Abstract
The scientific community is continuously putting efforts to improve the energy/power density of energy storage devices, which leads to development of novel materials with enhanced electrochemical properties. Polymer-in-salt electrolytes (PISEs) are expected to have faster ion transport and hence may result in improved power density. In the present study, Ca salt–based PISE is synthesized using glutaraldehyde (GA)–crosslinked arrowroot starch as host matrix. The synthesized PISE has high conductivity (~ 0.01 S/cm), wide electrochemical stability window (ESW > 3 V), and small characteristic relaxation time (τ ~ 17 µs) indicating the possibility of faster response in any device fabricated using synthesized PISEs. Fabricated supercapacitor, using the highest conducting PISE with rGO as electrode, has specific capacitance ~ 17 F/g at 1 mV/s and high power density 2.1 kW/kg with coulombic efficiency (CE) of > 90.05% and with CAC as electrode, specific capacitance ~ 125 F/g at 1 mV/s and high power density 2.1 kW/kg with coulombic efficiency (CE) of > 99%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The hunt for improved electrode and electrolyte materials, so that performance of energy devices can be enhanced, is an ever-lasting process. The progress in the field of electrode materials is quite appreciating [1,2,3,4], but to demonstrate the performance of any new electrode materials, the scientific community still uses liquid electrolytes [5,6,7,8]. The bigger size of the ions requiring larger space for movement leads to poor conductivity in solid physique [9, 10]. But with liquid electrolytes, the problems of lower electrochemical stability window, corrosion, and packaging-related issues have been faced. Few organic electrolytes and ionic liquid-based electrolytes have shown better performance but they are not cost-effective. Polymer electrolytes are widely being used at commercial level but additives are required to achieve the desired electrochemical properties [11,12,13,14]. After the work of Angell and co-authors in 1990s [15,16,17], polymer-in-salt electrolytes (PISEs) came as a possible alternate to have faster ion movement in solid-state electrolytes, but the efforts in the field still did not reach commercial applications. In PISEs, ion transport is not only faster but it may also have greater cationic transference number, a well-desired property of the electrolytes [18, 19]. PISEs offer a possibility to blend the fast-ionic conduction of glassy electrolytes with the required mechanical properties of rubbery polymers [20]. In the case of PISEs, the ion motion is decoupled from polymer segmental motion, and due to its diffusion through ion clusters, it is much faster. Even after so many promising characteristics, the PISEs are not reaching commercial level because of brittleness of lattice at higher salt concentration, recrystallization of salt, need of eutectic salt combination which can remain in molten state at room temperature so that by adding a small amount of polymer a better electrolyte can be achieved. Previous studies indicate [21, 22] that a loosely bonded host matrix may lead to better PISE. In few recent studies, it has also been pointed out that polymer hosts which can be broken into small molecules by addition of salts may also lead to better PISEs [23,24,25]. With Na and Mg salts, it has been proved that crosslinked starches are quite good host for ecofriendly, economical, and flexible PISEs which are free from aging effects/recrystallization problems, and hence in the present study, Ca salt–based PISE is being synthesized and characterized for their energy device fabrication. Calcium is the 5th most abundant element on the earth’s crust that can make energy devices more cost-effective [26], and studies on the development of Ca-based energy devices are in an early stage [27] relative to the well-studied electrolyte systems based on Li+ [28, 29], Na+ [30, 31], K+ [32], Mg2+ [33, 34], and Al3+ [35, 36]. In the present work, glutaraldehyde (GA)–crosslinked starch has been used as host matrix. Crosslinking it with glutaraldehyde not only makes the system fungal-free but it also leads to flexible films which can be molded into different sizes and shapes. Although crosslinking with GA leads to many favorable properties, there is an optimum value after which other problems are seen in the material [37, 38]. To the best of the author’s knowledge, understanding of the Ca-based PISEs is not reported till date and hence this work is an attempt to understand that the same Ca is the fifth most abundant element naturally found in earth’s crust which is attracting scientific community for its application in energy devices [39, 40]. CaCl2 is used as this can be dissolved in solvents like methanol due to its low dissociation energy [41] which makes electrolyte synthesis non-toxic in nature. The primary goal of our study is to develop environmentally benign and cost-effective systems, which is why it was chosen to employ CaCl2 as the material for energy device applications [42].
To develop an understanding of the electrochemical properties of calcium-based polymer electrolytes, a series of electrolytes have been synthesized from 25 wt% salt to 72 wt% salt with crosslinked starch as a host polymer. Since the aqueous electrolytes are the most promising electrolytes (if the smaller ESW and designing problems are neglected), efforts are being made to have water-based electrolytes in solid state [43, 44] and a new nomenclature “Water-in-Polymer Salt electrolytes (WiPSEs)” [45, 46] has recently appeared in literature. In the present study, efforts have also been made to enhance the moisture content of the synthesized PISE by exposing it in a high humidity atmosphere.
Experimental details
Materials
Arrowroot starch, calcium chloride dihydrate (Sigma-Aldrich, 99% purity), glutaraldehyde (Merck, Product Code 8.20603.0521), methanol (Qualigens, HPLC grade (assay percentage range 99.8%)), carbon black (Super P, 99% purity) (Alfa Aesar), polyvinyl alcohol (PVA) (Sigma-Aldrich, Product Code 363,081), distilled water (lab prepared).
Electrolyte preparation
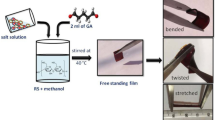
Arrowroot starch, calcium chloride, glutaraldehyde, and methanol were used to prepare the sample. Solid polymer electrolyte films were prepared at room temperature (40 ℃) using the solution cast technique as used previously in our lab for synthesis [47,48,49] as shown in Fig. 1a. A series of electrolytes were prepared using 0.5 g of starch with different amounts of salt 0.25 g, 0.5 g, 0.75 g, 1.0 g, and 1.25 g, and for further discussion, they are named 25wt%, 50wt%, 60wt%, 66wt%, and 72wt%. For the preparation of 25 wt% electrolyte, 0.5 g of starch was added to 10 ml methanol and left to continuously stir for 1 h at room temperature (40 ℃). In a separate beaker, 0.25 g of salt was added to 10 ml methanol, and the mixture was continuously stirred for 1 h at room temperature. Then, 1 ml glutaraldehyde is added to the starch mixture and left for continuous stirring for 30 min. Then, salt mixture was added to the starch mixture and stirred at a constant speed (350 rpm) and the whole mixture was left to stir till a homogenous mixture was obtained. After this, a homogeneous mixture was poured into the petri dish and was left to dry at ambient for almost 10 days to obtain a transparent, free-standing film as shown in Fig. 1. Same protocol is followed for all salt concentrations.
Preparation of electrodes
Commercial rGO and commercial activated carbon (CAC) were used as two types of electrode material for the comparative study of the charge storage mechanism by fabricating a supercapacitor. The slurry was prepared using distilled water and 80wt% of active material (CAC, rGO), 10wt% conducting agent (Super P), and 10wt% of binder (PVA, Sigma-Aldrich). Firstly, add PVA into distilled water and left for stirring till it dissolves. After dissolving PVA in distilled water, add Super P, active material (CAC or rGO), and stir for 3 h to get slurry. The prepared slurry was coated on carbon cloth using the doctor blade technique and was left to dry at 100 ℃ for 3 h. The weight of the loaded active material is 0.24 mg/cm2 for rGO and 0.24 mg/cm2 for CAC. The obtained electrodes were used for the characterization.
Structural and electrochemical characterization
XRD measurements were recorded over the range of diffraction angle 5° to 80° done using D2 Phaser Bruker. For moisture content analysis of electrolytes, the HS153 moisture analyzer machine of Metler Toledo was used. The electrochemical impedance spectroscopy (EIS), linear sweep voltammetry (LSV), cyclic voltammetry (CV), and galvanostatic charging discharging (GCD) were carried out using a two-electrode setup using CHI660E. The conductivity of the electrolyte was calculated using the following equation [50]:
Here, R denotes the ESR (ohm) value of the electrolyte, and l and A represent the thickness of the electrolyte and surface area of contact of electrode/electrolyte in cm2 respectively. σ represents the conductivity. The specific capacitance using GCD plot of the fabricated supercapacitor was calculated using the formula:
Here, C represents the specific capacitance of the fabricated supercapacitor in units of F/g. (I/m) is the current density. \(\Delta\) t is discharging time from GCD plot, m is the mass of the active material, and \(\Delta\) V is the voltage range.
The specific capacitance using the cyclic voltammetry curve of the fabricated supercapacitor was calculated using the formula:
Here, C represents the specific capacitance of the fabricated supercapacitor in units of F/g. ∫IdV is the area enclosed in CV curve. s is the scan rate (V/s), m is the mass of the active material, and V is the voltage range.
The values of specific energy density (E) and power density (P) were calculated using the following formula [51, 52]:
Here, C is specific capacitance (F/g), V is the potential change after IR drop, and t (s) is discharging time in GCD plot. Here, η represents coulombic efficiency (%) where td and tc are the time of discharging and charging of the supercapacitor.
Results
Structural characterization
The biggest hurdle in the path of PISEs reaching commercial level is recrystallization of salt at high salt concentrations. Hence, while characterizing PISEs, XRD comes as the most basic analysis as it provides information on whether the crystallinity is present in the electrolyte or not. Hence, XRD is used to understand the crystalline and amorphous nature of prepared electrolytes [53] from salt-in-polymer electrolyte (SIPE) to PISE range. Figure 2 shows the XRD spectra of pure salt (CaCl2.2H2O) and synthesized electrolytes from 25wt% salt to 72wt% salt. In XRD data, distinct XRD peaks could be seen only in pure salt which is found to be identical to the JCPDS data (ICCD-PDF 01–070-0385). It is observed that the crystalline nature of pure salt has completely disappeared at 25wt% salt itself. While shifting salt concentration from 25wt% to 72wt%, no recrystallization is detected in XRD spectra, which means complete acceptance of salt by crosslinked starch matrix without recrystallization.
The present system has an edge over the systems reported [54, 55] with synthetic polymers as host matrix, as the XRD data indicates that the amorphicity is continuously enhancing with salt concentration. The possible reasons for this may be (i) breaking of starch molecules into smaller molecules by salt [25] which leads to enhanced availability of ion-interacting moieties and also enhances the possibility of hydrogen bonding leading to loosely bonded structure (ii) enhance moisture content with salt concentration which also support better interaction of ions and starch through hydrogen bonding via these water molecules.
Electrochemical performance of synthesized electrolytes
Electrochemical impedance spectroscopy was used for analyzing the effect of salt concentration on the conductivity of the electrolytes with salt concentration performed in the range of 1 Hz to 1 MHz. Conductivity and ESW have been analyzed to understand the electrochemical behavior of synthesized electrolytes. Impedance data has been analyzed by Nyquist plots and is given in Fig. 3a.
a Nyquist plot for prepared materials having different salt concentrations (mentioned salt wt% in total material). b ESW for all synthesized polymer films with different wt% of salt present in electrolyte films as mentioned in the curve. c Variation of conductivity with variation of wt% of salt concentration in polymer films. d Ratio of parallel capacitance and series capacitance Cp/Cs graph with log frequency
The straight-line nature of Nyquist plot which intercepts at very low resistance indicates high conductivity of the synthesized PISE. Nyquist plots indicate that as the salt concentration is increased the resistance is continuously decreasing. The conductivity vs salt concentration plot given in Fig. 3c indicates a jump at nearly 50wt% salt concentration which may be correlated with transition from SIPE to PISE transport mechanism. LSV curves for all five synthesized electrolytes are given in Fig. 3b and the ESW estimated from these curves are summarized in Table 1.
ESW is quite wide, ~ 4 V for all electrolytes. A decrease in ESW with salt concentration is an obvious [48,49,50, 56,57,58] phenomenon because as the salt concentration increases, there are more number of fast moving ions and hence can be easily oxidized/reduced leading to a lowered value of ESW.
The value of ESW depends on the combination of electrolyte and electrode [59] resulting to the limits of the maximum energy density and power density of the system [60]. Here, it is evaluated by using an Al electrode to study the ESW of the prepared series of SIPE to PISE in order to understand the electrochemical stability of the material. Al/electrolyte/Al composition is used to estimate the ESW and Fig. 3b shows the LSV curves for electrolytes with different salt concentrations. It is observed that from SIPE to PISE, ESW has decreased.
The value of relaxation time is estimated from the frequency at which the value of Cp/Cs is 0.5 [61] as shown in Fig. 3d and values for different salt concentrations are summarized in Table 1. Although, for both SIPE and PISE, the relaxation time τ is in μs range, the resistance values of SIPE and PISE are different that can be associated with difference in the available number of charge carriers in two ranges. The estimated relaxation times are in μs range indicating faster ion transport in synthesized electrolytes, which can be associated with high moisture content in the system leading to aqueous type of electrolyte behaviors.
Humidity/moisture content (MC) effect on ESR
Aqueous electrolytes are well established, but due to leakage/designing problems, their solid-state alternates are always sought. In this hunt, for achieving aqueous electrolyte properties in solid state, with wider electrochemical stability window (ESW), a new class of electrolytes classified as “Water-In-Salt-Electrolytes”, or “Water-In-Polymer-Salt-Electrolytes” [43,44,45,46], are being explored by the researchers. Here, in the present system where the salt and starch both have an affinity for water, a novel approach has been adopted to stabilize water in the polymer matrix. Moisture content seems to play a favorable role in stopping the recrystallization by keeping the matrix flexible and easy to handle for design purposes.
Hence, to develop the understanding of synthesized electrolytes, MC and resistance of the synthesized samples have been measured at different relative humidities in series of five steps, as mentioned in Table 2. A difference between the behavior of SIPE and PISEs at different humidity levels is observed. In the case of SIPE, resistance drastically changes varying from ~ 3Ω (at 99% RH) to 9358 Ω at (22%) whereas in the case of PISEs the variation is very small. It indicates that in the case of SIPE either the H+ or OH- from absorbed water are moving or water is assisting the movement of cation/anion from salt.
ESW of these three samples were also measured at different humidity levels. The results are very interesting and in accordance to the previous patent from our group. In SIPE region at 99% RH, the ESW is almost zero indicating that the absorbed water molecules are easily dissociated [62], whereas on moving towards higher salt concentration water molecules are stabilized [46, 63,64,65]. At normal ambient humidity (~ 60% RH), the ESW is > 2.5 V which is quite wide for device fabrication. The effect of different humidity exposures on the moisture content and Nyquist are shown in Fig. 2S-12S.
Supercapacitor performance using activated carbon and rGO
To understand the possible application of synthesized electrolytes for energy device fabrication, two symmetric supercapacitors are fabricated, with commercially available active electrode materials namely (i) rGO and (ii) activated carbon (CAC). Active materials are coated on carbon cloths to be used as electrode. In both the cases, the configuration used is electrode/electrolyte/separator/electrolyte/electrode with commercial tissue paper (cellulose based) as a separator. The values of ESR estimated from Nyquist plots given in Fig. 4a are 9.25 Ω for CAC electrode and 8 Ω for rGO electrode. The electrochemical stability window (ESW) were estimated from LSV curve given in Fig. 4b. The ESW value obtained for rGO electrode is > 2.5 V but obtained ESW > 2 V for CAC electrode. The difference between the ESW for two systems can be correlated with the size of ion clusters with surface area as well as the pore size of electrode material [66].
CV curve of devices with rGO and CAC electrode are shown in Fig. 5 a and b at different scan rates ranging from 1 to 10 mV/s. The values of specific capacitance calculated using CV plots are found to be 17 F/g and 125 F/g respectively for rGO and CAC electrode materials using Eq. (3) at 1 mV/s scan rate as mentioned in Table 3. CV curves with rGO are more rectangular in comparison to CAC-based systems indicating EDLC type nature of rGO-based supercapacitor. Specific capacitance estimated as a function of scan rate is given in Fig. 5c, which shows quite a stable behavior, while CAC electrode based supercapacitor shows pseudocapacitive behavior which can state that the charge transfer between the electrode and electrolyte occurs as a result of a faradic current passing through the supercapacitor in pseudocapacitors, which store energy by faradaic reaction, intercalation, or electrosorption [5] that may have resulted in the higher specific capacitance in CAC-based supercapacitors. The variation of specific capacitance with scan rate for CAC electrode is shown in Fig. 5d.
Cyclic voltammograms (CV) of fabricated supercapacitor scan rate ranging from 1 to 10 mV/s using a rGO electrode and b CAC electrode. Value of variation in specific capacitance with variation in the scan rate using c rGO electrode and d CAC electrode. Ragone plot for comparing the category of energy device fabricated using e rGO electrode and f CAC electrode
There is a small variation in the value of specific capacitance with scan rate because at low scan rates (1 mV/s) the larger electrode surface is utilized for electrochemical reaction and at higher scan rates a limited surface area of electrodes can be utilized resulting in low value of capacitance [67]. At high scan rates, the high diffusion resistance which could be because of meager ion migration and ion diffusion affecting the ions’ movements between electrode and electrolyte results in decreased specific capacitance [68].
The two most popular electrochemical energy storage technologies among the different energy storage devices are batteries and supercapacitors, as seen in a graph known as a Ragone plot that shows energy density in Wh/kg vs. power density in W/kg as shown in Fig. 5 e and f. Using the Ragone plot, it can be found that in the studied energy storage systems power density lies in the range of 103–104 W/kg and energy density in the range of (10−1–10−2) Wh/kg, which belongs to the category of supercapacitors or ultracapacitors [69, 70].
Analysis of charging-discharge curve
Galvanostatic charging-discharging (GCD) has been carried out to access the charge and discharge characteristic of rGO- and CAC-based supercapacitors, ranging from current density 4 A/g to 37.5 A/g. Figure 6 a and b show the charging-discharging plot for rGO- and CAC-based supercapacitor. It is observed that time taken for the charging-discharging of cycles is ~ 0.5 s that may be due to the low relaxation time and high conductivity of electrolyte material. Charging-discharging test of rGO and CAC electrode is carried out till 5000 cycles to check the stability of the system as a supercapacitor at 4 A/g. Charging-discharging curve shows triangular shape cycles [71, 72] which indicates the fast ion transport in fabricated supercapacitor at different current densities in the 0–1 V voltage range.
The values of energy and power density are mentioned in Table 4 and are found to be 0.14 Wh/kg, 2.1 kW/kg, and 0.12 Wh/kg, 2.1 kW/kg that are comparable to the other reported systems [73,74,75] which is due to the short relaxation time of the synthesized electrolytes. Figure 6 c and d show the value of coulombic efficiency found to be (η %) > 90% for rGO-based supercapacitors and the value of coulombic efficiency is found to be (η %) > 99% in CAC-based supercapacitor.
Conclusions
GA-crosslinked arrowroot starch doped with CaCl2 salt–based polymer electrolytes is studied for the SIPE to PISE range, by using a simple synthesis protocol which satisfies the three “E” conditions (environment friendly, easy to prepare, economical) required for commercial application. Here, success has been achieved to keep it free from recrystallization problem without the need of any additives and/or the requirement of molten salt/salts which makes it an interesting material. High humidity exposure of PISE films resulted in ambient stabilized electrolyte with high conductivity, which could be considered aqueous electrolyte in solid-state physique. Small relaxation time (~ 17 μs) and high conductivity (10−2 S/cm) with high ESW range (> 3.5 V) make the synthesized polymer electrolyte film a potential candidate for future commercial testing. Supercapacitors are fabricated using commercially available activated carbon (CAC) and reduced graphene oxide (rGO). rGO-based supercapacitor is having EDLC behavior whereas CAC-based supercapacitor has pseudocapacitance type of nature. Faster charging-discharging cycles are obtained because of PISE films, which results in high power density values. Although the energy and power density estimated from GCD techniques results in almost similar energy and power density behavior for both the systems, CAC-based supercapacitor seems to have better coulombic efficiency (~ 99%) in comparison to rGO-based supercapacitor (~ 90%), which indicates long life and stable behavior. Achieved power density is of the order of kW/kg which adds up to favorable properties for commercial application.
Data availability
No datasets were generated or analysed during the current study.
References
Kim H, Kim H, Ding Z, Lee MH, Lim K, Yoon G, Kang K (2016) Recent progress in electrode materials for sodium-ion batteries. Adv Energy Mater 6:1600943. https://doi.org/10.1002/aenm.201600943
Jin H, Li J, Yuan Y, Wang J, Lu J, Wang S (2018) Recent progress in biomass-derived electrode materials for high volumetric performance supercapacitors. Adv Energy Mater 8. https://doi.org/10.1002/aenm.201801007
Xu Y, Yu S, Johnson HM, Wu Y, Liu X, Fang B, Zhang Y (2024) Recent progress in electrode materials for micro-supercapacitors. iScience 27. https://doi.org/10.1016/j.isci.2024.108786
Sharma SK, Sharma G, Gaur A, Arya A, Mirsafi FS, Abolhassani R, Rubahn HG, Yu JS, Mishra YK (2022) Progress in electrode and electrolyte materials: path to all-solid-state Li-ion batteries. Energy Adv 1:457–510. https://doi.org/10.1039/d2ya00043a
Anandhi P, Harikrishnan S, Senthil Kumar VJ, Lai WC, Mahmoud AED (2022) The enhanced energy density of rGO/TiO2 based nanocomposite as electrode material for supercapacitor. Electronics (Switzerland) 11:1792. https://doi.org/10.3390/electronics11111792
Sankar KV, Selvan RK (2015) Improved electrochemical performances of reduced graphene oxide based supercapacitor using redox additive electrolyte. Carbon N Y 90:260–273. https://doi.org/10.1016/j.carbon.2015.04.023
Rajasekaran SJ, Grace AN, Jacob G, Alodhayb A, Pandiraj S, Raghavan V (2023) Investigation of different aqueous electrolytes for biomass-derived activated carbon-based supercapacitors. Catalysts 13:286. https://doi.org/10.3390/catal13020286
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196:1–12
Li H, Han C, Huang Y, Huang Y, Zhu M, Pei Z, Xue Q, Wang Z, Liu Z, Tang Z, Wang Y (2018) An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ Sci 11:941–951. https://doi.org/10.1039/c7ee03232c
Wang J, Lei G, He T, Cao H, Chen P (2022) Defect-rich potassium amide: a new solid-state potassium ion electrolyte. J Energy Chem 69:555–560. https://doi.org/10.1016/j.jechem.2022.01.046
Liu W, Yi C, Li L, Liu S, Gui Q, Ba D, Li Y, Peng D, Liu J (2021) Designing polymer-in-salt electrolyte and fully infiltrated 3D electrode for integrated solid-state lithium batteries. AngewandteChemie 133:13041–13050. https://doi.org/10.1002/anie.202101537
Wu H, Gao P, Jia H, Zou L, Zhang L, Cao X, Engelhard MH, Bowden ME, Ding MS, Hu J, Hu D (2021) A polymer-in-salt electrolyte with enhanced oxidative stability for lithium metal polymer batteries. ACS Appl Mater Interfaces 13:31583–31593. https://doi.org/10.1021/acsami.1c04637
Ma Q, Liu D, Wang B, Liu W, Xiong G, Liu J (2023) Cellulose acetate-promoted polymer-in-salt electrolytes for solid-state lithium batteries. J Solid State Electrochem 27:1411–1421. https://doi.org/10.1007/s10008-023-05414-z
Chattopadhyay J, Pathak TS, Santos DMF (2023) Applications of polymer electrolytes in lithium-ion batteries: a review. Polymers 15:3907
Angell CA (2017) Polymer electrolytes—some principles, cautions, and new practices. Electrochim Acta 250:368–375. https://doi.org/10.1016/j.electacta.2017.07.118
Angell CA (1992) Mobile ions in amorphous solids. Annu Rev Phys Chem 43:693–717.https://doi.org/10.1146/annurev.pc.43.100192.003401
Angell CA, Liu C, Sanchez E (1993) Rubbery solid electrolytes with dominant cationic transport and high ambient conductivity. Nature 362:137–139. https://doi.org/10.1038/362137a0
Kumar M, Tiwari T, Srivastava N (2012) Electrical transport behaviour of bio-polymer electrolyte system: potato starch + ammonium iodide. Carbohydr Polym 88:54–60. https://doi.org/10.1016/j.carbpol.2011.11.059
Wischnewski A, Monkenbusch M, Willner L, Richter D, Kali G (2003) Direct observation of the transition from free to constrained single-segment motion in entangled polymer melts. Phys Rev Lett 90:058302. https://doi.org/10.1103/PhysRevLett.90.058302
Gao H, Grundish NS, Zhao Y, Zhou A, Goodenough JB (2021) Formation of stable interphase of polymer-in-salt electrolyte in all-solid-state lithium batteries. Energy Material Advances. https://doi.org/10.34133/2021/1932952
Wright PV (1976) An anomalous transition to a lower activation energy for dc electrical conduction above the glass-transition temperature. J Polym Sci Polym Phys Ed 14:955–957. https://doi.org/10.1002/pol.1976.180140516
Wright PV (1998) Polymer electrolytes - the early days. Electrochim Acta 43:1137–1143. https://doi.org/10.1016/S0013-4686(97)10011-1
Wang Z, Gao W, Chen L, Mo Y, Huang X (2002) Study on roles of polyacrylonitrile in “salt-in-polymer” and “polymer-in-salt” electrolytes. Solid State Ion 154:51–56. https://doi.org/10.1016/S0167-2738(02)00463-0
Moreau L, Bindzus W, Hill S (2011) Influence of salts on starch degradation: Part I - sodium chloride. Starch/Staerke 63:669–675. https://doi.org/10.1002/star.201100031
Moreau L, Bindzus W, Hill S (2011) Influence of salts on starch degradation: Part II - salt classification and caramelisation. Starch/Staerke 63:676–682. https://doi.org/10.1002/star.201100032
Monti D, Ponrouch A, Araujo RB, Barde F, Johansson P, Palacín MR (2019) Multivalent batteries-prospects for high energy density: Ca batteries. Front Chem 7:79. https://doi.org/10.3389/fchem.2019.00079
Arroyo-De Dompablo ME, Ponrouch A, Johansson P, Palacín MR (2019) Achievements, challenges, and prospects of calcium batteries. Chem Rev 120:6331–6357
Sun M, Zeng Z, Zhong W, Han Z, Peng L, Yu C, Cheng S, Xie J (2022) In situ prepared “polymer-in-salt” electrolytes enabling high-voltage lithium metal batteries. J Mater Chem A Mater 10:11732–11741. https://doi.org/10.1039/d2ta00093h
Ma L, Kim MS, Archer LA (2017) Stable artificial solid electrolyte interphases for lithium batteries. Chem Mater 29:4181–4189. https://doi.org/10.1021/acs.chemmater.6b03687
Delmas C (2018) Sodium and sodium-ion batteries: 50 years of research. Adv Energy Mater 8:1703137
Lu Y, Li L, Zhang Q, Niu Z, Chen J (2018) Electrolyte and interface engineering for solid-state sodium batteries. Joule 2:1747–1770
Gao H, Xue L, Xin S, Goodenough JB (2018) A high-energy-density potassium battery with a polymer-gel electrolyte and a polyaniline cathode. AngewandteChemie 130:5547–5551. https://doi.org/10.1002/anie.201802248
Thanisha F, Koliyoor J, Ismayil MJ (2024) Amorphous magnesium-doped chitosan films as solid polymer electrolytes for energy storage device applications. Mater Sci Eng, B 301:117149. https://doi.org/10.1016/j.mseb.2023.117149
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Maheshwari T (2020) Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym Bull 77:6299–6317. https://doi.org/10.1007/s00289-019-03071-9
Elia GA, Marquardt K, Hoeppner K, Fantini S, Lin R, Kinpping E, Peters W, Drillet JF, Passerini S, Hahn R (2016) An overview and future perspectives of aluminum batteries. Adv Mater 28:7564–7579. https://doi.org/10.1002/adma.201601357
Yang H, Li H, Li J, Sun Z, He K, Cheng HM, Li F (2019) The rechargeable aluminum battery: opportunities and challenges. AngewandteChemie 35:11978–11996
Tiwari T, Pandey K, Srivastava N, Srivastava PC (2011) Effect of glutaraldehyde on electrical properties of arrowroot starch + NaI electrolyte system. J Appl Polym Sci 121:1–7. https://doi.org/10.1002/app.33559
D Yadav A Pandey N Srivastava 2024 Effect of crosslinker on the electrochemical properties of starch-based water-in-polymer-salt electrolytes https://doi.org/10.1007/s11581-024-05689-w
Yan L, Yang W, Yu H, Zhang L, Shu J (2023) Recent progress in rechargeable calcium-ion batteries for high-efficiency energy storage. Energy Storage Mater 60:102822
Shakourian-Fard M, Kamath G, Taimoory SM, Trant JF (2019) Calcium-ion batteries: identifying ideal electrolytes for next-generation energy storage using computational analysis. J Phys Chem C 123:15885–15896. https://doi.org/10.1021/acs.jpcc.9b01655
Ropp RC (2013) Group 17 (H, F, Cl, Br, I) Alkaline earth compounds. In: Encyclopedia of the Alkaline Earth Compounds. Elsevier, pp 25–104
Gao Y, Qin Z, Guan L, Wang X, Chen GZ (2015) Organoaqueous calcium chloride electrolytes for capacitive charge storage in carbon nanotubes at sub-zero-temperatures. Chem Commun 51:10819–10822. https://doi.org/10.1039/c5cc03048j
Zhang J, Cui C, Wang PF, Li Q, Chen L, Han F, Jin T, Liu S, Choudhary H, Raghavan SR, Eidson N (2020) “Water-in-salt” polymer electrolyte for Li-ion batteries. Energy Environ Sci 13:2878–2887. https://doi.org/10.1039/d0ee01510e
Samanta P, Ghosh S, Kolya H, Kang CW, Murmu NC, Kuila T (2021) Molecular crowded “water-in-salt” polymer gel electrolyte for an ultra-stable Zn-ion battery. ACS Appl Mater Interfaces 14:1138–1148
Khan Z, Ail U, Nadia Ajjan F, Phopase J, Ullah Khan Z, Kim N, Nilsson J, Inganas O, Berggren M, Crispin X (2022) Water-in-polymer salt electrolyte for slow self-discharge in organic batteries. Adv Energy Sustain Res 3:2100165. https://doi.org/10.1002/aesr.202100165
Suo L, Borodin O, Gao T, Olguin M, Ho J, Fan X, Luo C, Wang C (1979) Xu K (2015) “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350:938–943. https://doi.org/10.1126/science.aab1595
Yadav M, Nautiyal G, Verma A, Kumar M, Tiwari T, Srivastava N (2019) Electrochemical characterization of NaClO4 –mixed rice starch as a cost-effective and environment-friendly electrolyte. Ionics 25:2693–2700. https://doi.org/10.1007/s11581-018-2794-x
Yadav M, Kumar M, Srivastava N (2018) Supercapacitive performance analysis of low cost and environment friendly potato starch based electrolyte system with anodized aluminium and Teflon coated carbon cloth as electrode. Electrochim Acta 283:1551–1559. https://doi.org/10.1016/j.electacta.2018.07.060
Yadav D, Basuroy S, Kumar R, Aggarwal K, Srivastava N (2024) Effect of high humidity exposure to wheat starch based high conducting flexible polymer-in-salt-electrolyte. Macromol Symp 413:2300068. https://doi.org/10.1002/masy.202300068
Tiwari T, Chauhan JK, Yadav M, Kumar M, Srivastava N (2017) Arrowroot + NaI: a low-cost, fast ion conducting eco-friendly polymer electrolyte system. Ionics 23:2809–2815. https://doi.org/10.1007/s11581-017-2028-7
Xiang C, Li M, Zhi M, Manivannan A, Wu N (2013) A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. J Power Sources 226:65–70. https://doi.org/10.1016/j.jpowsour.2012.10.064
Wu Q, Xu Y, Yao Z, Liu A, Shi G (2010) Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 4:1963–1970. https://doi.org/10.1021/nn1000035
Ali A, Chiang YW, Santos RM (2022) X-ray diffraction techniques for mineral characterization: a review for engineers of the fundamentals, applications, and research directions. Minerals 12:205. https://doi.org/10.3390/min12020205
Xu W, Wang LM, Angell CA (2003) “PolyMOB”-lithium salt complexes: from salt-in-polymer to polymer-in-salt electrolytes. Electrochim Acta 48:2037–2045
St-Onge V, Cui M, Rochon S, Daigle JC, Claverie JP (2021) Reducing crystallinity in solid polymer electrolytes for lithium-metal batteries via statistical copolymerization. Commun Mater 2:83
Yadav M, Kumar M, Srivastava N (2023) High-conducting, economical, and flexible polymer-in-salt electrolytes (PISEs) suitable for energy devices: a reality due to glutaraldehyde crosslinked starch as host. J Solid State Electrochem 27:1213–1226. https://doi.org/10.1007/s10008-023-05421-0
Yadav M, Verma A, Nautiyal G, Srivastava N (2019) Magnesium perchlorate mixed and glutaraldehyde crosslinked potato starch: an economical and flexible electrolyte membrane. Macromol Symp 388:1900033. https://doi.org/10.1002/masy.201900033
Chauhan JK, Yadav D, Yadav M, Kumar M, Tiwari T, Srivastava N (2020) NaClO4 added, corn and arrowroot starch based economical, high conducting electrolyte membranes for flexible energy devices. SN Appl Sci 2:1–12. https://doi.org/10.1007/s42452-020-2660-0
Pal B, Yang S, Ramesh S, Thangadurai V, Jose R (2019) Electrolyte selection for supercapacitive devices: a critical review. Nanoscale Adv 1:3807–3835. https://doi.org/10.1039/c9na00374f
Zhong C, Deng Y, Hu W, Qiao J, Zhang L, Zhang J (2015) A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 44:7484–7539
Oll O, Väärtnõu M, Gorbatovski G, Zhao J, Siimenson C, Siinor L, Lusk K, Romann T, Pikma P, Lust E (2019) Adsorption of anions on bismuth and cadmium single crystal plane electrodes from various solvents and ionic liquid mixtures. Electrochim Acta 319:895–908. https://doi.org/10.1016/j.electacta.2019.06.179
Srivastava Neelam, Kumar Manindra, Tiwari Tuhina, Electrolysis of starch based electrolyte. 372370. 31–05–2012
Groves TS, Perez-Martinez CS, Lhermerout R, Perkin S (2021) Surface forces and structure in a water-in-salt electrolyte. J Phys Chem Lett 12:1702–1707. https://doi.org/10.1021/acs.jpclett.0c03718
Shen Y, Liu B, Liu X, Liu J, Ding J, Zhong C, Hu W (2021) Water-in-salt electrolyte for safe and high-energy aqueous battery. Energy Storage Mater 34:461–474
Tot A, Zhang L, Berg EJ, Svensson PH, Kloo L (2023) Water-in-salt electrolytes made saltier by Gemini ionic liquids for highly efficient Li-ion batteries. Sci Rep 13:2154. https://doi.org/10.1038/s41598-023-29387-1
Avraham E, Yaniv B, Soffer A, Aurbach D (2008) Developing ion electroadsorption stereoselectivity, by pore size adjustment with chemical vapor deposition onto active carbon fiber electrodes. Case of Ca2+/Na+ Separation in water capacitive desalination. J Phys Chem C 112:7385–7389. https://doi.org/10.1021/jp711706z
Ranjithkumar R, Arasi SE, Sudhahar S, Nallamuthu N, Devendran P, Lakshmanan P, Kumar MK (2019) Enhanced electrochemical studies of ZnO/CNT nanocomposite for supercapacitor devices. Physica B Condens Matter 568:51–59. https://doi.org/10.1016/j.physb.2019.05.025
Chen YL, Hu ZA, Chang YQ, Wang HW, Zhang ZY, Yang YY, Wu HY (2011) Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J Phys Chem C 115:2563–2571. https://doi.org/10.1021/jp109597n
Raghavendra KV, Vinoth R, Zeb K, Gopi CV, Sambasivam S, Kummara MR, Obaidat IM, Kim HJ (2020) An intuitive review of supercapacitors with recent progress and novel device applications. J Energy Storage 31:101652
Beyers I, Bensmann A, Hanke-Rauschenbach R (2023) Ragone plots revisited: a review of methodology and application across energy storage technologies. J Energy Storage 73:109097
Du H, Wu Z, Xu Y, Liu S, Yang H (2020) Poly(3,4-ethylenedioxythiophene) based solid-state polymer supercapacitor with ionic liquid gel polymer electrolyte. Polymers 12:297. https://doi.org/10.3390/polym12020297
Kötz R, Carlen MJ (2000) Principles and applications of electrochemical capacitors. Electrochim Acta 45:2483–2498. https://doi.org/10.1016/S0013-4686(00)00354-6
Figueroa-Gonzalez E, Mendoza R, Oliva AI, Gomez-Solis C, Valle-Garcia LS, Oliva E, Rodriguez-Gonzalez V, Encinas A, Oliva J (2021) Highly efficient and biodegradable flexible supercapacitors fabricated with electrodes of coconut-fiber/graphene nanoplates. J Phys D Appl Phys 55:035501. https://doi.org/10.1088/1361-6463/ac2db5
Hoseinizadeh M, Salem KE, Gouda A, Belanger D, Santato C (2023) Tannins for sustainable semi-solid-state supercapacitors. Waste Biomass Valorization 14:3475–88. https://doi.org/10.1007/s12649-023-02125-4
Feng C, Jia B, Wang H, Wang Y, Wu X (2023) A novel strategy for high energy density supercapacitors: formation of cyanuric acid between Ti3C2Tx (MXene) interlayer hybrid electrodes. Chemical Eng J 465:142935. https://doi.org/10.1016/j.cej.2023.142935
Acknowledgements
Author NS is thankful to BHU for providing research grant for faculty (IOE Scheme) DeV scheme No 6031- sanction number R/DeV/D/IoE/Incentive/2021-22/32161 and also infra structure grant sanction number R/Dev/D/Object head 35/2018-19/73496. Authors acknowledge the CIL (central instrumentation lab) MMV, BHU for providing facility for XRD measurement. Author KA, DY is thankful to Banaras Hindu University for providing UGC research fellowship.
Funding
Author NS is thankful to BHU for providing research grant for faculty (IOE Scheme) DeV scheme No 6031- sanction number R/DeV/D/IoE/Incentive/2021–22/32161 and also infrastructure grant sanction number R/Dev/D/Object head 35/2018–19/73496. Authors KA and DY are thankful to Banaras Hindu University for providing UGC research fellowship. NS is thankful to SRICC credit research incentive for faculty members (SRICC/IOE/CRI(FM)/2022-23/2724).
Author information
Authors and Affiliations
Contributions
NS is Ph.D. supervisor of KA and DY and has framed the scientific work and finalized the manuscript. KA and DY have equal contribution in doing the experimental work and conceptualizing of scientific content. Kashish Tiwari and Pushpa Kushwaha did some experimental and calculation work as a summer intern.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aggarwal, K., Yadav, D., Tiwari, K. et al. Investigating Ca2+ salt–based polymer-in-salt electrolyte for future energy storage systems. Ionics (2024). https://doi.org/10.1007/s11581-024-05754-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05754-4