Abstract
Tin dioxide-based high-performance anode materials for lithium-ion batteries have been a hot research topic in recent years. In this study, nitrogen-doped and double-layered SnO2@NC hollow spheres were prepared via simple and convenient method using carbon spheres as template. A series of products were obtained by varying additive amount of dopamine. When tested in the current density of 400 mA g−1, SnO2@NC-3 can provide a robust reversible capacity of 697.7 mAh g−1 after 270 cycles. The discharge capacity can remain 640.8 mAh g−1 after 800 cycles at 1000 mA g−1. Above excellent electrochemical properties were attributed to the synergistic effect between nitrogen-doped carbon and nanosized-SnO2 particles. The hollow structure can not only effectively buffer the structure crushing of the electrode in the process of charge and discharge, but also facilitate the electron diffusion by improving the electronic conductivity. Therefore, the unique nitrogen-doped and double-layered tin dioxide is a promising anode material for lithium-ion battery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) are critical portable electronic power sources with high energy density, long cycle life and low self-discharge [1,2,3]. In order to meet the increasing demand for large-scale applications, especially in the field of transportation, there are still high expectations for new electrode materials with high energy density and long-term cycling stability [4, 5]. However, with the rapid development and growth of energy demand, the commercial graphite with a theoretical specific capacity of 372 mAh g−1 has been unable to meet the increasing demand for battery capacity [6, 7]. Thus, it is the pursuit of scientific researchers to explore novel anode materials with higher theoretical specific capacity to meet the urgent requirements.
As one of the most competitive candidates for lithium-ion batteries, SnO2 has attracted much attention on account of its abundant resources, high theoretical specific capacity, and low working potential [8,9,10]. Furthermore, the voltage window of SnO2 electrode matches well with the positive electrode material, which guaranteed wider application range [11, 12]. However, in the electrode reaction, poor electronic conductivity and serious volume change (~ 359%) will inevitably lead to electrode disintegration and other problems, further reducing its capacity, cycle performance, and rate performance [13]. In addition, the pulverization and shedding of active substances will occur in the process of discharge–charge cycle, which will destroy the solid electrolyte interface (SEI), resulting in rapid capacity attenuation, and severely limiting its application in life. [14]
Various strategies have been devoted to overcome above problems for decades and kinds of tactics have been proposed. One effective approach is to design materials with nano/microstructure hierarchies to take the advantages of relatively stable microstructure, short diffusion path, and large interfacial contact area, which is benefit for excellent rate capacity, better cycling performance, and improved lithium storage capacity. [15, 16] Tong group fabricated tube-in-tube SnO2 superstructure with high specific surface area and sufficient volume, which supplied enough room for buffering volume changing during Li insertion and extraction and exhibited excellent discharge capacity when evaluated as lithium-ion battery anode. [17]
Another approach is to enhance electronic conductivity of SnO2 electrode. In recent years, various carbon-based substrates have been widely reported for encapsulation or recombination of SnO2 active materials, including graphene, carbon black, carbon nanotubes, carbon nanofibers, and carbon frames [18,19,20]. Furthermore, the agglomeration of nanomaterials electrode is alleviated with the introduction of carbon component. Notohara group synthesized SnO2/SWCNT nanocomposite in a vacuumed glass using SWCNT and SnCl2 as raw material. The nanoconfinement structure of SnO2 nanoparticles in SWCNT limited the volume change and is benefit for reversible electrode reaction [21]. The introduction of nitrogen atoms into the carbon layer will produce more defects, providing more active sites for lithium storage, thus nitrogen-doped carbon (NC) can effectively improve electron conductivity in carbon materials [22]. Liang group reported a simple route to obtain nitrogen-doped graphene/SnO2 composite. Nitrogen doping in graphene give rise to good conductivity, simultaneously compensates defects of pure SnO2, and displayed higher lithium storage capacity and good cycling stability [23].
In this paper, nitrogen-doped and double-layered porous SnO2 hollow spheres are prepared by electrostatic adsorption between negatively charged carbonaceous microspheres (CMS) template and positive metal cation (Sn4+) followed by annealing treatment. The porous SnO2 hollow spheres are loaded with nitrogen-doped carbon (NC) by deposition reaction. The double-layered SnO2@NC-3 electrode materials delivered high specific capacity of 697.7 mAh g−1 at 400 mA g−1 after 270 cycles. What’s more, the SnO2@NC-3 composite materials reveal outstanding cycling stability under high current density (640.8 mAh g−1 at 1000 mA g−1 after 800 cycles).
Experimental section
Synthesis and treatment of carbon spheres
Synthesis of carbon spheres: 2 g glucose was dissolved in 32 mL water. Subsequently, the solution was transferred to a 40-mL autoclave at 170 °C for 12 h in an oven. After the reaction, the product was naturally cooled to room temperature, washed several times with water and ethanol, and dried at 80 °C in the oven. Alkali-treated carbonaceous microspheres: a certain quantity of carbon spheres was added to 0.05 M sodium hydroxide solution followed by stirring for a certain time at room temperature and drying at 80 °C for 12 h.
Synthesis of hollow SnO2
The 0.6 g alkali-treated carbonaceous microspheres were dispersed in 1 M SnCl4·5H2O solution for ultrasonic dispersion, and the resulting suspension was aged at room temperature for 4 h, filtered, washed and dried at 80 °C for 12 h. Then the obtained alkaline carbon spheres were calcined at 700 °C in the air to obtain white products, which was named SnO2.
Synthesis of double layered SnO2@NC
In a typical process, 0.08 g hollow SnO2 and 0.24 g dopamine were stirred in 25 ml of Tris buffer for 24 h. The SnO2@polydopamnine hollow balls were centrifuged and carbonized in an N2 atmosphere at 600 °C for 2 h (denoted as SnO2@NC-3). The SnO2@NC-2 and SnO2@NC-1 samples were synthesized via the identical process by adapting the content of dopamine.
Material characterization
The crystal structures of the as-prepared samples were studied using X-ray diffraction (XRD) on Shimadzu XRD-6100 powder with Cu Kα radiation (λ = 1.5406 Å). The morphology of the samples and elemental distribution was obtained by transmission electron microscope (TEM, JEOL JEM-2100). Scanning electron microscopy (SEM) images were obtained on Hitachi S-4800 field emission microscope equipped with an X-ray energy dispersive spectrometer (EDS), and X-ray photoelectrons (XPS) was conducted on ESCALAB 250 system to analyze element composition and surface element states.
Electrochemical measurements
First of all, active material, cochin black, and polyvinylidene fluoride (PVDF) were mixed with a mass ratio of 70: 20: 10 in N-methyl-2-pyrrolidone (NMP). The resultant slurry was coated on copper foil and dried in a vacuum cabinet at 100 °C for 12 h. The mass loading calculated was approximately 0.6–0.8 mg cm−2. Lithium sheet was used as the counter electrode and polyethylene (PE) film as the separator. 1 M LiPF6 dissolved in ethylene carbonate, dimethyl carbonate, and ethylmethyl carbonate (1:1:1, in volume ration) was employed as the electrolyte. The half cells were assembled in a glove box filled with highly pure argon (H2O, O2 < 0.3 ppm, Etelux) using CR 2032-coin cells. The charge–discharge measurement was tested on LAND CT-2001A (Wuhan, China). Cyclic voltammetry (CV) measurements were employed in the voltage range of 0.01–3.0 V with a scan rate of 0.1 mV s−1 in RST5000 electrochemical workstation. Electrochemical impedance spectroscopy (EIS) measurements of cells were also recorded on RST 5000 electrochemical workstation over a frequency range of 100 kHz to 0.01 Hz.
Results and discussion
Material synthesis and characterization
The synthesis diagram of double-layer SnO2@NC was shown in Fig. 1. Variation of dopamine from 0.08 g, 0.16 g, to 0.24 g give rise to three products, SnO2@NC-1, SnO2@NC-2, and SnO2@NC-3, respectively.
The XRD spectrums of the synthesized SnO2@NC composite and SnO2 were shown in Fig. 2a. The sharp diffraction peaks at 2θ values of 26.61°, 33.89°, 37.94°, 51.78°, 54.75°, and 62.59° were corresponded to (110), (101), (200), (211), (220), and (221) planes of tetragonal rutile SnO2, which matches well with JCPDS card No.41–4451 [24, 25]. After careful observation, there was still a small amount of carbon remaining in the SnO2 sphere, the typical bulging peak of carbon appears at about 20°. From the XRD results of SnO2@NC-3, SnO2@NC-2, and SnO2@NC-1, the intensity of the SnO2 peak became stronger than SnO2 sphere, which can be ascribed to better crystallinity at higher temperature after calcination under N2 atmosphere. No obvious coexisting carbon peaks were observed, which was supposed to amorphous carbon produced by the carbonization of polydopamine. The surface electronic state and composition of the as-prepared SnO2@NC-3 was detected by XPS in Fig. 2b–2f. The full XPS spectrum of SnO2@NC-3 in Fig. 2b included Sn, N, C, and O elements. Peaks at 495.3 eV and 486.9 eV in the fine spectrum of Sn 3d3/2 and Sn 3d5/2 manifested the formation of SnO2 in Fig. 2c. [26] A symbolic spin energy separation of 8.4 eV in Fig. 2c corresponds well to the Sn (IV) oxidation state of SnO2 [27,28,29] .High resolution peak of N in Fig. 2d demonstrated the three forms of nitrogen element, which are pyridinic N (398.2 eV), pyrrolic N (400.3 eV), and graphitic N (401.6 eV), respectively [30, 31]. N-doped carbon can provide sufficient defects and active sites, which inevitably improve lithium storage capacities [32]. As shown in Fig. 2e, the C1s spectrum can be divided into three peaks corresponding to the 284.6, 286, and 288.6 eV of C–C (sp2 C), C = O, and O-C = O groups, respectively [33, 34] .The peak of C1s was mainly due to the nitrogen-doped carbon outside SnO2 nanoparticle. In the Fig. 2f, the O1s binding energy of SnO2 was 532 eV, corresponding to the typical Sn–O bond binding energy. Meanwhile, the peak at 533.4 eV was ascribed to C–O–C bond. Full XPS spectrum and fine spectrums of SnO2 were shown in Fig. S1, which demonstrated the existing of Sn–O bonds.
The morphology and detail structure of the prepared SnO2 samples were characterized by SEM and TEM. It can be seen from Fig. 3a–b that the carbon spheres synthesized by hydrothermal method were of uniform size and good dispersion, with a diameter of 600–700 nm. From the SEM images of SnO2 formed by calcination of tin salt-soaked carbon sphere in Fig. 3c–d, uniformly SnO2 hollow spheres were detected clearly. Subsequent TEM images of SnO2 were presented in Fig. 3e–f, from which hollow sphere structures were confirmed and porous sphere shell accumulated by many small particles can also be observed distinctly. The crystal structure of SnO2 was measured by HRTEM in Fig. 3g. A clear lattice fringe of approximately 0.33 nm was detected in the image, which matches well with the (110) plane of SnO2. [35, 36] The selected area electron diffraction (SAED) patterns of SnO2 showed characteristic diffraction rings in Fig. 3h, which demonstrated the rutile structure of SnO2 and polycrystalline state of the material. Above results were in good accordance with XRD results, indicating the polycrystalline structure of the product.
Using dopamine as carbon source to carbonize polycrystalline SnO2, variation of dopamine additive amount leading to three products, as is shown in Fig. 4. From Fig. 4a, a layer of nitrogen doped carbon (NC) can be detected on the surface of hollow SnO2, which maintained the hollow morphology. As shown in Fig. 4b, when the amount of dopamine was 0.16 g, the NC coating layer loaded on SnO2 surface was thicker than that of 0.08 g dopamine. When the amount of dopamine was increased to 0.24 g, the hollow tin dioxide has been completely wrapped by nitrogen-doped carbon, forming an intact spherical structure in the Fig. 4c. From the above analysis, it can be speculated that with the continuous increase of dopamine content, the nitrogen doped carbon on the surface of hollow SnO2 spheres became more and more, until the hollow tin dioxide spheres were completely enveloped, forming a spherical double-layered tin dioxide hollow sphere structure. Corresponding TEM pictures in Fig. 4d–4f displayed the same tendency of thicker carbon layer with more dopamine additive amount. Selected area electron diffraction pattern of brown circle in TEM picture was presented in Fig. 4g–i. From Fig. 4g, typical polycrystal diffraction points were observed and amorphous diffraction ring appeared with the thickening of carbon layer as shown in Fig. 4h and i. The EDX spectrum SnO2@NC-3 in Fig.S2 revealed the presence of C, O, Sn, and N peaks in SnO2@NC-3, implying that nitrogen-doped carbon has been successfully loaded on the surface of SnO2. Figure 4j presents the elemental mapping images of SnO2@NC-3 electrode, from which uniform distribution of Sn, O, C, and N can be clearly observed, representing even carbon coating on the surface of SnO2 and good integrity of electrode materials.
The electrochemical characterization of the SnO2@NC-3 composite was investigated in Fig. 5. From Fig. 5a, the CV curves of the composite were obtained in a range from 0.1 to 3.0 V at a scanning rate of 0.1 mV s−1. During the initial anode scan, a sharp and wide peak at 0.5 V was observed, which was ascribed to dealloying, while the two peaks at 1.25 and 1.91 V corresponded to the oxidation of SnO2 [37, 38]. In the initial cathodic scanning, the reduction reaction at the peaks of 0.9 V represented the lithiation reaction of SnO2: \(\mathbf{S}\mathbf{n}{\mathbf{O}}_{2}+4{\mathbf{L}\mathbf{i}}^{+}+4{\mathbf{e}}^{-}\to \mathbf{S}\mathbf{n}+{2\mathbf{L}\mathbf{i}}_{2}\mathbf{O}\). The peak at about 0.2 V was attributed to the lithiation reaction of Sn:\(\mathbf{S}\mathbf{n}+{\mathbf{x}\mathbf{L}\mathbf{i}}^{+}+{\mathbf{x}\mathbf{e}}^{+}\to {\mathbf{L}\mathbf{i}}_{\mathbf{x}}\mathbf{S}\mathbf{n}(0\le \mathbf{x}\le 4.4)\) [39, 40]. In addition, the unique peak at about 0.71 V revealed the lithiation of SnO2 and the irreversible formation of the SEI (solid electrolyte intermediate phase) layer [41]. However, the peak density gradually decreases during the cycle, indicating the irreversible nature. The SEI film formed during the first cycle is beneficial to the stability of the anode material structure and improvement of electrochemical performance. The following CV curves were almost overlapping, thus verifying the good reversibility of the SnO2@NC-3 composite.
Figure 5b disclosed the first charge and discharge curves of SnO2@NC-1, SnO2@NC-2, SnO2@NC-3, and SnO2 electrodes at a current density of 100 mA g−1. The first cycle discharge specific capacities of SnO2@NC-3, SnO2@NC-2, SnO2@NC-1, and SnO2 electrode materials were 1944.3, 1894.8, 1479.5, and 1470.8 mAh g−1, respectively.
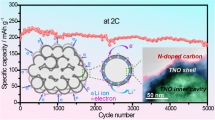
When tested at a current density of 400 mA g−1, SnO2@NC-3 can provide a high reversible capacity of 697.7 mAh g−1 after 270 cycles, which was distinctly higher than SnO2@NC-1 (294 mAh g−1), SnO2@NC-2 (362.9 mAh g−1), and SnO2 (137.7 mAh g−1) in Fig. 5c, showing excellent cycling stability. In order to further study the differences in the electrochemical performance of SnO2@NC-1, SnO2@NC-2, SnO2@NC-3, and SnO2, rate performance tests were carried out on four samples. The rate capabilities of the as-obtained samples were displayed in Fig. 5d, the SnO2@NC-3 electrode delivered discharge capacities of about 990.9, 689.2, 579.3, 478.4, and 433.8 mAh g−1 at increasing current rates of 100, 200, 400, 800, and 1000 mA g−1, respectively. Furthermore, a high capacity of the electrode can be backed to 650.4 mAh g−1 when the current was rebounded to 100 mA g−1, while SnO2@NC-2, SnO2@NC-1, and SnO2 showed the discharge specific capacity of 906, 593.6, 455.4, 313.8, 250.2 mAh g−1; 886.3, 676.9, 546.1, 390.6, 331.8 mAh g−1; and 604.8, 421.3, 349.4, 208.6, 204.2 mAh g−1 respectively. Rate performance test of SnO2@NC-3 electrode showed step-sensitive changes under different current densities; furthermore, higher discharge capacities implied better stability than SnO2@NC-2, SnO2@NC-1, and SnO2 electrode materials.
Long-cycle stability of all samples was tested at a high current density of 1000 mA g−1 in Fig. S3a; the double layered SnO2@NC-3 can provide a high capacity of 640.8 mAh g−1 after 800 cycles. The reversible capacity was significantly superior to SnO2@NC-2 (235.1 mAh g−1), SnO2@NC-1 (192.5 mAh g−1), and SnO2 (146.9 mAh g−1). It can be seen from Fig. S3a that the discharge specific capacity of the four electrode materials showed a downward trend before 100 cycles; however, after 100 cycles, the discharge specific capacity exhibited an upward trend. The full immersion of active material in electrolyte and activation of electrode in previous loop give rise to upward tendency, which is benefit for the maintenance of capacity [42]. The reversible capacity degradation of SnO2@NC-1, SnO2@NC-2, and SnO2 electrodes has a more severely decline than that of SnO2@NC-3, on account of the instability of the electrode structure and the formation of thicker SEI film. Finally, the long-cycle stability of SnO2@NC-3 under an ultra-high current density of 5000 mA g−1 was shown in Fig. S3b; the double layered SnO2@NC-3 still can maintain a discharge capacity of 212.7 mAh g−1 after 1000 cycles, with 100% Coulombic efficiency. According to the description of above results, SnO2@NC-3 exhibited excellent cycling stability, which was more suitable for large current charging and discharging.
There were two main reasons for the high reversible capacity of the SnO2@NC-3 electrode material: One reason was the advantage of the hollow sphere structure of interior SnO2, which was accumulated by small nanoparticles of SnO2. Nanosized SnO2 crystal can fully contact with the electrolyte to improve the reversibility of the reaction, and the hollow sphere structure can relieve the volume expansion in the process of charge and discharge. Another reason was the introduction of nitrogen doped carbon. Nitrogen doped carbon coated on the surface of SnO2 hollow spheres can not only inhibit the volume effect of SnO2 nanocrystals during cycling, but also prevent the accumulation of SnO2. Meanwhile, it can also improve the formation and decomposition of SEI films to prevent the capacity decline caused by the formation of thicker SEI films.
More importantly, nitrogen-doped carbon can significantly improve the conductivity of the electrode material, and ultimately improved the electrochemical performance of the SnO2@NC-3. It can be speculated from the above analysis that the amount of carbon content has a great influence on the electrochemical performance of battery materials. More amount of nitrogen-doped carbon coating on the surface of the hollow SnO2 is conducive to alleviate the large volume expansion and the crushing and shedding charge/discharge process of active materials. Thus, SnO2@NC-3 electrode material (dopamine addition was 0.24 g) has excellent electrochemical performances.
To further investigate the transmission kinetics of electrons and ions promoted by the designed multilayer hollow structure, electrochemical impedance spectroscopy was performed on half-cells under the test frequency ranges from 100 kHz to 0.01 Hz in Fig. 6a, which showed that each plot was composed of semicircles (high frequency region) and straight lines (low frequency region). After calculation, the Rct of SnO2@NC-3 electrode was about 84.86 Ω, which was lower than SnO2@NC-1 (103 Ω), SnO2@NC-2 (86.07 Ω), and SnO2 (193 Ω). The relatively low charge transfer resistance of SnO2@NC-3 can accelerate the ion migration between the electrolyte and the active material, which accelerated electrode reaction and generated better electrochemical performance.
a Nyquist diagram in the frequency range of 0.01 Hz–100 kHz and b Z΄-ω−1/2 curve in the low-frequency range of SnO2@NC-3, SnO2@NC-2, SnO2@NC-1, and SnO2; diagrams of SnO2@NC-3: c CV curves at different scanning rates; d The line relationship of log(i) vs. log(v); e capacitive contribution at the scan rate of 0.8 mV s−1; f Comparison of the pseudocapacitive contribution under different sweeping rates
In the low frequency region, the slope value σ in Fig. 6b can be obtained by Eq. (1): [43, 44].
After calculation, the slope of the SnO2@NC-3 was 25.44, which was smaller than SnO2@NC-2 (30.54), SnO2@NC-1 (186.43), and SnO2 (350.54), indicating SnO2@NC-3 was more conducive to the diffusion of Li+ between active material particles and electrolyte. This result was consistent with the excellent electrochemical performance of SnO2@NC-3.
The cyclic voltammetry curves at different scan rates of SnO2@NC-3 (in a voltage window of 0.01 to 3 V) were shown in Fig. 6c; the pseudocapacitance behavior was discussed and capacitance contribution can be evaluated by Eq. (2): [45, 46].
The value of a is an empirical parameters and b can be calculated from the slope of the linear relationship between logi and logv in Fig. 6d. The b value close to 0.5 discloses a diffusion-controlled behavior and the b value close to 1 demonstrates an excellent pseudocapacitive behavior. As shown in Fig. 6d, the b-value for peak 1 and peak 2 was 0.869 and 0.897, respectively, manifesting that the diffusion-controlled and pseudocapacitive behaviors were responsible for the high capacity of SnO2@NC-3. Therefore, we can reasonably infer that the two processes contributed to the total capacity.
The contribution of capacitance to total lithium storage capacity can be quantitatively calculated by Eq. (3): [47,48,49].
In Eq. (3), i(v) is the total current value, meanwhile the values of k1v and k2v1/2 reflect the pseudocapacitive and diffusion-controlled process, respectively. From Fig. 6e, the capacitive contribution of SnO2@NC-3 electrode was ~ 71.4% at a scan rate of 0.8 mV s−1. When the scan rates ascended from 0.2 to 1.6 mV s−1 in Fig. 6f, the percentage of the pseudocapacitive contribution increased from 62 to 83.4%. It can be concluded that capacitive behavior was beneficial to predominant capacity for the enhanced cycling stability and rate performances.
Conclusions
SnO2@NC composite material was synthesized by simple hydrothermal and deposition reactions. Polydopamine was successfully loaded on the surface of SnO2 through a deposition reaction and carbonized under the protection of N2 atmosphere. Carbon stabilized the electrode structure and improved the charge transfer ability of active material, which are responsible for better electrochemical performance. The introduction of nitrogen atoms in carbon skeleton can bring in more defects and active sites. N-doped carbon coating on the surface of SnO2 prominently alleviate volume effect, which is beneficial to the propagation and diffusion of Li+. Moreover, hollow interior provided sufficient room for volume change because of insertion and extraction of Li+ during electrode reaction. In conclusion, SnO2@NC-3 electrode can retain a reversible capacity of 640.8 mAh g−1 after 800 cycles at a current density of 1000 mA g−1 and displayed steady cycling stability. Furthermore, rate evaluation of SnO2@NC-3 electrode at different current densities also demonstrated best and most flexible response. The above results well proved that the double layered SnO2@NC-3 hollow sphere was a potential and prospective anode material for lithium-ion batteries.
Data availability
The data presented in this paper are available on request from the corresponding author.
References
Liu X, Zhang S, Zhang P, Zheng ZM, Bai F, Li Q (2023) Integrated structure design and synthesis of a pitaya-like SnO2/N doped carbon composite for high-rate lithium storage capability. Nanoscale 15:1669–1675
Tu Z-Y, Choudhury S, Zachman M-J, Wei S-Y, Zhang K-H, Kourkoutis L-F, Archer L-A (2018) Fast ion transport at solid-solid interfaces in hybrid battery anodes. Nat Energy 3:310–316
Kim H, Kim H, Ding Z, Lee M-H, Lim K, Yoon G, Kang K (2016) Recent progress in electrode materials for sodium-ion batteries. Adv Energy Mater 16:943–981
Benedek P, Forslund O, Nocerino E, Yazdani N, Matsubara N (2020) Quantifying diffusion through interfaces of lithium ion batteries active materials. ACS Appl Mater Interfaces 12:16243–16249
Yin S-J, Zhang X-Q, Liu D-D, Huang X-X, Wang Y-S, Wen G-W (2024) Synthesis of heterointerfaces in NiO/SnO2 coated nitrogen-doped graphene for efficient lithium storage. Phys Chem Chem Phys 26:3415–3423
Liu Y-W, Sun S-W, Han J, Gao C, Fan L, Guo R (2021) Multi-yolk–shell MnO@Carbon nanopomegranates with internal buffer space as a lithium ion battery anode. Langmuir 37:2195–2204
Tian W-H, Bai P, Wang Z-H, Ling G-Q, Ren J, Ren R-P, Lv Y-K (2023) CdS@C nanowires with rich sulfur vacancies for high-performance lithium storage anodes. Ionics. https://doi.org/10.1007/s11581-023-05345-9
Cui Z-P, Sun M, Liu H-Q, Li S-J, Zhang Q-Y (2020) Double-shell SnO2@Fe2O3 hollow spheres as a high-performance anode material for lithium-ion batteries. CrystEngComm 22:1197–1208
Zhang X-Q, Huang X-X, Zhang X-D, Xia L, Zhong B, Zhang T, Wen G-W (2016) Flexible carbonized cotton covered by graphene/Co-doped SnO2 as free-standing and binder-free anode material for lithium-ions batteries. Electrochim Acta 222:518–527
Hu R-Z, Ouyang Y-P, Chen D-C, Wang H, Chen Y, Zhu M, Liu M-L (2016) Inhibiting Sn coarsening to enhance the reversibility of conversion reaction in lithiated SnO2 anodes by application of super-elastic NiTi films. Acta Mater 109:248–258
Zhan L, Zhou X, Luo J, Ning X (2019) Binder-free multilayered SnO2/graphene on Ni foam as a high-performance lithium ion batteries anode. Ceram Int 45:6931–6936
Wu C-H, Zhu G-J, Wang-Q W-H, Zhang H-J (2021) A novel route to prepare N-graphene/SnO2 composite as a high-performance anode for lithium batteries. Energy Storage Mater 43:430–462
Etacheri V, Seisenbaeva G-A, Caruthers J, Daniel G, Nedelec J-M, Kessler V-G, Pol V-G (2015) Ordered network of interconnected SnO2 nanoparticles for excellent lithium-ion storage. Adv Energy Mater 5:1401289
Dai Y, Li F, Fu Y-X, Mo D-C, Lyu S-S (2021) Carbon-coated SnO2 riveted on a reduced graphene oxide composite (C@SnO2/RGO) as an anode material for lithium-ion batteries. RSC Adv 11:8521–8529
Wang M-K, Chen T-R, Liao T-H, Zhang X-L, Zhu B, Tang H, Dai C-S (2021) Tin dioxide-based nanomaterials as anodes for lithium-ion batteries. RSC Adv 11:1200–1221
Zhao K, Zhang L, Xia R, Dong Y, Xu W, Niu C, He L, Yan M, Qu L, Mai L (2016) SnO2 quantum dots@ graphene oxide as a high-rate and long-life anode material for lithium-ion batteries. Small 12:588–594
Tong Y-L, Dang L-Y, Wang Z-R, Zhang H, Gao F, Lu Q-Y (2019) Tube-in-tube tin dioxide superstructures with enhanced lithium storage performance. Chem Commun 55:2222
Xie F-R, Zhao S-Q, Bo X-X, Li G-H, Fei J-M (2023) A robust solvothermal-driven solid-to-solid transition route from micron SnC2O4 to tartaric acid-capped nano-SnO2 anchored on graphene for superior lithium and sodium storage. J Mater Chem A 11:53–67
Abe J, Takahashi K, Kawase K, Kobayashi Y, Shiratori S (2018) Self-standing carbon nanofiber and SnO2 nanorod composite as a high-capacity and high-rate-capability anode for lithium-ion batteries. ACS Appl Nano Mater 1:2982–2989
Boya V, Parakandy MP, Khasim SB, Pavan SV, Mantripragada RK, Bulusu VS (2022) Oxygen vacancies enable excellent electrochemical kinetics of carbon coated mesoporous SnO2 nanoparticles in lithium ion batteries. Mater Adv 3:1617–1628
Hiroo N, Koki U, Isamu M (2023) Direct evidence of reversible SnO2−Li reactions in carbon nanospaces. ACS Appl Mater Interfaces 15:30600–30605
Du F-X, Liu S-L, Li Y, Wang J-K, Zhang P (2023) Facile synthesis of MoS2/N-doped carbon as an anode for enhanced sodium-ion storage performance. Ionics 29:5183–5193
Liang K, Zhao Z-W, Zhou X, Xu A-W (2018) A novel route to prepare N-graphene/SnO2 composite as a high-performance anode for lithium batteries. Dalton Trans 47:10206–10212
Kang Y-R, Li Z, Xu K, He X-J, Wei S-X, Cao Y-M (2019) Hollow SnO2 nanospheres with single-shelled structure and the application for supercapacitors. J Alloy Compd 779:728–734
Han M-S, Mu Y-B, Yu J (2020) Nanoscopically and uniformly distributed SnO2@TiO2/C composite with highly mesoporous structure and bichemical bonds for enhanced lithium ion storage performances. Mater Adv 1:421–429
Korusenko P-M, Nesov S-N, Bolotov V-V et al (2019) Structure and electrochemical characterization of SnOx/Sn@MWCNT composites formed by pulsed ion beam irradiation [J]. J Alloy Compd 793:723–731
Chen W-H, Song K-N, Mi L-W, Feng X-M, Zhang J-M, Cui S-Z, Liu C-T (2017) Synergistic effect induced ultrafine SnO2/graphene nanocomposite as an advanced lithium/sodium-ion batteries anode. J Mater Chem A 5:10027–10038
Hu R-Z, Ouyang Y-P, Liang T, Wang H, Liu J, Chen J, Yang C-H, Yang L-C, Zhu M (2017) Stabilizing the nanostructure of SnO2 anodes by transition metals-a route to achieve high initial Coulombic efficiency and stable capacities for lithium storage. Adv Mater 29:1605006
Xiao X-W, Wang Z-Q, Yao W-L, Rao X-F, Zhang Q (2023) Nano Sn-SnOx embedded in multichannel hollow carbon nanofibers: microstructure, reversible lithium storage property and mechanism. Appl Surf Sci 635:157739
Eng A-Y, Wang Y, Nguyen D-T, Tee S-Y, Lim C-Y-J et al (2021) Tunable nitrogen-doping of sulfur host nanostructures for stable and shuttle-free room-temperature sodium−sulfur batteries [J]. Nano Lett 21:5401–5408
Wang J, Zhu Y-H, Zhang C, Kong F-J, Tao S, Qian B, Jiang X-F (2019) Bimetal phosphide Ni1.4Co0.6P nanoparticle/carbon@nitrogendoped graphene network as high-performance anode materials for lithium-ion batteries. Appl Surf Sci 485:412–422
Xiao X-W, Yao W-L, Yan T-T, Zhang W-Y, Zhang Q (2023) Hybrid CuSn nanosphere-functionalized Cu/Sn co-doped hollow carbon nanofibers as anode materials for sodium-ion batteries. Nanoscale 156:15405–15414
Huang Z-Q, Gao H-Y, Ju J, Yu J-G, Zhao Y (2020) Sycamore-fruit-like SnO2@C nanocomposites: rational fabrication, highly reversible capacity and superior rate capability anode material for Li storage. Electrochim Acta 331:135297
Korusenko P-M, Nesov S-N, Bolotov V-V, Povoroznyuk S-N, Sten’kin Y-A, Pushkarev A-I, Fedorovskaya E-O, Smirnov D-A (2019) Structure and electrochemical characterization of SnOx/Sn@MWCNT composites formed by pulsed ion beam irradiation. J Alloy Compd 15:723–731
Lu H, Wan Y, Wang T, Jin R, Ding P, Wang R, Wang Y, Teng C, Li L, Wang X, Zhou D, Xue G (2019) A high performance SnO2/C nanocomposite cathode for aluminum-ion batteries. Mater Chem A 7:7213–7220
Notohara H, Urita K, Moriguchi I (2023) Direct evidence of reversible SnO2–Li reactions in carbon nanospaces [J]. ACS Appl Mater Interfaces 15:30600–30605
Ke J, Feng Y-F, Yang B-W, Wu K-D, Deng X-Q, He M (2020) A sheet-like SnO2@SiO2/graphite composite as anode material with excellent performance for lithium-ion batteries. Int J Electrochem Sci 15:10173–10183
Zhang L, Wu H-B, Liu B, Lou X-W (2014) Formation of porous SnO2 microboxes via selective leaching for highly reversible lithium storage. Energy Environ Sci 7:1013–1017
Feng Y-F, Bai C, Wu K-D, Dong H-F, Ke J, Huang X-P, Xiong D-P, He M (2020) Fluorine-doped porous SnO2@C nanosheets as a high performance anode material for lithium ion batteries. J Alloy Compd 843:156085
Ma Q, Chen M-N, Fang Z (2024) Tunable composition of Sn/SnO2@C composites for lithium-ion batteries. Mater Lett 357:135716
Zheng Y, Zhou T-F, Zhang C-F, Mao J-F, Liu H-K, Guo Z-P (2016) Boosted charge transfer in SnS/SnO2 heterostructures: toward high rate capability for sodium-ion batteries. Angew Chem Int Edit 10:3408–3413
Cheng J-P, Xiong D-P, Jiang W-Q, Ye W-B, Song P (2024) SnO2-MoO2 nanoparticles coated on graphene oxide as a high-capacity, high-speed, long-life lithium-ion battery anode. Chem Phys Lett 835:140994
Zhou Y, Zhang M, Wang Q, Yang J, Luo X, Li Y, Du R, Yan X, Sun X, Dong C, Zhang X, Jiang F (2020) Pseudocapacitance boosted N-doped carbon coated Fe7S8 nanoaggregates as promising anode materials for lithium and sodium storage. Nano Res 13:691–700
Lin X-T, Li P, Shao L-Y, Shui M, Wang D-J, Long N-B, Ren Y-L, Shu J (2015) Lithium barium titanate: a stable lithium storage material for lithium ion batteries. J Power Sources 278:546–554
Fang G-Z, Wu Z-X, Zhou J, Zhu C-Y, Cao X-X, Cao T-Q, Chen Y-M, Wang C, Pan A-Q, Liang S-Q (2018) Observation of pseudocapacitive effect and fast ion diffusion in bimetallic sulfides as an advanced sodium-ion battery anode. Adv Energy Mater 8:1703155
Zhang S-G, Zhao H-P, Ma W-Y, Mi J, Zhu J-F, Liu J-L (2022) Insight to Se-doping effects on Fe7S8/carbon nanotubes composite as anode for sodium-ion batteries. J of Power Sources 536:231458
Sun R-M, Liu S-J, Wei Q-L, Sheng J-Z, Zhu S-H, An Q-Y, Mai L-Q (2017) Mesoporous NiS2 nanospheres anode with pseudocapacitance for high-rate and long-life sodium-ion battery. Small 13:1701744–1701748
Cook J-B, Kim H-S, Yan Y, Ko J-S, Robbennolt S, Dunn B, Tolbert S-H (2016) Mesoporous MoS2 as a transition metal dichalcogenide exhibiting pseudocapacitive Li and Na-Ion charge storage. Adv Energy Mater 6:1501937
Ge P, Hou H, Li S, Yang L, Ji X (2018) Tailoring rod-like FeSe2 coated with nitrogen-doped carbon for high-performance sodium storage. Adv Funct Mater 28:1801765–1801712
Funding
This study was funded by College students’ innovation and entrepreneurship training (202311765015), Henan Key Science and Technology Research (242102230104), and Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2023JD65).
Author information
Authors and Affiliations
Contributions
Jin'an Zhao: Methodology, Software, Investigation, Writing-original draft. Liyun Dang: Conceptualization, Supervision. Jiyong Hu: Resources, Validation, Formal analysis. Yan Guo: Software, Methodology.
Corresponding authors
Ethics declarations
Ethics approval
This work did not include any studies involving humans or animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Dang, L., Hu, J. et al. Double-layered SnO2@NC hollow spheres as anode materials for high-performance lithium-ion batteries. Ionics 30, 3745–3753 (2024). https://doi.org/10.1007/s11581-024-05530-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05530-4