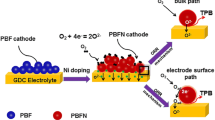
Abstract
Double-perovskite oxide PrBaFe2O5+δ (PBF) is considered as a potential electrode material because of its superior oxygen reduction reaction (ORR) activity in air and excellent stability in wet hydrogen atmospheres. However, the electrochemical activities of Fe-based electrode materials are constrained by the oxygen vacancy concentration and oxy-ion transport properties. Herein, PrBaFe2-xNbxO5+δ (PBFNx, x = 0, 0.05, 0.1, 0.15) oxides are synthesized and evaluated as electrodes for symmetrical solid oxide fuel cell (SSOFC). X-ray diffraction (XRD) indicates that PBFNx samples have an orthorhombic structure and good chemical compatibility with electrolyte. Among all the samples, the PBFN0.1 symmetrical half-cell shows the lowest polarization resistance at 800 °C, which decreases by 29.2% compared with that of PBF in air and decreases by 59.9% compared with that of PBF in wet hydrogen atmospheres. The output performance of the single cell with PBFN0.1 as symmetrical electrodes achieves 197.10 mW cm−2 in wet hydrogen atmospheres at 800 °C, which is an improvement of 31.97% compared with that of PBF. The enhanced electrochemical performance can be attributed to an increase in oxygen vacancy concentrations. The results suggest that the PBFN0.1 material is a potential candidate for SSOFC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solid oxide fuel cell (SOFC) is a high-efficiency energy conversion device that can directly convert chemical energy in various fuels into electrical energy. Considering its excellent fuel flexibility, high efficiency, and environmental friendliness, SOFC has shown great application potential in the electrochemical field [1,2,3]. A conventional SOFC configuration consists of three components, namely, porous anode, dense electrolyte, and porous cathode, and the electrodes normally use different materials because they are exposed to different working environments [4,5,6]. In recent years, symmetrical SOFC (SSOFC) with the new configuration of the A│B│A structure, where the cathode and anode use the same electrode material, has attracted significant attention. The preparation process can be greatly simplified to decrease the cost for operating and maintenance, thereby minimizing compatibility problems and improving thermal and long-term operational stability. Furthermore, this cell configuration eliminates carbon deposition and sulfur poisoning by switching the direction of gas flow [7,8,9,10,11]. However, the performance of SSOFC is limited by the electrodes. For SSOFC to work well, the electrode materials of the SSOFC require good structural and chemical stability in oxidizing and reducing atmospheres as well as excellent electrocatalytic activity for the oxygen reduction reaction (ORR) and hydrogen oxidation reaction (HOR) [12,13,14]. Recently, single-perovskite oxides (ABO3 type) and double-perovskite oxides (AA′BO5+δ or AA′B′B″O6-δ type) are primarily used as electrode materials for SSOFC [15], including Mn-, Cr-, Sr-, Fe-, and Co-based oxides [16]. Although Sr- and Co-based oxides show excellent catalytic activity and high electrical conductivity, they also encounter some issues. Co-based perovskite materials may suffer some problems, including poor stability and relatively high price of cobalt element [17]. Sr cations often show surface segregation or secondary phases with critical impurities [18]. Several recent studies have shown that Co-free and Sr-free layered perovskite oxides may be suitable candidates, which can overcome these complex shortcomings. In particular, Fe-based perovskites have attracted considerable interest due to their good catalytic activity [19], low cost of iron, and suitable TEC, such as single-perovskite oxides (SrFeO3 and BaFeO3) [20] and double-perovskite oxides (Sr2Fe1.5Mo0.5O6−δ and LnBaFe2O5+δ [LnBFO, Ln = La, Pr, Sm, and Gd]) [21, 22].
In the LnBaFe2O5+δ series, PrBaFe2O5+δ (PBF) oxides have been reported as electrode materials for SSOFC because of their rapid oxygen ion diffusion, excellent ORR activity, and superior stability in H2 atmospheres [23]. The PrBaFe2O5+δ sample with symmetrical electrodes exhibits superior redox stability and high peak power density (PPD). The stability has been investigated, and the PrBaFe2O5+δ shows excellent chemical stability in CO2 atmosphere [24, 25]. Compared with Co-based perovskite materials, the electrochemical activity of Fe-based perovskites lags behind a long distance. For example, at 700 °C, the interfacial polarization resistance of LaBaFe2O5+δ is 1.12 Ω cm2, approximately 3 times higher than the value of LaBaCo2O5+δ, which can be attributed to higher energy barriers for the formation of oxygen vacancies and diffusion of oxygen ions [26]. Therefore, the electrochemical catalytic activity still needs to be further improved. Based on literature, b-site cations and oxygen vacancies may be the main factors influencing electrochemical catalytic activity [27, 28]. As is known to all, elemental doping is an effective method for improving the electrochemical activity [29, 30]. PrBa(Fe0.8Sc0.2)2O5+δ was prepared and applied as symmetrical electrodes for SSOFC, and the Sc-doped electrode showed low polarization resistance (Rp) of 0.18 Ω·cm2 in wet H2 [31]. W-doped PrBaFe2O5+δ greatly improves the electrochemical performance and maintains excellent stability in air and hydrogen atmospheres [23]. Mo-doped PrBaFe2O5+δ has shown higher power density than the parent phase PBF [32]. In our previous work, Ta-doped PrBaFe2O5+δ perovskite achieved the Rp of 0.171 and 0.503 Ω cm2 in air and H2 at 800 °C, respectively [33].
Nb is an effective dopant for the B-site of perovskite oxides, which can significantly enhance their structural stability and constrain the oxidation state changes of other ions on the B-site due to the formation of strong Nb-O bond [34, 35]. In addition, the ionic radius of Nb5+ (0.064 nm) is very close to that of Fe3+ (0.0645 nm), making it easier for Nb ions to partially replace Fe ions on the B-site [36]. The performance of the Nb-doped LaBaFe2-xNbxO6-δ cathode has been evaluated, and the electrochemical performance and electrocatalytic activity were significantly improved by Nb-doped [37]. In addition, perovskite La0.6Sr0.4Fe1−xNbxO3−δ oxides as fuel electrodes have been investigated in our previous study. Nb doping can improve the electrochemical catalytic activity in CO2 atmospheres [38]. However, few studies have been conducted on Nb-doped PrBaFe2O5+δ as the symmetrical electrode of SSOFC.
In this paper, Nb-doped PrBaFe2O5+δ (PBFNx, x = 0, 0.05, 0.1, 0.15) oxides were synthesized and analyzed as symmetrical electrodes of SSOFC. The effect of Nb-doped PBF on the crystal structure, conductivity, electrochemical performance, and output performance was also investigated.
Experimental
Preparation of materials
The powders of PrBaFe2-xNbxO5+δ (PBFNx, x = 0, 0.05, 0.1, 0.15) were synthesized via a conventional solid-state reaction method. Stoichiometric amounts of Pr6O11 (99.99%, Shanghai Macklin Biochemical Co., Ltd.), BaCO3 (99.99%, Sinopharm Chemical Reagent Co., Ltd.), Fe2O3 (99.99%, Sinopharm Chemical Reagent Co., Ltd.), and Nb2O5 (99.99%, Sinopharm Chemical Reagent Co., Ltd.) powders were fully mixed and then homogenized in ethanol by ball milling for 24 h. After drying the milled solutions, the obtained powders were then calcined at 1200 °C for 6 h. The resultant oxides were grounded and dried using the abovementioned method and then passed through a sieve (80 mesh). Subsequently, the powders were pressed into pellets or rectangular bars using a cuboid mold and sintered at 1350 °C for 5 h. GDC (Gd0.1Ce0.9O2-δ) and YSZ (yttria-stabilized zirconia) powders were bought from Ningbo SOFCMAN Energy Technology Co., Ltd., China.
Material characterization
X-ray diffraction (XRD, Empyrean, PANalytical, The Netherlands) with Cu-Ka radiation accurately analyzed the crystal structure of all samples. A scanning speed of 10° per minute was used in the diffraction angle range of 20–80° at room temperature. X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha) was utilized to assess the composition and chemical elemental state of Fe2p and O1s ions in the PBFNx samples. The microstructure of the PBFN0.1|GDC|YSZ|GDC|PBFN0.1 symmetrical cell was observed by scanning electron microscopy (SEM, JSM-7600F, JEOL, Japan). For the electrical conductivity, the PBFNx sample materials were measured in air and humidified H2 by using a four-probe direct DC method equipped with a Keithley 2420 source meter with a temperature range of 300–800 °C and under an atmosphere with p(O2) = 0.21 atm.
Cell fabrication and electrochemical performance
The Rp of half cells (with the configuration of PBFNx|GDC|PBFNx in air or PBFNx|GDC|YSZ|GDC|PBFNx in H2) was measured using the CHI660e workstation with a frequency range of 106 to 0.1 Hz from 600 to 800 °C in a step of 50 °C. Furthermore, half cells with the configuration of PBFNx|GDC|PBFNx (x = 0, 0.1) were used to study the ORR kinetics of the PBF and PBFN0.1 electrodes at 800 °C. The electrolyte-supported single cells with the configuration of PBFNx|GDC|YSZ|GDC|PBFNx were fabricated to obtain the current density versus the voltage (I–V) and current density versus power output (I–P) curves. A half cell was prepared with a 0.5-mm-thick GDC electrolyte, and a full cell was prepared with a 0.3 mm-thick YSZ electrolyte. First, the YSZ electrolyte powders were ground and shaped into disk-shaped pellets at 100 MPa using a 18-mm-diameter cylindrical stainless-steel die. Then, the pellets were sintered at 1450 °C for 5 h in air to obtain dense pellets. The GDC slurry was deposited onto both sides of the YSZ by using a conventional screen-printing technique and was subsequently sintered at 1300 °C for 2 h. The as-prepared electrode slurry was painted on both sides of the GDC interlayer and sintered at 1000 °C in air for 2 h to form symmetrical cells. The electrode has an active area of 0.5 cm2. The single cells were assembled and sealed on the top of an alumina tube using a conductive resin (DAD-87, Shanghai Research Institute of Synthetic Resins) as sealant. The electrochemical performance of the single cell was measured using an electrochemical workstation (CHI660e) at a temperature range of 600–800 °C with humidified hydrogen (~3% H2O) as the fuel and ambient air as the oxidant. The flow rate of humidified hydrogen was 50 mL·min−1. After measuring the single cell, the short-term stability of the symmetrical cell with the PBFN0.1 electrode was evaluated in humidified hydrogen atmospheres by using the same electrochemical workstation.
Results and discussion
Crystal structure
Figure 1a shows the XRD patterns of the as-prepared PBFNx (x = 0, 0.05, 0.1, 0.15) powders calcined at 1200 °C for 6 h in air. PBFNx materials are an orthorhombic double-perovskite structure (space group: Pmmm) with no impurity phases, indicating that Nb element has been successfully doped into the PBF powder, and it is consistent with that of the PrBaFe2O5+δ materials in the previous study [39]. In addition, as shown in the enlarged view of the (110) peak of PBFNx (Fig. 1b), Nb doping slightly shifts the diffraction peaks of PBF to a lower angle, which can be attributed to the fact that the radius of Nb5+ (0.70 Å) is larger than that of Fe3+ (0.65 Å)/Fe4+ (0.59 A˚) [38]. In obtaining structural information of PBFNx samples, Rietveld refinements of XRD data are shown in Fig. 1c–f, and Table 1 shows the detailed refinement results. The lattice parameters of the parent PBF powder are a = 3.9351 Å, b = 3.9364 Å, and c = 7.8514 Å, which are close to the results reported by Lü et al. [40]. Doping Nb slightly increases the lattice volume from 121.81 to 123.60 Å3. The lattice parameter of the PBFN0.1 powder is a =3.9571 Å, b =3.9460 Å, and c = 7.8752 Å.
Chemical and thermal compatibility
Good chemical compatibility between electrode and electrolyte is important to the application of SSOFC [41]. Therefore, to evaluate the chemical compatibility between electrode and electrolyte materials, the electrode materials were sintered at 1000 °C for 2 h with a 1:1 mass ratio of GDC or YSZ electrolytes (Fig. 2). Figure 2a shows that new diffraction peaks were observed, indicating the formation of new phases. Consequently, the YSZ electrolyte has poor compatibility with PBFNx electrodes, and this electrolyte cannot be used directly as the support electrolyte material. Figure 2b shows no any new diffraction peaks and shifts, indicating that PBFN0.1 has good chemical compatibility with the GDC electrolyte. Thus, GDC can be used as an electrolyte for symmetrical cells or as a buffer layer to prevent the reaction between the electrolyte and electrode.
XPS analysis
XPS can be utilized to analyze the composition and surface chemical states of Fe2p and O1s ions for the PBFNx samples in the air. Figure 3a displays the high-resolution XPS spectra of Fe2p in the PBFNx samples. The fitting peaks located at ∼709.9 /723.5 eV and ∼711.8/725.0 eV can represent Fe3+ and Fe4+ ions, respectively. Furthermore, the satellite peak at ∼717.7 eV is assigned to the Fe3+ species [42]. In Table 2, with the increase of Nb substitution, the ratios of Fe4+/Fe3+ induce an obvious decline, implying the reduction of Fe ions from Fe4+ to Fe3+ (\(2{\text{Fe}}_{\text{Fe}}^{ \bullet }+{\text{O}}_{\text{O}}^{\text{X }}\iff {2\text{Fe}}_{\text{Fe}}^{\text{ X}}+ {\text{V}}_{\text{o}}^{\bullet \bullet }+\frac{1}{ 2}\) O2) to achieve electrical neutrality and the formation of more oxygen vacancies [37]. The increase of Fe3+ and oxygen vacancies results in the expanding lattice, which is consistent with the XRD result (Fig. 1b). As depicted in Fig. 3b, it was observed that the two fitting peaks in the spectra are lattice oxygen and absorbed oxygen. The lattice oxygen peak is located at ∼528.5 eV, while the absorbed oxygen peak is located at ∼531.3 eV [43]. One criterion for evaluating the relative content of oxygen vacancy in materials is the ratio of Oads/Olat [44]. The Oads/Olat values are 1.29, 1.94, 1.77, and 1.5 for PBFNx at x = 0, 0.05, 0.1, and 0.15, respectively, suggesting the formation of more oxygen vacancies after Nb doping. For PBFNx (x = 0.05, 0.1, 0.15) oxides, the oxygen vacancies exhibit a downward trend with increasing the amount of Nb doping, which is attributed to the stronger ionic affinity of Nb with O than of Fe. Based on literature, oxygen vacancies are important for the transport of oxygen ions during the oxygen reduction reaction (ORR) [45]. Consequently, the XPS results show that the Nb doping of PBF samples is beneficial for achieving good electrochemical performance.
Electrical conductivity
For mixed ionic–electronic conductor materials, electron conduction and ion conduction exist simultaneously. However, electronic conductivity is usually at least two orders of magnitude higher than ion conductivity, so the conductivity primarily refers to the electronic conductivity [46]. Figure 4a shows the conductivity of PBFNx (x = 0, 0.05, 0.1, 0.15) at different temperatures, ranging from 300 to 800 °C in the air. At below 450 °C, the conductivity of each composition in PBFNx increases because of the existence of a small polaron hopping conduction mechanism, indicating a semiconductor-like behavior. At about 450 °C in air, the conductivities of PBFNx reach maximum values. With the further increase in temperature, the oxygen lattice may lose along with the generation of oxygen vacancies and a mobility barrier of electronic conduction carriers. Consequently, the conductivity drops and shows a metallic-like behavior [47]. With increasing the amount of Nb doping, the conductivity exhibits a downward trend, which is ascribed to the increase in non-conducting Nb-O bonds and the decrease in the concentration of Fe4+. For further study, the conductivity of PBFNx (x = 0, 0.05, 0.1, 0.15) is measured in hydrogen. As shown in Fig. 4b, the conductivity of all samples increases with the increase of temperature. However, the electrical conductivity of PBFNx samples in H2 is lower than that of samples in air because of the presence of reduction reactions. Zhao et al. reported that \({\text{Fe}}_{\text{Fe }}^{\cdot }\)(Fe4+/Fe3+) are the mobile electronic holes, providing a significant contribution to electronic conduction. Under reducing conditions, electronic holes in PBFNx would reduce, leading to a decrease in electrical conductivity (A novel layered perovskite as symmetrical electrode for direct hydrocarbon solid oxide fuel cells). Furthermore, appropriate amount of Nb doping improves the conductivity of PBF in H2 atmosphere, which is attributed to the high-valence Nb5+ replacing Fe4+ and Fe3+ and promoting the concentration of free electrons [48]. The maximum conductivity of PBFNx samples is 46.35 S cm−1 in air and 3.74 S cm−1 in H2, which is higher than that of symmetrical electrodes, such as SrFe0.8W0.2O3 (1.16 S cm−1 in H2) [49], La0.5Sr0.5Fe0.9Nb0.1O3- δ (0.47 S cm−1 in H2) [48], and Sr2TiFe0.9Mo0.1O6 (0.90 and 0.26 S·cm−1 in air and H2) [50].
Half-cell performance
To study the electrochemical performance of PBFNx (x = 0, 0.05, 0.1, 0.15) symmetrical electrodes, the impedance of the electrodes was performed on the cells with the configuration of PBFNx|GDC|PBFNx in air at 600–800 °C. The corresponding EIS results were obtained by using Z-view fitting. The impedance of the electrodes primarily contains ohmic resistance (Ro) and polarization resistance (Rp). Ro is defined as the intercept of the semicircle on the real axis, including GDC electrolyte, PBFNx electrodes, and other contact resistance [51]. Rp is defined as the distance between semicircles on the real axis, which is primarily due to charge transfer, surface adsorption, and oxygen diffusion, and is an essential index to determine the electrochemical catalytic activity of SSOFC electrodes [52]. For the convenience of comparison, Ro is usually normalized to zero for clarity, and the intersection of two semi-circular arcs and the x axis is the polarization resistance. As shown in Fig. 5a, the Rp value decreases significantly after Nb doping, indicating that the ORR process has been improved. The Rp value of PBFN0.1 is 0.109 Ω cm2 at 800 °C, which decreases by 29.2% compared with that of PBF. The Rp consists of high-frequency resistance (RHF, >103 Hz) and low-frequency resistance (RLF, 10−1−103 Hz). The RHF response corresponds to the charge transfer among the interface between the electrode and the electrolyte. The RLF response is associated with gas diffusion and gas transformations (adsorption and desorption) [53]. As shown in Fig. 5b, RHF of the PBFN0.1 electrode is decreased by 22.69% to 0.01025 Ω cm2 at 800 °C, whereas RLF is decreased by 28.09% to 0.09931 Ω cm2 at 800 °C. The result indicates that Nb doping promotes electrochemical processes of PBF electrode by the increase in gas diffusion and gas transformations. Figure 5c shows the Rp values of PBFNx at different temperatures, ranging from 600 to 800 °C in air. The PBFN0.1 sample obtains the lowest Rp of 2.501, 0.985, 0.476, 0.229, and 0.109 Ω cm2 at 600 °C, 650 °C, 700 °C, 750 °C, and 800 °C, respectively. Figure 5d shows the Arrhenius curves of Rp of PBFNx electrodes in air. The activation energy (Ea) of the PBFN0.1 electrode is lower than that of PBF, which further indicates that Nb doping improves the catalytic activity of ORR in the PBF electrode.
Based on some GDC literature reports, GDC cannot be used directly as an electrolyte under a hydrogen atmosphere because its structure change (Ce4+→Ce3+) leads to performance degradation [32]. When the YSZ is used as the electrolyte, GDC is required as a buffer layer to prevent reactions between the electrodes and electrolyte (Fig. 2b). The impedance of the electrodes was performed on the cells with the configuration of PBFNx|GDC|YSZ|GDC|PBFNx in hydrogen at 600–800 °C. As shown in Fig. 6a, the Rp value decreases significantly after Nb doping. Among PBFNx electrodes, the x = 0.1 sample shows the lowest Rp, which is decreased by 59.9% to 0.391 Ω cm2 at 800 °C in hydrogen atmospheres. This result indicates that Nb doping can improve the HOR activity. As shown in Fig. 6b, RHF of the PBFN0.1 electrode is decreased by 30.7% to 0.0009 Ω cm2 at 800 °C, whereas RLF is decreased by 61.33% to 0.3633 Ω cm2 at 800 °C, suggesting that the HOR is primarily determined by the charge transfer between the electrode and the electrolyte. As shown in Fig. 6c, PBFN0.1 sample has the lowest Rp, which is 6.001, 3.592, 1.877, 0.841, and 0.391 Ω·cm2 at different temperatures, ranging from 600 to 800 °C in hydrogen atmospheres. As shown in Fig. 6d, the Ea value of the PBFN0.1 electrode is lower than that of the PBF electrode, which further indicates that the introduction of Nb doping into the PBF electrode improves the HOR activity. Based on the abovementioned results, Nb doping can improve the ORR and HOR activity of PBF electrode. In addition, the performance of PBFN0.1 symmetrical electrodes is higher than that of other iron-based perovskite, and the Rp results in the literature are listed in Table 3.
To obtain more insights into the ORR mechanism of electrode, the PBF and PBFN0.1 electrodes with GDC as the electrolyte were measured under different oxygen pressure levels at 800 °C, and the EIS data are shown in Fig. 7a, b. Based on literature, the Rp value of the electrodes satisfies a certain relationship with the oxygen partial pressure p(O2) by using the following expression:
where n represents the number of reaction steps of the electrochemical ORR, satisfying the following correspondence [58, 59]:
The impedance spectrum can be separated into two arcs, namely, RHF and RLF, indicating the rate of ORR reaction consists of two electrochemical processes as illustrated in Fig. 7c, d.
Figure 7e, f takes logarithms of Rp and oxygen partial pressure value p(O2) to obtain the relationship diagram. The slope of the straight line corresponds to the number of reaction levels n in Eq. (1), where the n value size can be inferred from the reaction steps of the corresponding electrode. As shown in Fig. 7e, the n corresponding to RHF of the PBF electrode is 0.09, which indicates that RHF during the electrode reaction is primarily affected by the transfer of oxygen ions from the double-phase boundaries and triple-phase boundaries reaction to the interior of the electrolyte (Eq. (5)), and the n corresponding to RLF is 0.42, which indicates that RLF is primarily due to the diffusion of adsorbed oxygen on the electrode surface (Eq. (3)). For the PBFN0.1 electrode (Fig. 7f), where the values of n corresponding to RHF and RLF are 0.22 and 0.56, RHF of PBFN0.1 is due to the charge transfer reactions (Eq. (2)), whereas RLF is primarily due to the adsorption and desorption process of oxygen (Eq. (3)). It is observed that RLF is higher than RHF in Table 4, suggesting that the gas diffusion and gas transformation are rate-determining influences for the ORR process. The result is consistent with the EIS analysis.
Single-cell performance
Single cell
To investigate the output performance of PBF and PBFN0.1 symmetrical electrodes in SSOFCs, electrolyte-supported single cells with the configurations of PBF|GDC|YSZ|GDC|PBF and PBFN0.1|GDC|YSZ|GDC|PBFN0.1 were prepared. With humidified hydrogen as fuel and air as an oxidant, the single cell was measured in the temperature range of 600–800 °C. Figure 8a and b shows the I-V and I-P curves of the single cell with PBF and PBFN0.1 as symmetrical electrodes, respectively. As shown in Fig. 8, the open-circuit voltage at 800 ℃ is 1.09 V, which is approximated the theoretical value, indicating that the single cell has good sealing performance and density. At 800 °C, 750 °C, 700 °C, 650 °C, and 600 °C, the PPD of PBF is 134.07, 82.68, 60.64, 32.54, and 15.17 mW cm−2, and the PPD of doped Nb is 197.10, 145.65, 97.38, 60.50, and 31.18 mW cm−2, respectively. The PPD of the electrolyte-supported single cell with PBFN0.1 symmetrical electrodes is higher than that of PBF, indicating that the Nb-doped PBF electrode can effectively promote ORR activity in the electrode. This remarkable improvement was achieved by reducing the Rp of the electrodes. The enhanced output performance of a PBFN0.1 single cell further proves that Nb doping improves the electrochemical performance.
Stability
Stability is also an important index to evaluate the working state of SSOFC. Therefore, in this experiment, the short-term performance stability of a single cell with PBFN0.1 symmetrical electrodes exposed to 3% H2O–97% H2 was maintained at a current density of 0.14 A/cm2 for 32 h at 800 °C. As shown in Fig. 9, the single cell maintained a relatively stable performance with no degradation under the operating condition, indicating that the PBFN0.1 electrode has good short-term stability.
SEM
Figure 10 shows the morphology of the PBFN0.1|GDC|YSZ|GDC|PBFN0.1 symmetrical cell before and after the stability test at 800 °C. No evident change for electrodes is observed before and after the test. The GDC interlayer is well attached between the porous electrode and the YSZ electrolyte, which promotes the transport of oxygen ions and reduces interfacial resistance. In addition, the electrode region presents a porous structure, which facilitates ion diffusion and transport. The elemental mapping of the single-cell cross-section is shown in Fig. 10e, f, which confirms the presence of Pr, Ba, Fe, Ce, Gd, Zr, Y, Nb, and O elements. In addition, the elements are uniformly distributed as expected. Since the atomic number of Nb is close to that of Zr, the distribution of Nb and Zr elements overlap.
Figure 11 shows the schematic diagram of the cathode and anode after Nb doping. Nb-doped PBF electrodes increase the concentration of oxygen vacancies in air and H2, which improves the electrochemical catalytic activity of ORR and HOR. However, the mechanism of performance improvement is different. In PBF materials, the oxygen reduction reaction occurs at the cathode, which mainly reduces oxygen and electrons to oxygen ions and transports them to the anode [60]. Doping Nb promotes the adsorption and desorption processes of surface oxygen, which makes it easier for oxygen molecules adsorbed on the surface to be desorbed and enter the interior of the material. This adsorption and desorption process helps to increase the concentration of oxygen vacancies, thereby enhancing the ORR of the material. The function of the anode is to receive electrons from the oxygen ions supplied to the cathode and react with the fuel. Doping Nb promotes electron transport, which leads to the formation of more oxygen vacancies at the anode. Oxygen vacancies could facilitate the increase of the anode surface area for reaction with oxygen, thus improving the electrochemical catalytic activity of HOR (Fig. 11).
Conclusion
Double-perovskite PrBaFe2-xNbxO5+δ (PBFNx, x = 0, 0.05, 0.1, 0.15) is synthesized by the solid-state reaction and investigated. The XRD results exhibit that PBFN0.1 shows good chemical compatibility with GDC electrolyte. The XPS results show that Nb doping can increase the concentration of oxygen vacancies and promote oxygen ion transport. In the EIS test, the PBFN0.1 sample shows the lowest Rp values of 0.109 and 0.391 Ω cm2 in air and H2, respectively. Furthermore, the PPD of the single cell increases by 31.97% from 134.07 to 197.10 mW cm−2. Moreover, the single cell with the PBFN0.1 cathode exhibits good short-term stability during 32 h after operation. Thus, the novel material PBFN0.1 is a potential candidate electrode for SSOFC.
Data availability
No datasets were generated or analysed during the current study.
References
Li Z, Wan Z et al (2021) A robust network binder via localized linking by small molecules for high-areal-capacity silicon anodes in lithium-ion batteries. Nano Energy 79:105430. https://doi.org/10.1016/j.nanoen.2020.105430
Kim J, Rehman SU et al (2023) Vertical-cavity surface-emitting laser (VCSEL)-based ultrafast photonic sintering of solid oxide fuel cells (SOFCs): prospects for time-efficient/two-dimensional scalability to large-sized SOFCs. J Mater Chem A 11(17):9474–9484. https://doi.org/10.1039/d3ta00602f
Shah M, Lu YZ et al (2023) Semiconductor-membrane fuel cell (SMFC) for renewable energy technology. Renew Sust Energ Rev 185:24. https://doi.org/10.1016/j.rser.2023.113639
Yang Y, Chen ZP et al (2023) A highly efficient bismuth substitution induced A-site ordered layered perovskite electrode for symmetrical solid oxide fuel cells. J Mater Chem A 11(15):7995–8002. https://doi.org/10.1039/d2ta08892d
Zhao X, Yang X et al (2021) Tailoring an interstitial oxygen conducting electrode by in situ fabrication for quasi-symmetrical solid oxide fuel cells. Ionics 27(1):259–268. https://doi.org/10.1007/s11581-020-03790-4
Qin HC, Cheng Z et al (2023) Thermoelectrical comprehensive analysis and optimization of multi-stack solid oxide fuel cell system. Energy Conv Manag 291:18. https://doi.org/10.1016/j.enconman.2023.117297
Shen YF, Song Y et al (2022) Mo-doped Ba0.5Sr0.5Co0.8Fe0.2O3-δ as a high-performance symmetric electrode for solid oxide fuel cells. J Alloy Compd 928:9. https://doi.org/10.1016/j.jallcom.2022.167029
Zhang M, Du ZH et al (2022) Progress of Perovskites as Electrodes for Symmetrical Solid Oxide Fuel Cells. ACS Appl Energ Mater 5(11):13081–13095. https://doi.org/10.1021/acsaem.2c02149
Zhang Y, Zhang X et al (2023) Pr-Doped SrTi0.5Mn0.5O3-δ as an electrode material for a quasi-symmetrical solid oxide fuel cell using methane and propane fuel. Acs Appl Mater Interfaces 15(3):3974–3984. https://doi.org/10.1021/acsami.2c18530
Zhu K, Luo B et al (2022) Recent advances and prospects of symmetrical solid oxide fuel cells. Ceram Int 48(7):8972–8986. https://doi.org/10.1016/j.ceramint.2022.01.258
Zamudio-García J, Caizán-Juanarena L et al (2022) A review on recent advances and trends in symmetrical electrodes for solid oxide cells. J Power Sources 520:24. https://doi.org/10.1016/j.jpowsour.2021.230852
Zhang Y, Zhao HL et al (2023) Boosting the electrode reaction kinetics of SSOFCs by the synergistic effect of nanoparticle codecoration on both the cathode and anode. Chem Mat 35(2):499–510. https://doi.org/10.1021/acs.chemmater.2c02861
Ling YH, Li T et al (2021) Oxygen vacancies-rich iron-based perovskite-like electrodes for symmetrical solid oxide fuel cells. Ceram Int 47(9):12916–12925. https://doi.org/10.1016/j.ceramint.2021.01.154
Zhang JH, Lei LB et al (2021) A practical approach for identifying various polarization behaviors of redox-stable electrodes in symmetrical solid oxide fuel cells. Electrochim Acta 384:15. https://doi.org/10.1016/j.electacta.2021.138340
Beshiwork BA, Teketel BS et al (2022) Nanoengineering electrode for yttria-stabilized zirconia-based symmetrical solid oxide fuel cells to achieve superior output performance. Sep Purif Technol 295:14. https://doi.org/10.1016/j.seppur.2022.121174
Qian B, Wang S et al (2023) Cobalt-free double-perovskite oxide Sr2Ti0.9FeNi0.1O6 as a promising electrode for symmetric solid oxide electrolysis cells. J Eur Ceram Soc 43(16):7561–7572. https://doi.org/10.1016/j.jeurceramsoc.2023.08.006
Ding HP, Lin B et al (2008) High performance protonic ceramic membrane fuel cells (PCMFCs) with Ba0.5Sr0.5Zn0.2Fe0.8O3-δ perovskite cathode. Electrochem Commun 10(9):1388–1391. https://doi.org/10.1016/j.elecom.2008.07.016
Shen LY, Du ZH et al (2021) Medium-entropy perovskites Sr(FeαTiβCoγMnζ)O3-δ as promising cathodes for intermediate temperature solid oxide fuel cell. Appl Catal B-Environ 295:10. https://doi.org/10.1016/j.apcatb.2021.120264
Zhang Y, Li JB et al (2023) CO2-induced reconstruction for ORR-enhanced solid oxide fuel cell cathode. Chem Eng J 462:7. https://doi.org/10.1016/j.cej.2023.142216
Beshiwork BA, Wan XY et al (2024) A defective iron-based perovskite cathode for high-performance IT-SOFCs: tailoring the oxygen vacancies using Nb/Ta co-doping. J Energy Chem 88:306–316. https://doi.org/10.1016/j.jechem.2023.09.015
Gao JX, Zhang YJ et al (2021) Nitrogen-doped Sr2Fe1.5Mo0.5O6-δ perovskite as an efficient and stable catalyst for hydrogen evolution reaction. Mater Today Energy 20:7. https://doi.org/10.1016/j.mtener.2021.100695
Chen DJ, Wang FC et al (2012) Systematic evaluation of Co-free LnBaFe2O5+δ (Ln = Lanthanides or Y) oxides towards the application as cathodes for intermediate-temperature solid oxide fuel cells. Electrochim Acta 78:466–474. https://doi.org/10.1016/j.electacta.2012.06.073
Zhang BZ, Wan YH et al (2021) Tungsten-doped PrBaFe2O5+δ double perovskite as a high-performance electrode material for symmetrical solid oxide fuel cells. ACS Appl Energ Mater 4(8):8401–8409. https://doi.org/10.1021/acsaem.1c01618
Kim D, Son SJ et al (2021) PrBaFe2O5+δ promising electrode for redox-stable symmetrical proton-conducting solid oxide fuel cells. J Eur Ceram Soc 41(12):5939–5946. https://doi.org/10.1016/j.jeurceramsoc.2021.05.031
Son SJ, Kim D et al (2021) Investigation of oxygen ion transport and surface exchange properties of PrBaFe2O5+δ. J Eur Ceram Soc 41(4):2691–2698. https://doi.org/10.1016/j.jeurceramsoc.2020.11.022
Zhang K, Ge L et al (2008) Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater 56(17):4876–4889. https://doi.org/10.1016/j.actamat.2008.06.004
Zhang Q, Wei T et al (2012) Electrochemical performance of double-perovskite Ba2MMoO6 (M=Fe Co, Mn, Ni) anode materials for solid oxide fuel cells. J Power Sour 198:59–65. https://doi.org/10.1016/j.jpowsour.2011.09.092
Jo S, Jeong HG et al (2021) Stability and activity controls of Cu nanoparticles for high-performance solid oxide fuel cells. Appl Catal B-Environ 285:7. https://doi.org/10.1016/j.apcatb.2020.119828
Zhang SW, Wan YH et al (2020) Bismuth doped La0.75Sr0.25Cr0.5Mn0.5O3-δ perovskite as a novel redox-stable efficient anode for solid oxide fuel cells. J Mater Chem A 8(23):11553–11563. https://doi.org/10.1039/d0ta03328f
Chen FZ, Zhou DC et al (2023) Doping strategy on improving the overall cathodic performance of double perovskite LnBaCo2O5+δ (Ln=Pr, Gd) as potential SOFC cathode materials. J Materiomics 9(5):825–837. https://doi.org/10.1016/j.jmat.2023.02.004
He W, Wu XL et al (2017) A novel layered perovskite electrode for symmetrical solid oxide fuel cells: PrBa(Fe0.8Sc0.2)2O5+δ. J Power Sources 363:16–19. https://doi.org/10.1016/j.jpowsour.2017.07.059
Zhou J, Xu LY et al (2019) Layered perovskite (PrBa)0.95(Fe0.9Mo0.1)2O5+δ as electrode materials for high-performing symmetrical solid oxide electrolysis cells. Mater Lett 257:4. https://doi.org/10.1016/j.matlet.2019.126758
Liu CH, Wang F et al (2023) Ta-doped PrBaFe2O5 double perovskite as a high- performance electrode material for symmetrical solid oxide fuel cells. Int J Hydrog Energy 48(26):9812–9822. https://doi.org/10.1016/j.ijhydene.2022.11.237
Xiao GL, Liu Q et al (2012) Synthesis and characterization of Mo-doped SrFeO3-δ as cathode materials for solid oxide fuel cells. J Power Sources 202:63–69. https://doi.org/10.1016/j.jpowsour.2011.11.021
Jiang SS, Sunarso J et al (2015) Cobalt-free SrNbxFe1-xO3-δ (x=0.05, 0.1 and 0.2) perovskite cathodes for intermediate temperature solid oxide fuel cells. J Power Sources 298:209–216. https://doi.org/10.1016/j.jpowsour.2015.08.063
Wang SB, Xu JS et al (2021) Cobalt-free perovskite cathode BaFe0.9Nb0.1O3–δ for intermediate-temperature solid oxide fuel cell. J Alloy Compd 872:10. https://doi.org/10.1016/j.jallcom.2021.159701
Li H, Wei B et al (2020) Novel cobalt-free layered perovskite LaBaFe2-xNbxO6-δ (x=0-0.1) as cathode for solid oxide fuel cells. J Power Sources 453:10. https://doi.org/10.1016/j.jpowsour.2020.227875
Wang S, Qian B et al (2021) High catalytic activity of Fe-based perovskite fuel electrode for direct CO2 electroreduction in SOECs. J Alloy Compd 888:10. https://doi.org/10.1016/j.jallcom.2021.161573
Lu CL, Niu BB et al (2020) Efficient symmetrical electrodes of PrBaFe2-xCoxO5+δ (x=0, 0.2,0.4) for solid oxide fuel cells and solid oxide electrolysis cells. Electrochim Acta 358:12. https://doi.org/10.1016/j.electacta.2020.136916
Lü SQ, Zhu YZ et al (2022) A-site deficient Fe-based double perovskite oxides PrxBaFe2O5+δ as cathodes for solid oxide fuel cells. J Alloy Compd 911:12. https://doi.org/10.1016/j.jallcom.2022.165002
Gao JT, Liu YY et al (2023) Cobalt-free fluorine doped Bi0.7Sr0.3FeO3-δ oxides for energetic cathodes of low-temperature solid oxide fuel cells. Chem Eng J 452:11. https://doi.org/10.1016/j.cej.2022.139584
Qiu P, Li C et al (2023) Materials of solid oxide electrolysis cells for H2O and CO2 electrolysis: a review. J Adv Ceram 12(8):1463–1510. https://doi.org/10.26599/jac.2023.9220767
Liu SB, Sun C et al (2020) A high-performance Ruddlesden-Popper perovskite for bifunctional oxygen electrocatalysis. ACS Catal 10(22):13437–13444. https://doi.org/10.1021/acscatal.0c02838
Mao XB, Wang WB et al (2015) A novel cobalt-free double-perovskite NdBaFe1.9Nb0.1O5+δ cathode material for proton-conducting IT-SOFC. Ceram Int 41(8):10276–10280. https://doi.org/10.1016/j.ceramint.2015.03.326
Wang SZ, Fin FJ et al (2017) Stability, compatibility and performance improvement of SrCo0.8Fe0.1Nb0.1O3+δ perovskite as a cathode for intermediate-temperature solid oxide fuel cells. Int J Hydrog Energy 42(7):4465–4477. https://doi.org/10.1016/j.ijhydene.2016.11.015
Lee J, Bark H et al (2023) Size-selective ionic crosslinking provides stretchable mixed ionic–electronic conductors. Angewandte Chemie Int Edition 62:06520. https://doi.org/10.1002/ange.202306994
Han X, Ling YH et al (2023) Utilizing high entropy effects for developing chromium-tolerance cobalt-free cathode for solid oxide fuel cells. Adv Funct Mater 33(43):9. https://doi.org/10.1002/adfm.202304728
Bian LZ, Duan CC et al (2018) Electrochemical performance and stability of La0.5Sr0.5Fe0.9Nb0.1O3-δ symmetric electrode for solid oxide fuel cells. J Power Sources 399:398–405. https://doi.org/10.1016/j.jpowsour.2018.07.119
Cao YX, Zhu ZW et al (2020) Development of tungsten stabilized SrFe0.8W0.2O3-δ material as novel symmetrical electrode for solid oxide fuel cells. J Power Sources 455:11. https://doi.org/10.1016/j.jpowsour.2020.227951
Niu BB, Jin FJ et al (2018) Performance of double perovskite symmetrical electrode materials Sr2TiFe1-xMoO6-x (x = 0.1, 0.2) for solid oxide fuel cells. Electrochim Acta 263:217–227. https://doi.org/10.1016/j.electacta.2018.01.062
Lü SQ, Meng XW et al (2012) Performances of SmBaCoCuO5+δ-Ce0.9Gd0.1O1.95 composite cathodes for intermediate-temperature solid oxide fuel cells. Int J Hydrog Energy 37(23):18319–18325. https://doi.org/10.1016/j.ijhydene.2012.09.043
Li GD, Gou YJ et al (2021) Enhanced electrochemical performance of the Fe-based layered perovskite oxygen electrode for reversible solid oxide cells. Acs Appl Mater Interfaces 13(29):34282–34291. https://doi.org/10.1021/acsami.1c08010
Liu B, Jia LC et al (2020) A novel PrBaCo2O5+δ-BaZr0.1Ce0.7Y0.1Yb0.1O3+δ composite cathode for proton-conducting solid oxide fuel cells. Compos Pt B-Eng 191:7. https://doi.org/10.1016/j.compositesb.2020.107936
Zhang Y, Zhang BZ et al (2021) Electrochemical performance and structural durability of Mg-doped SmBaMn2O5+δ layered perovskite electrode for symmetrical solid oxide fuel cell. Catal Today 364:80–88. https://doi.org/10.1016/j.cattod.2020.05.057
Tian D, Lin B et al (2016) Enhanced performance of symmetrical solid oxide fuel cells using a doped ceria buffer layer. Electrochim Acta 208:318–324. https://doi.org/10.1016/j.electacta.2016.04.189
Luo XY, Yang Y et al (2018) Reduced-temperature redox-stable LSM as a novel symmetrical electrode material for SOFCs. Electrochim Acta 260:121–128. https://doi.org/10.1016/j.electacta.2017.11.071
Dos Santos-Gómez L, Porras-Vázquez JM et al (2020) Investigation of PO43- oxyanion-doping on the properties of CaFe0.4Ti0.6O3−δ for potential application as symmetrical electrodes for SOFCs. J Alloy Compd 835:8. https://doi.org/10.1016/j.jallcom.2020.155437
Li M, Zheng MH et al (2017) Improving electrochemical performance of lanthanum strontium ferrite by decorating instead of doping cobaltite. Electrochim Acta 230:196–203. https://doi.org/10.1016/j.electacta.2017.02.014
Zhang Y, Shen LY et al (2022) Enhanced oxygen reduction kinetics of IT-SOFC cathode with PrBaCo2O5+δ/Gd0.1Ce1.9O2−δ coherent interface. J Mater Chem A 10(7):3495–3505. https://doi.org/10.1039/d1ta09615j
Wang S, Jiang HG et al (2020) Mo-doped La0.6Sr0.4FeO3-δ as an efficient fuel electrode for direct electrolysis of CO2 in solid oxide electrolysis cells. Electrochim Acta 337:11. https://doi.org/10.1016/j.electacta.2020.135794
Funding
This work was supported by the National Natural Science Foundation of China (No. 21978133 and No. 21506100), the Natural Science Foundation of Jiangsu Province (BK 20211260), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Author information
Authors and Affiliations
Contributions
Feng Wang: Conceptualization, Validation, Investigation, Visualization, Formal analysis, Writing - Original Draft. Jinyan Qi: Conceptualization, Investigation, Validation. Pengkai Shan:Conceptualization, Visualization. Bin Qian: Investigation. Lishuai Xie: Validation. Yifeng Zheng: Conceptualization, Resources, Supervision, Writing - Review & Editing. Han Chen: Formal analysis. Lin Ge: Methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Qi, J., Shan, P. et al. Co-free and Sr-free double-perovskite oxide PrBaFe1.9Nb0.1O5+δ as a potential electrode material for symmetrical solid oxide fuel cells. Ionics (2024). https://doi.org/10.1007/s11581-024-05724-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05724-w