in vivo study has revealed that the scan time could be half of that of the previous one without any significant differences in the mean contrast-to-noise ratio between the endolymph and perilymph [10]. However, the degree of increased signal intensity (SI) by longer repetition time (TR) has not yet been evaluated in detail. Although there are theoretical equations for concentration of Gd, relaxivity, T1 and T2 relaxation times, and signal intensity, which is well known [11, 12], there has been no study on the signal behavior in the range of low concentrations, which has not been used in clinical practice until now, and the effect of varying TR on each concentration range.
The purpose of this study was to quantify the degree of increased SI of low-concentration Gd solution by varying the TR in hT2w-3D-FLAIR using a diluted-Gd phantom and to clarify the usefulness of longer TR for detecting low-concentration Gd, such as those in the perilymph and cerebrospinal fluid (CSF) at 4 h after IV.
Materials and methods
Phantom measurements
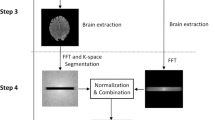
All scans were performed on a 3-T MR scanner (Verio, Siemens, Erlangen, Germany) using a 8-channel array head coil to reduce the effect of inhomogeneous coil sensitivity as much as possible; however, a 32-channel array head coil was used in clinical examinations. We designed a phantom using disposable plastic 20-mL syringes (Terumo, Tokyo, Japan) filled with diluted gadodiamide (Gd-DTPA-BMA; Omniscan, DaiichiSankyo Pharm, Tokyo, Japan). The original Gd-DTPA-BMA (500 mM solution) was diluted with saline to 0.250-, 0.125-, 0.063-, 0.031-, 0.016- and 0.008-mM to simulate the Gd-DTPA-BMA concentration in the perilymph at 4 h after IV (Fig. 1a, b, d) [1]. The measured syringes were surrounded by agar to minimize motion by vibration and eliminate the influence of surrounding air-induced artifacts, and placed the phantom in the center of the coil to minimize the effect of inhomogeneous coil sensitivity (Fig. 1c).
The layout of phantom experiment. We prepared disposable plastic 20-mL syringes filled with diluted gadodiamide. The original Gd-DTPA-BMA (500 mM solution) was diluted with saline to 0.250-, 0.125-, 0.063-, 0.031-, 0.016- and 0.008-mM solution (a). The periphery of the syringe was fixed with agar (b) and placed in the center of the 8-channel array head coil (c). Schematic diagram. Numbers indicate the concentration (d) and an example image of the phantom (e)
While maintaining their temperature at approximately 24 °C, the phantom images were then obtained five times in hT2w-3D-FLAIR by varying the TR from 9000 to 16,000 ms at 1000-ms intervals and by modifying the TI according to the TR to minimize the signal from saline (an example image shown in Fig. 1e). A TR of 16,000-ms was the maximum value possible on the scanner. Detailed TIs are indicated in Table 1. The other parameters were set as follows: echo time (TE), 544 ms; echo train duration, 1968 ms; 180° initial refocusing flip angle, rapidly decreased to 120° constant flip angle for the turbo spin-echo refocusing echo train in SPACE sequence (constant); echo train length, 144; matrix size, 320 × 270; 72 axial, 0.8-mm-thick slices; 16-cm × 16-cm field of view; generalized auto-calibrating partially parallel acquisitions (GRAPPA) technique; acceleration factor, 2; and number of excitations (NEX), 1. Non-selective inversion pulses and slab-selective excitation pulses were used. We equalized the TE and echo train duration and GRAPPA to the clinical settings to equalize the recovery of longitudinal magnetization. Constant receiver gain was employed throughout the experiment.
In the phantom images, we measured the SI of five slices around the center for each concentration. We normalized the SI by the 9000-ms TR, defining normalized SI (SInorm) values as the SI value of each TR divided by the SI value of 9000-ms TR for all concentrations. The SInorm values were obtained using the following formula:
We then compared the mean SI (SImean) values and each SInorm value. In addition, SInorm values were compared between the group of 0.250 mM and the groups of each concentration to clarify the significant difference between concentrations. Because data were not normally distributed, a Mann–Whitney U test with Bonferroni-correction was performed. Because we conducted the test in five groups, P < 0.01 was considered to indicate a significant difference. Statistical analyes were performed using SPSS software (SPSS for Windows, version 25, IBM).
Simulations
We performed a numerical simulation to validate the relationship between the concentrations of Gd and signal behavior, determined by varying the TR. Signal intensity on FLAIR images was approximated using the following formula [13]:
The TR and TE were used the parameters described above. The TIs for each TR were calculated using online tool [14]. The T1 and T2 values for each concentration of Gd were calculated using the following formula:
where Ti is the T1 or T2 relaxation times at concentration C; Ti(0) is the relaxation time of solution without Gd, and C is the concentration. Relaxivities for T1 and T2 were adopted from [12]. We then calculated signal intensity on hT2w-3D-FLAIR images and compared it to the measurement value.
Results
Figure 2 shows the mean ± standard deviation (SD) of SI from five slices in five measurement values in each diluted solution by varying the TR. The measurement values of each diluted solution were calculated from 600 pixels in a circular region of interest manually drawn on each image to exclude edge pixels. The SImean values of all diluted solutions increased as the TR increased. Figure 3 shows the measured (a) and simulated (b) SInorm values by varying the concentrations of Gd. The SInorm values tended to increase with decreasing the concentrations of Gd or increasing TR. The measured SInorm (TR16,000/TR9000) value was 1.07 on 0.250 mM solution, indicating that the SImean of 16,000-ms TR was 1.07-times greater than that of 9000-ms TR. The SInorm (TR16,000/TR9000) value was 1.19 on 0.125 mM solution, 1.33 on 0.063 mM solution, 1.46 on 0.031 mM solution, 1.57 on 0.016 mM solution, and 1.67 on 0.008 mM solution. Therefore, the measured SImean of 16,000-ms TR was 1.67-times greater than that of 9000-ms TR. Although there were some differences in the SInorm value, the measurement value showed the same trend as simulations. With statistical analysis, there was no significant difference between the 0.250 mM group and the 0.125 mM group (P = 0.021); however, there were significant differences between the 0.250 mM group and others lower concentration groups (P = 0.007).
Discussion
In previous studies on the use of hT2w-3D-FLAIR for visualizing endolymphatic hydrops, 9000-ms TR was utilized after optimization [1]. According to our results, longer TR increased the SI. We found that the degree of increased SI by longer TR depended on the Gd concentration. The recovery of longitudinal magnetization would be a major factor in this result. On FLAIR images, the signal increases with increasing TR [14, 15]. The recovery of longitudinal magnetization is sufficient in 9000-ms TR at relatively high concentrations used in normal clinical practices. On the other hand, the recovery could be insufficient at lower concentrations due to weaker T1 shortening as the SI of low concentrations was greatly increased by longer TR. As can be seen from the simulation curve (Fig. 3b), if TR longer than 16,000-ms TR can be set, further increase in signal intensity can be expected in the future.
The SInorm of measurements and simulations were different due to temperature and setting TI. We used the relaxivity data at 37°C in the simulation. It is well known that the relaxivities of Gd are temperature-dependent (12); however, we experimented at room temperature (approx. 24 °C) because it was difficult to maintain the temperature of the solutions in the clinical MRI scanner. In the experiment, TI was set to minimize (almost zero) the signal from saline; in contrast, the measured values were not used in simulations to obtain the data at more than 16,000-ms TR. Although our measured values were smaller than simulation values, we clarified the relationship concentration of Gd and TR by showing the same tendency as simulations.
In the present study, actual SI was adopted for correctly evaluating the degree of increased SI due to the longitudinal magnetization recovery, not signal-to-noise ratio (SNR) or contrast ratio (CR), because SNR cannot evaluate the degree of actual longitudinal magnetization recovery. Also it should be note that a high SNR image is not always a good image. Even when the SNR is decreased by reducing NEX, the diagnostic ability can be improved by increasing the SI of low Gd concentrations by means of longer TR. On the other hand, in the case of CR, the use of extremely small values as the denominator in the ratio may increase the error in the results. Therefore, we could not use the value of saline that was close to zero.
Before the present study, the actual Gd concentration in patients is unknown, which was a limitation. Therefore, we prepared multiple dilutions of Gd that could simulate the Gd concentration in the perilymph at 4 h after IV. We could determine the actual Gd concentration in patients by obtaining the images of 9000-ms TR and 16,000-ms TR in the clinical examination and fitting the SInorm value to the graph of our result for each patient. This quantitative value of Gd concentration in each patient may provide new information and facilitate the clarification of the pathology of endolymphatic hydrops.
This study has some limitations. As mentioned earlier, it is impossible to obtain the actual Gd concentrations in each patient before scanning. Because the optimal TR could vary for each patient, not all patients would benefit from longer TR. Thus, it may be a waste of time to extend the TR for some patients. In the present study, the scan time was not considered; in fact, the use of a long TR means a long total scan time. In other words, we used only SI that did not vary when the scan time was controlled by NEX in the evaluation. In future study, it is essential to consider the scan time in clinical practice.
In conclusion, our study revealed that the SI of 16,000-ms TR was 1.67-times greater than that of 9000-ms TR in a 0.008 mM solution, which was useful in detecting low-concentration Gd, such as those in the perilymph and CSF at 4 h after IV.
References
Naganawa S, Kawai H, Sone M, Nakashima T. Increased sensitivity to low concentration gadolinium contrast by optimized heavily T2-weighted 3D-FLAIR to visualize endolymphatic space. Magn Reson Med Sci. 2010;9:73–80.
Naganawa S, Suzuki K, Yamazaki M, Sakurai Y. Serial scans in healthy volunteers following intravenous administration of gadoteridol: time course of contrast enhancement in various cranial fluid spaces. Magn Reson Med Sci. 2014;13:7–13.
Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci. 2017;16:61–5.
Deike-Hofmann K, Reuter J, Haase R, Paech D, Gnirs R, Bickelhaupt S, et al. Glymphatic pathway of gadolinium-based contrast agents through the brain: overlooked and misinterpreted. Investig Radiol. 2018. https://doi.org/10.1097/RLI.0000000000000533.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Investig. 2013;123:1299–309.
Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44:S93–S9595.
Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107.
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93.
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–61.
Naganawa S, Kawai H, Taoka T, Sone M. Improved HYDROPS: imaging of endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci. 2017;16:357–61.
Uematsu H, Dougherty L, Takahashi M, Butler NS, Song HK, Ohno Y, et al. A direct comparison of signal behavior between 4.0 and 1.5 T: a phantom study. Eur J Radiol. 2003;45:154–9.
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig Radiol. 2005;40:715–24.
Rydberg JN, Riederer SJ, Rydberg CH, Jack CR. Contrast optimization of fluid-attenuated inversion recovery (FLAIR) imaging. Magn Reson Med. 1995;34:868–77.
Kita M, Sato M, Kawano K, Kometani K, Tanaka H, Oda H, et al. Online tool for calculating null points in various inversion recovery sequences. Magn Reson Imaging. 2013;31:1631–9.
Wong EC, Liu TT, Luh WM, Frank LR, Buxton RB. T(1) and T(2) selective method for improved SNR in CSF-attenuated imaging: T(2)-FLAIR. Magn Reson Med. 2001;45:529–32.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kato, Y., Bokura, K., Taoka, T. et al. Increased signal intensity of low-concentration gadolinium contrast agent by longer repetition time in heavily T2-weighted-3D-FLAIR. Jpn J Radiol 37, 431–435 (2019). https://doi.org/10.1007/s11604-019-00828-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-019-00828-0