Abstract
During the operation progress of solid oxide fuel cell (SOFC), the performance and endurance are two major concerns significantly affected by gas flowing, charge transport, and chemical reaction. This paper presents a thorough research on the key parameters related to syngas and charge transport in the SOFC to reveal the intrinsic influence mechanism, including electro conductibility, gas mixture concentration, CH4 component ratio, temperature, and anode thickness, which is instrumental in improving the operational efficiency and applicability of SOFC. Firstly, the theoretical models of charge transport and multi-component mass transfer are established, respectively, and the two are coupled using the reaction rate calculation method. Then, employing an innovative combination of the representative elementary volume (REV) scale lattice Boltzmann method (LBM) and the finite-difference LBM, the potential and multi-component gases distributions are simulated to calculate the evaluated indicators, namely activation and concentration overpotential. Finally, considering various operational conditions, the simulation experiments are conducted to investigate the parametric effect on the performance of SOFC fueled by syngas. The results demonstrate that compared to the direct reforming way, the external syngas with lower CH4 component ratio is more favorable to the SOFC and the optimal ratio should be controlled within 0.2. The higher concentration of gas mixture and lower anode thickness both contribute to weakening the effect of concentration polarization. Especially, the performance of SOFC is improved when the concentration is 15 mol‧m−3 and the anode thickness is below 1.05 mm. With the increment of conductivity and operating temperature, the consumption of H2 gradually increases, enhancing the efficiency of reaction gas and reducing the economic cost. And the optimal operation temperature of SOFC is about 1073 K. Moreover, the anode thickness is a trade-off between the electrochemical reaction conditions of anode and cathode, as its variation affects both of them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
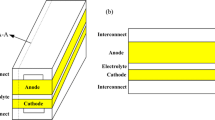
Underwater unmanned vehicle (UUV) with long endurance plays a core role in the deep-sea exploration. To meet its the power requirements, the solid oxide fuel cell (SOFC) with advantages of high electric efficiency and stable performance stands out as a promising energy device. One characteristic of the SOFC is simple structure, mainly consisting of the anode channels, porous anode, cathode channels, porous cathode and electrolyte [1]. According to the different thickness of porous anode and cathode, the SOFC can be classified into two types: anode-supported SOFC and cathode-supported SOFC. Especially, the former has gained increasing attention due to its superior operational performance [2]. The other characteristic is high fuel flexibility. Apart from the hydrogen (H2), the anode can also be supplied with a gas mixture of methane (CH4) and steam (H2O), as well as syngas.
When fuel gases are fueled to the cathode and anode, they flow into the triple phase boundary (TPB), that is, the interface among gas species, charge conductor and catalyzer. At the TPB, an electrochemical reaction takes place to produce the electrons (e−), which are collected to meet the energy demand of external loads. In the electrolyte, only oxygen ions (O2−) flow, while in the porous anode and cathode, both mass transfer and charge transport occur, resulting in a multi-physics field coupling and thus affecting the performance of SOFC.
The performance of SOFC can be improved by supplying a gas mixture of CH4 and H2O or syngas in comparison to the H2 only. However, the possibility of carbon deposition may increase owing to the gas mixture of CH4 and H2O, which leads to the structural damage of SOFC [3]. Therefore, the syngas obtains extending application. To enhance the performance and efficiency of SOFC, it is crucial to investigate the coupled physical and chemical processes in the porous anode and cathode fueled by syngas, which is also beneficial to promote the application of SOFC in the engineering.
Nonetheless, it is very difficult to directly monitor the charge transport and mass transfer in the porous cathode and anode [4, 5]. In order to study the characteristic of multi-physics field coupling in the porous cathode and anode, the computational fluid dynamic (CFD) based on the simulation model of SOFC proves to be an effective tool, including physical model and numerical method.
The physical models are generally employed to simulate the charge transport and mass transfer, such as dusty gas model (DGM), Stefan Maxwell model (SMM), Brinkman model and Fick’s model (FM) [6]. And the models, solved by adopting the traditional numerical methods like the finite difference method (FDM), the finite volume method (FVM) and the finite element method (FEM) are called the macroscopic model. Kasra Nikooyeh et al. developed a three-dimensional (3D) macroscale model to explore the thermal and electrochemical behavior of SOFC by directly reforming the CH4 internally. The results concluded that the gas mixture of CH4 and H2O can dilute the fuel concentration and the performance of SOFC is decreased. Even worse, there is a carbon deposition in the porous anode [7]. Through the combination of the DGM and the FVM, Meng Ni researched the transport and reaction processes of SOFC fueled by syngas. They found that compared to the H2-fueled SOFC, the performance of SOFC is slightly decreased with an increase of gas velocity [8], but the component ratio of CH4 remained higher.
Since the mass transfer mainly involves Knudsen and molecular diffusion, which belong to the mesoscale mechanisms, the simulation results based on the macro-scale models exhibit comparatively low accuracy and are unsuitable for the in-depth study of mass transfer. Thus, adopting the lattice Boltzmann method (LBM) to solve the physical model attracts more and more prominence, which could improve the simulation accuracy of mesoscale models.
To validate the feasibility of LBM-based model for simulating the SOFC, Grew conducted a study of electrochemical and gas transfer in the anode using the LBM, which contributed to enhancing the accuracy of simulation results [9]. Aiming at the representative elementary volume (REV) of SOFC and concentration polarization, Xu et al. designed a 2D multi-component LBM-based model to simulate mass transfer in both the porous cathode and anode [10]. Furthermore, the LBM can be combined with other numerical methods to simulate the process of multi-component gas transport. Dang designed a binary gas mixture finite-difference LBM to study the H2 transport, which has been proved to simulate the flow with larger ratio of molecular weights [11]. Xu built a pore scale LB model to predict the gas species distributions in the anode, fed with the gas mixture of CH4 and H2O [45]. However, the direct internal reforming in the anode would cause carbon deposition, which is detrimental to the performance of SOFC.
The LBM-based models can also be used to simulate other physical fields. For example, Xu established a numerical LBM-based model to predict the potential distribution of the pattern anode and calculate the reaction current density at TPB [12], but the coupling between mass transfer and charge transport was not considered.
As previously mentioned, the mixture of CH4 and H2O is harmful to the performance of SOFC [20]. A more favorable approach is to externally reform CH4 to produce the syngas, including CH4, H2, H2O, CO and CO2, and then supply the gas mixture to the anode, namely the external syngas, which can help to prevent the carbon deposition and maintain the performance of SOFC. Although the mesoscale models are widely employed to simulate the charge transport and multi-component mass transfer in SOFC, there still exist some deficiencies. One of deficiencies is that there are limited researches on the external syngas flowing in the porous anode. The other is that the mesoscale model is usually used to model the charge transport or mass transfer in the porous electrode, while few researches focus on the multi-physics field coupling.
Aiming at the SOFC fueled by external syngas, this paper conducts a thorough research on the effect of key parameters on potential distributions and concentration polarization of SOFC, which can provide a theoretical support for the operation strategy and structure optimization of SOFC. Firstly, the physical models of charge transport and multi-component mass transfer are established and coupled by means of the reaction rate calculation method. Secondly, innovatively combining the REV scale LBM with finite-difference LBM, the potential and gas species distributions in the porous cathode and anode are predicted. Based on this, three indicators, namely activation polarization, concentration polarization and ohmic polarization, are calculated to quantitatively evaluate the operational performance of SOFC. Finally, taking different operational cases into account, the operation mechanism and performance of SOFC fueled by syngas are investigated comprehensively.
Mathematical models
At present, the numerical model is based on the following assumptions: isothermal, steady state, isotropic, and laminar flow in the gas mixture. By adding the reformer in the SOFC system, the syngas is supplied into anode. In the external syngas, there are five components, that is, CH4, H2, carbon monoxide (CO), H2O and carbon dioxide (CO2). The methane reforming reaction (MRS) occurs between H2O and CH4 [13, 14], and the water gas shift reaction (WGS) also occurs between H2O and CO [15,16,17,18,19], expressed as follows.
According to the electrochemical reaction between H2 and oxygen (O2) [20, 43, 44], H2 is consumed to produce the H2O due to the O2− flowing, which can be written as follows.
The charge transport and multi-component gas transfer are shown in Fig. 1. The gas concentration at the TPB is lower than at the inlet, which results in a decrease in potential. The drop potential is known as the concentration overpotential. Compared to lower current densities, the impact of higher current densities on the concentration polarization is more significant. Another two types of polarization are activation overpotential and ohmic overpotential.
Charge transport model
It is appropriate to describe the process of charge transport by using the Poisson equation, which is as follows [1],
where φ is the electrostatic potential.
Multi-component mass transfer model
In order to describe the mass transfer in the cathode and anode, the Brinkman model is employed. The continuity, momentum and species equation are as follows [10],
where \(\overset\rightharpoonup u\) is the velocity of gas mixture, p is the pressure, K is the permeability, ν is the viscosity of gas, νeff is the effective viscosity, \({\overset\rightharpoonup I}_A\) is the mass flux of gas species A, \(\dot{S}\) is the source term.
Reaction rate calculation method
The current density at TPB is written as Eq. (5) [21],
where iTPB is the current density at TPB, iex is the exchange current density, α is the transfer coefficient, n is the electronic exchange coefficient, R is the universal gas constant, T is the operation temperature, F is the Faraday constant.
Because of electrochemical reaction, the consumption of H2 is calculated based on the Faraday’s law, as follows [12],
where j is the molar flux of H2,
The reaction rates of MSR and WGS are calculated by Eqs. (7) -(8) [15],
where
The reaction rates of five components at TPB are formulated as follows [20].
Lattice Boltzmann method
The charge and multi-component conversation equations are solved by the REV scale LBM and the finite-difference LBM, respectively. In the finite-difference LBM, the continuity gas is regarded as a lot of gas molecules, and the gas flow is regarded as the irregular motion of gas molecules [22, 23].
LBM for charge transport
The individual movements of large number electrons and ions are paid more attention in the LBM. There are two steps to using the LBM, which are the collision and streaming. In this research, the D2Q9 (2-dimensional 9-velocity) model is adopted [24, 25].
During the process of collision, Eq. (1) is solved by the following equation [26],
where fy is the distribution function with velocity \({\overset\rightharpoonup e}_y\) at the lattice site \({\overset\rightharpoonup x}_y\) and time t, \({\uptau }_{{\text{c}}}\) is the collision time. The equilibrium distribution function fyeq is calculated as,
where y is the streaming direction of electrons and ions, ωy is the weight function of every direction. For the D2Q9 model, the streaming direction is shown in Fig. 2.
In different directions, the streaming numbers of electrons and ions are different. The weight function ωy is given in the following equations [4],
According to the specific direction, the distribution function at the different nodes can be obtained with the help of electrons and ions streaming processes. In order to recover Eq. (1), the moment of distribution functions is employed via Chapman-Enskog expansion, which is as follows,
At the interface between the porous electrodes and electrolyte, the voltage difference is the activation polarization which is obtained [1],
where ηact,an is the activation polarization voltage of anode, ηact,c is the activation polarization voltage of cathode.
Finite-difference LBM for multicomponent flow
The relative molecular mass is different in the gas mixture. For example, the ratio of relative molecular weight between the H2 and CO2 is the largest which is 22. In order to improve the LBM, the finite-difference LBM is used to simulate the mass transfer. The Boltzmann equation of every component is as follows [11],
where f is the distribution function of gas species A, \(\overset\rightharpoonup\xi\) is the velocity and \(\mathop{a}\limits^{\rightharpoonup}\) is the acceleration, \(J^{AA}\) is the self-collision term which indicates the effect of collision between the same gas molecules, \(J^{AB}\) is the cross-collision term which indicates the effect of collision between the different gas molecules.
In the D2Q9 model, the gas molecular with smallest relative molecular mass has the fastest lattice speed which is given in the following equation [5],
where c is the lattice speed of gas species A, \({\updelta }_{{\text{x}}}\) is the streaming distance in the lattice unit, \({\updelta }_{{\text{t}}}\) is the lattice time step, \(\overset\rightharpoonup e_\text{y}^A\) is the discrete velocity. The lattice velocities and discrete velocities of other gas molecules have the following form [11]:
where B represents the other gas species. The speed of sound of each gas species is as follows,
The Boltzmann equation of each gas species is discretized which is given in the following form,
where
In the above equations, \(\tau_{f}^{A}\) is the self-collision relaxation time, and \(\tau_{f}^{AB}\) is the cross-collision relaxation time. The moments of the distribution function are used to obtain the concentration and speed of each gas species, and the equations are as following [27],
where m is the concentration of gas species A, \({\overset\rightharpoonup u}_A\) is the velocity of gas species A. The concentration and velocity of gas mixture is calculated by the following equations.
The finite-difference theory and LBM are coupled to improve the accuracy of simulation result. First, Eq. (23) is divided into two parts which are collision and streaming term, respectively. The equations are as follows.
The explicit first-order Euler scheme is adopted to discretize Eq. (33).
Equation (34) is discretized by a second-order Lax-Wendroff scheme which is given in the following equation.
Concentration and ohmic polarization model
Due to the concentration difference, the polarization loss is described as follows [33],
where η_con_a is the concentration polarization voltage of anode, η_con_c is the concentration polarization voltage of cathode, γ is the molar fraction.
In order to establish the comprehensive simulation model of SOFC, the ohm’s law is used to calculate the ohmic polarization. Because there is the higher electron conductivity of the electrode, the ohmic loss of the electrode is ignored [28,29,30,31]. Equation (42) is used to calculate the ohmic polarization voltage of electrolyte [32].
where η_ohm is the ohmic polarization voltage of SOFC, Oe is the ohmic resistance of electrolyte, anode, and cathode.
Boundary conditions
In the charge transport model, the input boundaries of anode and cathode are given in the following form [1],
where Etheory is the theory potential. The equations of top and bottom boundary are as follows [1],
At the TPB, the current density is continuous which can be described as follows [1],
where σ is the electrical conductivity.
As shown in Fig. 1, the total concentrations of external syngas and air are specified at the input of anode and cathode. At the TPB, the mass flux is specified using Eq. (11). As for the top and bottom boundary, the periodic boundary is employed [34,35,36,37,38].
Model validation
The model validation consists of two parts. Firstly, the characteristics of SOFC voltage-current between the numerical model and specific experimental setups are compared. Secondly, the relative error between the simulation and measured values is calculated. The model validation and subsequent simulation experiments are conducted by means of MATLAB.
Other parameters for planar anode-supported SOFC are listed in Table 1 [41, 42].
The comparison between the simulated and measured values is shown in Fig. 3, where the gas mixture only contains H2 and H2O. The initial molar fractions of two components are 0.97 and 0.03, respectively [39, 40]. In Fig. 3, it is found that the simulation data closely overlaps with the experimental data. When the current density is 0.75 A cm−2, the experimental and simulation data are 0.6775 V and 0.64755 V, respectively, and the voltage difference is largest. Figure 3 also depicts the relative error between the simulated and measured values. The maximum relative error is about 4.42%, implying that this numerical model can accurately simulate the operation progress of SOFC.
Comparison of volt-ampere characteristics between the experimental data [20] and predictions values by the present model with two components
Result and analysis
In order to investigate the operation mechanism of SOFC fueled with external syngas, the effect of operation and structure parameters on performance of SOFC is discussed.
Effect of external syngas on performance of SOFC
In the direct reforming experiment, the gas mixture includes the CH4 and H2O, with component ratios of 0.97 and 0.03 [31], respectively. The initial component ratios of external syngas for H2, H2O, CH4, CO, and CO2 are 0.263, 0.493, 0.171, 0.029, and 0.044, respectively [40,41,42,43]. The simulation of performance with five components is performed, as is shown in Fig. 4. The voltage difference range is larger when the current density is from 0.33 A cm−2 to 0.75 A cm−2, about from 0.0577 V to 0.07 V. In comparison to supplied with the gas mixture of H2O and H2, the output voltage of SOFC supplied with the gas mixture of H2O and CH4 and the external syngas degrades. But compared to supplied with the gas mixture of H2O and CH4, the output voltage of SOFC supplied with the external syngas is higher. One of the possible reasons is that due to the existence of MRS and WGS, the partial chemical energy will be consumed during the direct internal reforming, which results in the decrease of chemical energy converting into electric potential. Meanwhile, the MRS and WGS processes are endothermic reactions leading to the lower performance of SOFC. Another reason may be that there is more H2 present in the external syngas than a gas mixture of H2O and CH4. More H2 can arrive at the TPB, which can reduce the effect of concentration polarization. Thus, the use of external syngas can enhance the performance of SOFC.
Comparison of output voltage with different current densities with gas mixture H2O and CH4 [31] and external syngas
Owing to the existence of reforming reaction, the component ratio of CH4 plays a core role on the performance of SOFC. The effect of CH4 molar fraction on the concentration overpotential is shown in Fig. 5. The X-axis in Fig. 5 is along the thickness of porous electrode, which have been normalized. The equation is as follow,
where x is the distance between the inlet of porous electrode and calculation point, d is the thickness of porous electrode.
When the component ratio of CH4 is 30%, the concentration overpotential is maximum at the TPB, about 0.0382 V. With the increment of initial molar fraction, the concentration overpotential becomes larger. Because the loss of reactant will cause the output voltage to be lower than the theoretical voltage at the specific current density. By changing the molar fraction of CH4, the concentration of H2 will decrease. To meet the output power, the CH4 is consumed to generate more H2. Nevertheless, the reaction rate of reforming is lower, which makes it difficult to meet the consumption of H2, leading to the higher concentration polarization voltage. In Fig. 5, it is found that the lower molar fraction of CH4 is beneficial for improving the performance of SOFC.
Effect of electro conductibility
Figure 6 illustrates the potential distribution of SOFC, which clearly demonstrates that the potential drops in both the anode and cathode are minimal. In the electrolyte, the potential drop ranges from 0.943 V to 0.115 V. Additionally, the potential drops across the anode and cathode are approximately 0.002 V and 0.004 V. The potential difference at the interface between the anode and electrolyte measures 0.054 V, while the potential difference at the interface between the electrolyte and cathode is significantly larger, approximately 0.1112 V. The different materials of the electrolyte, the porous anode and the porous cathode leads to different electro conductivities, which results in the potential difference in the interface between electrodes and electrolyte. In Fig. 6, there is a rapid potential drop in the electrolyte because of lower conductivity compared to the anode and cathode. There are larger potential differences at the interfaces, particularly at the interface between the electrolyte and cathode. It is beneficial to decrease the potential difference at the interface by improving the electrolyte conductivity.
Figure 7 depicts the effect of electro conductivity of electrolyte on the electrochemical reaction. It is found in Fig. 7 that the reaction current density at the TPB increases with the increment of electro conductivity. At the middle region of TPB, the current density is lower. Especially, the minimum current density is about 3583.94 A m−2 when the electro conductivity is 2.382 × 104 S m−1. As the electro conductivity becomes larger, the performance of charge transport will become better, which can also promote the electrochemical reaction rate at the TPB. Nevertheless, the voltage difference will become larger by increasing the electro conductivity of the anode/cathode. Now, it is more difficult to improve the performance of charge transport for the electrolyte by decreasing the thickness of electrolyte during the manufacturing process. Thus, there are the technological barriers for the novel material of electrolyte.
Standard case
The initial component ratios of external syngas are reset, which is shown in Table 2.
Figure 8 demonstrates the change of five components in the standard case. The molar fractions of CH4, CO, and H2 all decrease along the direction of gas mixture flow, while the molar fractions of CO2 and H2O increase. At the TPB, the molar fraction of CH4, H2, H2O, CO2 and CO are about 0.00804, 0.37997, 0.41601, 0.11867 and 0.07731, respectively. The maximum concentration overpotential is about 0.00419.
In the standard case, the simulation results for the cathode, including the concentration polarization and the molar fractions of N2 and O2 along the gas flow direction, are shown in Fig. 9. The maximum of concentration overpotential is about 1.3 × 10–4 V and the minimum of O2 molar faction is about 0.2074. Because the supplied gas is usually excessive in the cathode, the effect of concentration polarization is lower.
Parametric analysis of anode
Several other key parameters also significantly influence mass transfer, including total concentration of external syngas, operation temperature and thickness of anode.
Effect of gas mixture concentration
The effect of total concentration of external syngas on mass transfer is shown in Fig. 10. By analyzing the curve of five components along the gas flowing, it can be found that the molar fractions of the CH4, CO and H2 all increase with the increment of total concentration. The mole fractions of the H2O and CO2 become lower by increasing the total concentration. Moreover, the concentration overpotential becomes lower with the increment of total concentration. The consumption of H2 and the concentration overpotential are two important parameters of simulations. When the total gas mixture concentration is 9 mol‧m−3, the H2 molar fraction and concentration overpotential are about 0.3746 V and 5.321 × 10–3 V at TPB. The molar fraction of CO changes from 0.1 to 0.07, indicating that the consumption of CO is also larger.
As the external syngas concentration increases, a greater number of reactant gas molecules can flow into the TPB, enhancing the potential for participation in both chemical and electrochemical reactions. Simultaneously, the concentration difference between the inlet and TPB decreases with the increasing gas mixture concentration. However, the total concentration of the gas mixture cannot be added without restrictions, which will cause the waste of reactant gas leading to the lower working efficiency of SOFC.
Effect of anode temperature
The influence of operation temperature on mass transfer is illustrated in Fig. 11. With the increment of the operation temperature, there are significant changes about the molar fractions of CH4, H2 and H2O. For example, when the operation temperature is 1073 K, the molar fractions of CH4, H2 and H2O are about 0.0076, 0.336 and 0.465 at the TPB. The variations of another two gases are insignificant by increasing the operation temperature.
The possible reason is that the temperature requirements for different chemical reactions are different. By increasing the temperature, the electrochemical reaction rates between H2 and O2 become larger, consuming more H2 and producing more H2O. At higher temperature, the reforming reaction of CH4 is intense, which results in the molar fraction drop of CH4 with the increase of temperature. The chemical reaction between CO and H2O can take place at the lower temperature (< 973 K). Thus, the effect of increasing temperature on the chemical reaction is insignificant. However, at the higher temperature, the solid particles with catalysis may drop, leading to the decrease of SOFC performance. The temperature of SOFC cannot also be increased without limitations, because the thermal management plays an important role on the maintaining the output voltage of SOFC.
Effect of anode thickness
The effect of anode thickness on the concentration overpotential is shown in Fig. 12. When the thickness of anode becomes larger, the concentration overpotential increases. The concentration polarization voltage is 0.038 V when the anode thickness is 1.55 mm. The main reason is that the resistance flowing along the horizontal direction becomes larger by increasing the anode thickness. Compared to increasing the anode thickness, the change of performance of SOFC is less by decreasing the thickness of anode although the resistance of gases flowing becomes lower.
Parametric analysis of cathode
In the cathode, the electrochemical reaction is simpler compared to the anode. Two main parameters are explored, namely the operation temperature and thickness of anode.
Effect of temperature
The simulation results at different temperatures are described in Fig. 13, which illustrate that the consumption of O2 becomes larger and the component ratio of N2 increases by improving the temperature. The concentration polarization voltage increases with the increment of temperature. When the operation temperature is 1073 K, the molar fraction and concentration overpotential of cathode are about 0.1992 × 10–4 V and 5.558 × 10–4 V. The main reason is that it is useful to improve the electrochemical reaction rate between the H2 and O2 by increasing the temperature. In Fig. 13, it is also found that the effect of changing the operation temperature from 1023 to 1073 K on the mass transfer is more significant. The possible reason is that the electrochemical reaction between H2 and O2 is close to the theory ideal state at 1073 K.
Effect of anode thickness on mass transport of cathode
Figure 14 describes the effect of increasing the anode thickness on the mass transfer in the cathode. By adjusting the thickness of anode, there is a change in the consumption of O2. When the anode thicknesses are 0.55 mm, 1.05 mm and 1.55 mm, the concentration overpotentials are 2.702 × 10–4 V, 2.452 × 10–4 V and 1.467 × 10–4 V, respectively. In Fig. 14, it is found that the consumption of O2 and the concentration overpotential of cathode both decreases with the increase of anode thickness. It is because that the resistance of gases flow in the anode becomes larger with the increasing of thickness, resulting in the decrease of reactant gas molecular quantity at the TPB of anode. The consumption of O2− and the consumption of O2 both decreases in the anode, leading to the drop of concentration polarization voltage. The change of operation performance in the cathode should be considered if the researchers want to improve the performance of SOFC by employing the strategy of adjusting the anode thickness.
Conclusions
To gain a deeper understanding of the multi-physics fields coupling in SOFC fueled by external syngas, a comprehensive numerical model for SOFC is designed and validated. In this model, the charge transport and mass transfer are simulated based on the REV scale LBM and the finite-difference LBM, respectively. Based on the parameter analysis of SOFC, some important conclusions are as follows:
-
(1)
The multi-physics fields coupling model is useful to predict the gas components, potential and polarization loss distributions in the SOFC.
-
(2)
Due to the low electric conductivity, there is a significant drop in electrostatic potential within the electrolyte, and increasing conductivity of electrolyte is beneficial for improving the electrochemical reaction rate.
-
(3)
Compared to the gas mixture of H2O and CH4, supplying the external syngas to the anode helps to improve the performance of SOFC. To enhance the output voltage of SOFC, the CH4 component ratio should be controlled within a lower range, which is less than 0.2.
-
(4)
By increasing the concentration of the external syngas, the concentration polarization decreases. However, the consumption of H2 and production of H2O will become larger by increasing the temperature, which results in a decrease in the performance of SOFC.
-
(5)
The gas transfers in both the cathode and anode are influenced by changing the anode thickness. The consumption of O2 decreases with an increase of anode thickness.
Findings
This study was funded by the National Natural Science Foundation of China (Grant nos. 52276026, 52006136) and Fundamental Research Funds for the Central Universities (2023JBMC016).
Nomenclature
- a:
-
Acceleration (m‧s.−2)
- c:
-
Lattice sound speed (m‧s.−1)
- Etheory:
-
Theory potential (V)
- e:
-
Discrete lattice velocity
- F:
-
Faraday constant (96,485 A‧s‧mol.−1)
- f:
-
Lattice Boltzmann distribution function
- I:
-
Mass flux (kg‧m−2‧s.−1)
- i:
-
Current density (A‧m.−2)
- J:
-
Collision term
- j:
-
Molar flux (mol‧m−2‧s.−1)
- k:
-
Reaction coefficient (mol‧m−3‧Pa−2‧s.−1)
- M:
-
Molecular weight (kg‧mol.−1)
- m:
-
Lattice molar concentration of gas
- n:
-
Electronic exchange coefficient
- O:
-
Ohmic resistance (Ω)
- Q:
-
External force term
- p:
-
Pressure (pa)
- R:
-
Universal gas constant (8.314 J‧mol−1‧K.−1)
- S:
-
Source term
- T:
-
Operation temperature (K)
- t:
-
Time (s)
- u:
-
Velocity of gas (m‧s.−1)
- x:
-
Coordinate along the direction of electrode thickness
Greek symbols
- γ:
-
Molar fraction
- ν:
-
Kinematic viscosity (m2‧s.−1)
- φ:
-
Electrostatic potential (V)
- η:
-
Polarization voltage (V)
- τ:
-
Relaxation time
- α:
-
Transfer coefficient
- ξ:
-
Lattice velocity of gas (m‧s.−1)
Data availability
No datasets were generated or analysed during the current study.
References
Xu H, Chen Y, Kim J H, Dang Z, Liu M (2019) Lattice Boltzmann modelling of the coupling between charge transport and electrochemical reactions in a solid oxide fuel cell with a patterned anode [J]. Int J Hydrogen Energy 44(57):30293–30305. https://doi.org/10.1016/j.ijhydene.2019.09.086
Park YM, Lee HJ, Bae HY et al (2012) Effect of anode thickness on impedance response of anode-supported solid oxide fuel cells [J]. Int J Hydrogen Energy 37(5):4394–4400. https://doi.org/10.1016/j.ijhydene.2011.11.152
Grew KN, Joshi AS, Peracchio AA, Chiu WKS (2012). Pore-scale investigation of mass transport and electrochemistry in a solid oxide fuel cell anode [J]. J Power Sources 195:2331–2345. https://doi.org/10.1016/j.jpowsour.2009.10.067
Joshi AS, Peracchio AA, Grew KN, Chiu WKS (2007) Lattice Boltzmann method for multi-component, non-continuum mass diffusion [J]. J Phys D Appl Phys 40:7593–7600. https://doi.org/10.1088/0022-3727/40/23/053
Joshi A S, Grew K N, Peracchio A A, Chiu WKS (2007). Lattice Boltzmann modeling of 2D gas transport in a solid oxide fuel cell anode [J]. J Power Sources 164(2):631–638. https://doi.org/10.1016/j.jpowsour.2006.10.101
Chan SH, Khor KA, Xia ZT (2001) A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness [J]. J Power Sources 93(1–2):130–140. https://doi.org/10.1016/s0378-7753(00)00556-5
Kasra Nikooyeh, Ayodeji A. Jeje, Josephine M, Hill (2007). 3D modeling of anode-supported planar SOFC with internal reforming of methane [J]. J Power Sources 171(2):601–609. https://doi.org/10.1016/j.jpowsour.2007.07.003
Meng N (2012) Modeling of SOFC running on partially pre-reformed gas mixture [J]. Int J Hydrogen Energy 37(2):1731–1745. https://doi.org/10.1016/j.ijhydene.2011.10.042
Grew KN, Joshi AS, Peracchio AA et al (2006) Detailed electrochemistry and gas transport in a SOFC anode using the lattice Boltzmann method [C]. ASME 2006 International Mechanical Engineering Congress and Exposition. Advanced Energy Systems, Chicago, pp.285–290. https://doi.org/10.1115/IMECE2006-13621
Xu H, Dang Z, Bai BF (2012) Numerical simulation of multispecies mass transfer in a SOFC electrodes layer using lattice Boltzmann method [J]. J Fuel Cell Sci Technol 9(6):061004-. https://doi.org/10.1115/1.4007791
Dang Z, Xu H (2016) Pore scale investigation of gaseous mixture flow in porous anode of solid oxide fuel cell [J]. Energy 107:295–304. https://doi.org/10.1016/j.energy.2016.04.015
Xu H, Dang Z, Bai BF (2014) Electrochemical performance study of solid oxide fuel cell using lattice Boltzmann method [J]. Energy 67:575–583. https://doi.org/10.1016/j.energy.2014.02.021
Yakabe H, Hishinuma M, Uratani M, Matsuzaki Y, Yasuda I (2000). Evaluation and modeling of performance of anode-supported solid oxide fuel cell [J]. J Power Sources 86(1–2):423–431. https://doi.org/10.1016/s0378-7753(99)00444-9
Yan M, Zeng M, Chen QY, Wang QW (2012) Numerical study on carbon deposition of SOFC with unsteady state variation of porosity [J]. Appl Energy 97:754–762. https://doi.org/10.1016/j.apenergy.2012.02.055
Baldinelli A, Barelli L, Bidini G (2015) Performance characterization and modelling of syngas-fed SOFCs (solid oxide fuel cells) varying fuel composition [J]. Energy 90(2):2070–2084. https://doi.org/10.1016/j.energy.2015.07.126
Ni M, Leung MKH, Leung DYC (2007) Parametric study of solid oxide fuel cell performance [J]. Energy Convers Manage 48(5):1525–1535. https://doi.org/10.1016/j.enconman.2006.11.016
Pramuanjaroenkij A, Kakac S, Zhou X (2008). Mathematical analysis of planar solid oxide fuel cells [J]. Int J Hydrogen Energy 33(10):2547–2565. https://doi.org/10.1016/j.ijhydene.2008.02.043
Ebbesen SD, Knibbe R, Mogensen M (2012) Co-electrolysis of steam and carbon dioxide in solid oxide cells [J]. J Electrochem Soc 159(8):482–9. https://doi.org/10.1149/2.076208jes
Kazempoor P, Braun RJ (2014) Model validation and performance analysis of regenerative solid oxide cells for energy storage applications: Reversible operation [J]. Int J Hydrogen Energy 39(11):5955–71. https://doi.org/10.1016/j.ijhydene.2014.01.186
Delavar MA, Farhadi M, Sedighi K (2010) Numerical simulation of direct methanol fuel cells using lattice Boltzmann method [J]. Int J Hydrogen Energy 35(17):9306–9317. https://doi.org/10.1016/j.ijhydene.2010.02.126
Guo Z, Zheng C, Shi B (2002) Discrete lattice effects on the forcing term in the lattice Boltzmann method [J]. Phys Review E 65(4):046308-. https://doi.org/10.1103/PhysRevE.65.046308
Shan X, Doolen G (1996). Diffusion in a multicomponent lattice Boltzmann equation model [J]. Phys Rev E 54(4):3614–3620. https://doi.org/10.1103/PhysRevE.54.3614
Yahya A, Naji H, Dhahri H (2022) A lattice Boltzmann analysis of the performance and mass transport of a solid oxide fuel cell with a partially obstructed anode flow channel [J]. Fuel 334(1):126537. https://doi.org/10.1016/j.fuel.2022.126537
Guo Z (2002). Lattice Boltzmann model for incompressible flows through porous media [J]. Phys Rev E 66(3):36304-. https://doi.org/10.1103/physreve.66.036304
Zhao F, Armstrong TJ, Virkar AV (2003) Measurement of O2-N2 effective diffusivity in porous media at high temperatures using electrochemical cell [J]. J Electrochem Soc 150(3):249–56. https://doi.org/10.1149/1.1540156
Chiu WKS, Joshi AS, Grew KN (2009) Lattice Boltzmann model for multi-component mass transfer in a solid oxide fuel cell anode with heterogeneous internal reformation and electrochemistry [J]. Eur Phys J Spec Top 171(1):159–165. https://doi.org/10.1140/epjst/e2009-01024-8
Zou QS, He XY (1997) On pressure and velocity boundary conditions for the lattice Boltzmann BGK model [J]. Phys Fluids 9(6):1591–0. https://doi.org/10.1063/1.869307
Blesznowski M, Sikora M, Kupecki J, Makowski L, Orciuch W (2022) Mathematical approaches to modelling the mass transfer process in solid oxide fuel cell anode [J]. Energy 239:121878. https://doi.org/10.1016/j.energy.2021.121878
Grew KN, Chiu WKS (2012). A review of modeling and simulation techniques across the length scales for the solid oxide fuel cell [J]. J Power Sources 199:1–13. https://doi.org/10.1016/j.jpowsour.2011.10.010
Chan SH, Khor KA, Xia ZT (2001) A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness [J]. J Power Sources 93(1–2):130–140. https://doi.org/10.1016/s0378-7753(00)00556-5
Xie Y, Ding H, Xue X (2013) Multi-physicochemical modeling of direct methane fueled solid oxide fuel cells [J]. J Power Sources 241:718–27. https://doi.org/10.1016/j.jpowsour.2013.06.028
Guo Z, Zheng C, Shi B (2002) Non-equilibrium extrapolation method for velocity and pressure boundary conditions in the lattice Boltzmann method [J]. Chin Phys 11(4):366–374. https://doi.org/10.1088/1009-1963/11/4/310
Zhao F, Virkar AV (2005) Dependence of polarization in anode-supported solid oxide fuel cells on various cell parameters [J]. J Power Sources 141(1):79–95. https://doi.org/10.1016/j.jpowsour.2004.08.057
Joshi AS, Peracchio AA, Grew KN, Chiu WKS (2007) Lattice Boltzmann method for continuum, multi-component mass diffusion in complex 2D geometries [J]. J Phys D Appl Phys 40(9):2961–2971. https://doi.org/10.1088/0022-3727/40/9/044
Luo L, Girimaji SS (2002) Lattice Boltzmann model for binary mixtures [J]. Phys Rev E 66(3):035301–.https://doi.org/10.1103/PhysRevE.66.035301
Luo L, Girimaji SS (2003) Theory of the lattice Boltzmann method: Two-fluid model for binary mixtures [J]. Phys Rev E 67(3):036302–. https://doi.org/10.1103/PhysRevE.67.036302
McCracken ME, Abraham J (2005) Lattice Boltzmann methods for binary mixtures with different molecular weights [J]. Phys Rev E 71(4):046704–.https://doi.org/10.1103/PhysRevE.71.046704
Hussain MM, Li X, Dincer I (2008) A general electrolyte-electrode-assembly model for the performance characteristics of planar anode-supported solid oxide fuel cells [J]. J Power Sources 189(2):916–928. https://doi.org/10.1016/j.jpowsour.2008.12.121
Mastropasqua L, Donazzi A, Campanari S (2018) Development of a multiscale SOFC model and application to axially-graded electrode design [J]. Fuel Cells 19(2):125–140. https://doi.org/10.1002/fuce.201800170
Yahya A, Hammouda S, Slimene S et al (2022) Effect of cathode pulsating flow on mass transport and performance of solid oxide fuel cell [J]. Int J Thermal Sciences 174:107437. https://doi.org/10.1016/j.ijthermalsci.2021.107437
Huang J, Li ZY, Li N et al (2021) An approach combining the lattice Boltzmann method and Maxwell-Stefan equation for modeling multi-component diffusion [J]. Phys Fluids 33:1–17. https://doi.org/10.1063/5.0059073
Mahmood MA, Chaudhary TN, Farooq M et al (2023) Sensitivity analysis of performance and thermal impacts of a single hydrogen fueled solid oxide fuel cell to optimize the operational and design parameters [J]. Sustain Energy Technol Assess 57:1–10. https://doi.org/10.1016/j.seta.2023.103241
Zhao HF, Zhou J, Zong Z et al (2024) Three-dimensional reconstruction and optimization of porous fuel electrode in reversible solid oxide cells based on the Lattice Boltzmann method [J]. Electrochim Acta 476:1–14. https://doi.org/10.1016/j.electacta.2023.143702
Yahya A, Rabhi R, Dhahri H et al (2018). Numerical simulation of temperature distribution in a planar solid oxide fuel cell using lattice Boltzmann method [J]. Power Technol 338:402–415. https://doi.org/10.1016/j.powtec.2018.07.060
Xu H, Dang Z (2016) Lattice Boltzmann modeling of carbon deposition in porous anode of a solid oxide fuel cell with internal reforming [J]. Appl Energy 178:294–307. https://doi.org/10.1016/j.apenergy.2016.06.007
Author information
Authors and Affiliations
Contributions
The statements of author contributions are as follows:
Yongqi Wei: Writing and Editing, Methodology, Software, Investigation, Writing original draft preparation, Data curation;
Zhi Ning: Investigation and Validation, Investigation.
Chunhua Sun: Editing, Data curation, Writing-Reviewing.
Ming Lv: Investigation.
Yechang Liu: Investigation.
All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, Y., Ning, Z., Sun, C. et al. Parametric analysis of solid oxide fuel cell fueled by syngas based on lattice Boltzmann method. Ionics 30, 2729–2745 (2024). https://doi.org/10.1007/s11581-024-05452-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05452-1