Abstract
Whether a brain abscess is apparent by imaging depends on the stage of the abscess at the time of imaging, as well as the etiology of the infection. Because conventional magnetic resonance imaging (MRI) is limited in its ability to distinguish brain abscesses from necrotic tumors, advanced techniques are required. The management of these two disease entities differs and can potentially affect the clinical outcome. We report a case having atypical imaging features of a pyogenic brain abscess on advanced MRI, in particular, on diffusion-weighted and perfusion imaging, in a patient with osteosarcoma undergoing chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain abscesses are caused by intracerebral infections that begin with a localized region of cerebritis and evolve into a discrete collection of pus surrounded by a well-vascularized capsule. Differential diagnosis between brain abscesses and necrotic tumors is not always possible by gadolinium-enhanced conventional magnetic resonance imaging (MRI). The management of these two disease entities is different, so efficient diagnosis could affect clinical outcome. Bacillus cereus (B. cereus) is an opportunistic gram-positive, spore-forming, rod-shaped bacterium that is ubiquitous in the environment. In immunosuppressed patients, including those receiving treatment for cancer, invasive infection can occur and is associated with significant morbidity and mortality. The purpose of this case report is to increase the awareness of the spectrum of brain abscesses by reporting MRI findings of an uncommon case of pyogenic brain abscess caused by B. cereus.
Case report
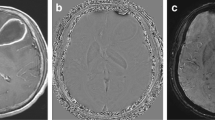
A 17-year-old female was diagnosed with osteosarcoma of the left thigh 2 years ago. A central venous catheter was inserted and she began chemotherapy. During a third episode of febrile neutropenia, the patient complained of headache and confusion. At this point, blood cultures obtained through both lumens of the catheter and a peripheral vein tested positive for a gram-positive bacillus. The organism produced large and green-colored colonies, and was identified as B. cereus on the basis of Gram staining, motility, colony morphology, and lecithinase activity. Laboratory work demonstrated a severely decreased white blood cell count. The patient had no recent history of gastrointestinal symptoms. In view of the patient’s immunosuppressed status and her clinical presentation, an MRI of the brain was ordered. The MRI revealed a rim-enhanced mass with surrounding edema in the left temporal lobe (Fig. 1a). A T2-weighted axial MRI revealed a well-defined hyperintense lesion with a hypointense rim (Fig. 1b). The center of the lesion displayed decreased signal on diffusion-weighted imaging (DWI) sequences (Fig. 1c) and increased signal on the apparent diffusion coefficient (ADC) (Fig. 1d) map, in keeping with non-restricted diffusion. On axial susceptibility-weighted imaging (SWI), the lesion was bordered by two concentric rims, with the outer rim being hypointense and the inner hyperintense (Fig. 1e). We performed proton MR spectroscopy (PMRS) to exclude metastasis. PMRS revealed only lactate and amino acid (AA) peaks; the absence of N-acetylacetate and choline is consistent with an abscess (Fig. 1f). Contrast-enhanced perfusion-weighted MRI showed an increased relative cerebral blood volume (rCBV) in the capsular wall, with no perfusion in the center of the abscess (Fig. 1g). In light of this, we began appropriate antibiotic treatment. A marked clinical improvement was observed within 1 week, at which time DWI showed restricted diffusion (Fig. 1h) and decreased signal on the ADC in the wall of the lesion (Fig. 1i). The patient continued therapy, and an MRI after 3 months showed almost complete resolution. The patient continues to be followed-up, but is not taking any medication at present.
Axial contrast-enhanced T1-weighted image showing a rim-enhancing mass with surrounding edema in the left temporal lobe (a), while the axial T2-weighted image shows an incomplete hypointense rim at the lesion margin and perifocal edema (b). Diffusion-weighted image (DWI) showing hypointensity; apparent diffusion coefficient (ADC) image shows hyperintensity, indicating non-restricted diffusion (c, d). On axial susceptibility-weighted imaging (SWI), the abscess cavity is bordered by two concentric rims, with the outer rim being hypointense (arrow), and the inner one hyperintense (arrowhead), forming the dual rim sign (e). Single-voxel proton MR spectroscopy from the center of the lesion to identify lipid and amino acid resonances (f). Perfusion-weighted imaging (PWI) shows increased relative cerebral blood volume (rCBV) in the abscess wall (g). Diffusion-weighted image (DWI) after 1 week of treatment showing hyperintensity, while the apparent diffusion coefficient (ADC) image shows hypointensity in the wall of lesion, indicating restricted diffusion (h, i)
Discussion
Even in the era of MRI, it is still difficult to differentiate between brain abscesses and intracranial tumors. The presence of a hypointense rim at the lesion margin on T2-weighted images is a common feature of brain abscesses and necrotic glioblastomas [1]. SWI produces high-resolution, three-dimensional gradient-echo sequences that provide an excellent capacity for the detection of hemoglobin breakdown products, iron, or calcifications. One study demonstrated the importance of SWI in the differentiation of necrotic glioblastomas and pyogenic abscesses, emphasizing the “dual-rim sign,” visible on SWI, as the most specific imaging feature differentiating between the two [2]. It has been postulated that granulation tissue lines the inner part of the fibrocollagenous abscess capsule, forming the histological basis of the hyperintense rim; while magnetic susceptibility in the abscess capsule resulting from the production of paramagnetic free radicals by macrophages forms the hypointense rim [1, 2]. More recently, Antulov et al. [3] reported that the dual-rim sign was a unique feature of pyogenic abscesses on SWI, as no fungal abscesses exhibit it, thus allowing differentiation between those two pathologies. Axial SWI revealed this sign in our case: two concentric rims, with the outer rim being hypointense and the inner one hyperintense. We identified very prominent hypointense signals on SWI in the enhancing walls of the abscess. Gupta et al. [4] estimated that diffuse hypointense rims identified by SWI were due to microhemorrhage in 73 % of all brain abscesses.
We initially suspected a metastatic brain tumor in our case, because the T1-weighted Gd-DTPA-enhanced brain MRI revealed a ring-enhanced lesion. However, in the absence of restriction, PMRS is mandatory in distinguishing brain abscesses from cystic tumors. A number of PMRS studies have been used to characterize intracranial mass lesions [5]. Brain abscesses characteristically demonstrate resonances from cytosolic AAs (leucine, isoleucine, and valine), lipids, lactate, alanine, acetate, succinate, and glycine but not N-acetylacetate, choline, or creatine, whereas cystic tumors show resonances only from lactate and choline. AA resonance is a sensitive marker of pyogenic abscesses and permits differentiation between cystic brain tumors and intracranial bacterial abscesses, but its absence does not rule out a pyogenic etiology [6]. Succinate (Suc) and acetate (Ac) are not usually observed in brain abscesses secondary to aerobic and facultative anaerobic microorganisms [7]. In accordance with the literature, we did not observe these resonances in the lesion.
Perfusion-weighted MRI allows non-invasive measurement of vascularity. rCBV maps derived from perfusion-weighted MR images can be used to quantify areas of neovascularization. Several studies have shown that the gadolinium-enhancing capsules of brain abscesses have an rCBV value lower than those of necrotic tumors, due to the lack of neoangiogenesis in brain abscesses [8]. However, these results should be considered preliminary, given the limited number of reported cases and etiologies of brain abscesses [9]. In our case, rCBV was increased in the wall of the lesion and in the perilesional edema, mimicking a necrotic tumor. A possible explanation is that in the early capsular stage, the brain abscess has a high capillary density, and thus the high rCBV values found result from the young age of the lesion [10].
Brain tumors generally have a low-intensity area at their core; in contrast, brain abscesses tend to be categorized as high intensity on DWI [11]. These differences likely result from differences in content: pus in the cavity of a brain abscess consists of inflammatory cells, bacteria, necrotic tissue, and highly viscous proteinaceous exudates; in contrast, metastatic tumors usually consist of necrotic tissue debris, fewer inflammatory cells, and much clearer serous fluid. However, not all abscesses follow this rule. Fungal and tuberculous abscesses may have elevated diffusivity and a low signal on DWI [12]. Mishra et al. [13] reported that pus cultures from five out of eight patients with high ADC were sterile, and that antibiotic drug therapy had already been initiated in these patients. High diffusion in abscesses might result from changes in pus composition, and probably reflects increasing pus liquefaction as a result of adequate antibiotic therapy. In our patient, the abscess cavity initially had a low signal intensity on trace DWI and high ADC values, inconsistent with restricted diffusion. We infer that extensive neutrophilic infiltration was not yet present, indicating that the abscess was not yet completely formed. In addition to antimicrobial chemotherapy, variable concentrations of inflammatory cells, different pathogenic organisms, the host immune response, and the age of an abscess might influence pus viscosity, resulting in variations in ADC values. The diffusivity of water molecules is affected in the organized abscess environment containing microorganisms, macromolecules, and intact inflammatory cells. The relative number of intact inflammatory cells in the abscess cavity is inversely proportional to the mean ADC value of corresponding abscess regions showing hyperintensity on DWI. The total absence of an inflammatory response may be explained by the combination of the patient’s severely immuno-deficient state and the character of the B. cereus infection itself, which causes little inflammatory reaction [14].
References
Haimes AB, Zimmerman RD, Morgello S, Weingarten K, Becker RD, Jennis R, et al. MR imaging of brain abscesses. Am J Neuroradiol. 1989;10:279–91.
Toh CH, Wei KC, Chang CN, Hsu PW, Wong HF, Ng SH, Castillo M, Lin CP. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33(8):1534–8.
Antulov R, Dolic K, Fruehwald-Pallamar J, Miletic D, Thurnher MM. Differentiation of pyogenic and fungal brain abscesses with susceptibility-weighted MR sequences. Neuroradiology. 2014;56(11):937–45.
Gupta RK, Tomar V, Awasthi R, Yadav A, Husain N, Bharadwaj V, et al. T2*-weighted MR angiography substantially increases the detection of hemorrhage in the wall of brain abscess: implications in clinical interpretation. Neuroradiology. 2012;54(6):565–72.
Kim SH, Chang KH, Song IC, et al. Brain abscess and brain tumor: discrimination with in vivo H-1 MR spectroscopy. Radiology. 1997;204:239–45.
Pal D, Bhattacharyya A, Husain M, Prasad KN, Pandey CM, Gupta RK. In vivo proton MR spectroscopy evaluation of pyogenic brain abscesses: a report of 194 cases. Am J Neuroradiol. 2010;31(2):360–6.
Garg M, Gupta RK, Husain M, et al. Brain abscesses: etiologic categorization with in vivo proton MR spectroscopy. Radiology. 2004;230:519–27.
Holmes TM, Petrella JR, Provenzale JM. Distinction between cerebral abscesses and high-grade neoplasms by dynamic susceptibility contrast perfusion MRI. AJR Am J Roentgenol. 2004;183:1247–52.
Cianfoni A, Calandrelli R, De Bonis P, Pompucci A, Lauriola L, Colosimo C. Nocardia brain abscess mimicking high grade necrotic tumour on perfusion-MRI: a case report. J Clin Neurosci. 2010;17:1080–2.
Muccio CF, Leonini S, Esposito G, Cerase A. Pyogenic abscess from Providencia stuartii mimicking necrotic tumour at perfusion-weighted imaging. Neurol Sci. 2011;32(5):919–23.
Nakaiso M, Uno M, Harada M, Kageji T, Takimoto O, Nagahiro S. Brain abscess and glioblastoma identified by combined proton magnetic resonance spectroscopy and diffusion-weighted magnetic resonance imaging—two case reports. Neurol Med Chir Tokyo. 2002;42:346–8.
Mueller-Mang C, Castillo M, Mang TG, Cartes-Zumelzu F, Weber M, Thurnher MM. Fungal versus bacterial brain abscesses: is diffusion-weighted MR imaging a useful tool in the differential diagnosis? Neuroradiol On-line. 2007;49:651–7.
Mishra A, Greaves R, Massie J. The limitations of sweat electrolyte reference intervals for the diagnosis of cystic fibrosis: a systematic review. Clin Biochem Rev. 2007;28:e60–76.
Burdon KL, Davis JS, Wende RD. Experimental infection of mice with Bacillus cereus: studies of pathogenesis and pathologic changes. J Infect Dis. 1967;117:307–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Ozbayrak, M., Ulus, O.S., Berkman, M.Z. et al. Atypical pyogenic brain abscess evaluation by diffusion-weighted imaging: diagnosis with multimodality MR imaging. Jpn J Radiol 33, 668–671 (2015). https://doi.org/10.1007/s11604-015-0472-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0472-8