Abstract
The non-precious metal-based materials show excellent catalytic activity for overall water splitting. However, it is still a challenge to meet the requirements of fabricating catalysts with cost-effective, high-performance, and large-scale production. In this paper, we report an energy-efficient, low-cost, scaled-up redox-corroding, and boronizing engineering method for transforming inexpensive nickel foam into highly active, flexible, and durable self-supported electrodes for overall water splitting. This work adopts a desirable redox-corroding and one-pot NaBH4 reduction reaction of nickel foam in aqueous solutions containing trivalent cations (Fe3+) under ambient temperature. This process results in well-distributed amorphous iron–nickel borides and hydroxides active materials that are grown in situ on the surface of porous nickel foam. The NiFeBx/NF//NiFe-OH/NF electrode pair shows low cell voltages of 1.63 V to achieve 10 mA cm−2 in 1 M KOH. More importantly, the NiFeBx/NF//NiFe-OH/NF electrode exhibits ultrahigh catalytic activities at high current densities, outperforming the benchmark electrode pair of Pt-C/NF//RuO2/NF. This inexpensive and simple controllable strategy provides Ni foam-substrate-derived electrodes with excellent catalytic activities and opens up new avenues for the rapid and simple fabrication of highly efficient electrodes for overall water splitting with large-scale applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen, due to its environmentally friendly and high gravimetric energy density characteristics, offers enormous potential to address increasing energy demands in the future [1,2,3]. Large-scale electrocatalytic water splitting technology is a key way to obtain high-purity hydrogen gas [4,5,6]. As an unfavorable reaction due to its thermodynamic barrier, water splitting always works at much larger overpotentials relative to the theoretical value [7]. The use of highly active catalysts can effectively reduce the energy barrier in hydrogen/oxygen evolution processes (HER/OER) and thus decrease the overpotential. It is known that noble metal-based materials (e.g., Pt-based and Ir/Ru-based) are the most active benchmark catalysts for water splitting. Unfortunately, their rarity and poor stability make their widespread application impractical [8, 9]. Accordingly, great efforts have been devoted to non-precious electrocatalysts using earth-enriched materials, such as hydroxides/layered double hydroxides [10,11,12], borides [13, 14], sulfides [15,16,17], carbides [18, 19], nitrides [20, 21], selenides [22,23,24], and phosphides [25,26,27,28,29].
Despite significant progress in the improvement of catalytic activity for HER/OER, notable challenges remained in using these promising non-precious electrocatalysts for large-scale and practical applications [30, 31]. On the one hand, most of the current powdered electrocatalysts can only be used when cast on the substrate with adhesives, which inevitably obstructs the active sites, increases the contact resistance, and hinders the translocation of the electrolyte to the active sites [32]. On the other hand, the material easily peels away from the current collector during large-current or long-term electrocatalysis for coated active materials, owing to the poor adhesive force between the active material and the current collector [33]. Thus, these electrocatalyst powders can only work at low current densities and cannot be adapted to industrial production in high current density states. As a result, to obtain more practical and highly efficient electrodes for large-scale applications, electrodes should be prepared using common materials and the strategy should be as simple as possible [34].
Currently, self-supported electrodes with active material directly growing on conductive substrates are more desirable because they offer many intrinsic properties compared with conventional powdery electrocatalysts [35, 36]. First, the direct growth of electroactive species on substrates avoids the post-coating procedure with an assistance binder and extra conductive agent, which improves the efficiency of the electrode preparation procedure and reduces the cost. Second, the binding of electroactive species and their underlying substrates offers thorough contact without an extra binder. Direct contact assures rapid electron transfer and prevents the catalyst from shedding over long-term usage. Typical synthetic strategies for self-supported electrodes include hydro/solvothermal, electrodeposition, and vapor deposition, which require additional energy inputs (heat or electricity) [37]. Therefore, the challenge of minimizing energy consumption during the preparation process, simplifying technological conditions, and fabricating highly active self-supported electrodes, particularly at large catalytic current densities, persists in large-scale applications.
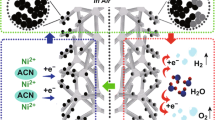
In this work, we present amorphous iron-nickel borides and iron–nickel hydroxide nanostructures grown in situ on surface-redox-corroding Ni foam. The self-supported electrocatalyst has shown great superiority in terms of cost control, manufacturing strategy, energy consumption, catalytic activity, etc. Firstly, the NiFe-OH/NF self-supported electrode is grown on the low-cost commercial Ni foam via a redox-corroding aqueous solution of Fe3+. Following this, the NiFeBx/NF electrode could be well-obtained via a one-pot NaBH4 reduction reaction. It is evident that this synthesis requires only a slice of Ni foam, a certain volume of Fe3+ solution, and NaBH4, which is inexpensive to produce. Secondly, in the redox-corroding process, the inexpensive Ni foam not only serves as a 3D conductive current collector but also acts as a slow-releasing Ni source, which is induced by redox-corroding of Fe3+ in solution [38]. Therefore, it can be accomplished at room temperature without extra external environment applied, such as high temperatures, high pressures, electric fields, etc. At the same time, since the reduction reaction of NaBH4 is exothermic, the subsequent boronization process can also be successfully completed at room temperature without additional heat input. More importantly, the NiFeBx/NF//NiFe-OH/NF as an asymmetric electrode couple showed small cell voltages of 1.63 V to achieve 10 mA cm−2 in 1 M KOH, and the NiFeBx/NF//NiFe-OH/NF electrode exhibited ultrahigh catalytic activities at high current densities, outperforming the benchmark electrode pair of Pt-C/NF//RuO2/NF. The superb HER/OER activity would be attributed to the enhanced catalytic performance and electroconductivity enabled by the synergistic interaction of Ni and Fe elements in NiFeBx and NiFe-OH nanostructures [39], the full utilization of catalytically active materials, the accelerated electrolyte infiltration and generated H2/O2 diffusion empowered by the high porosity within the surface-redox-corroding Ni foam support, and the prominent inherent activity empowered by the amorphous native of NiFeBx/NiFe-OH nanostructures. In conclusion, the self-supported electrocatalyst shows several characteristics of excellent commercial electrocatalysts: low cost, ease of fabrication, zero energy consumption, and good catalytic performance at high current densities, which is very important for large-scale practical applications.
Results and discussion
The 3D NiFe-OH/NF and NiFeBx/NF self-supported electrodes were fabricated by a controllable redox-corroding and boronizing engineering strategy as well as in situ growth mode. Low-cost Ni foam not only served as a conductive current collector but also acted as a slow-releasing Ni source. Figure S1 displayed the evolution of self-supported electrode colors after room-temperature redox-corroding and boronizing engineering strategy. The significant change in colors, from initial bright silvery grey to yellow and then to silvery grey, demonstrated that the NiFe-based materials were directly grown on the surface of the Ni foam.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed more detailed structural and micromorphological characteristics of the as-synthesized NiFe-OH and NiFeBx on Ni foam. Loose porous sponge-like NiFeBx were homogeneously deposited on Ni foam through the redox-corroding and boronizing engineering routes (Fig. 1a). TEM image clearly represented that the as-obtained NiFeBx samples were a sphere-like aggregated morphology enwrapped with irregularly shaped nanosheets (Fig. 1b). Furthermore, the selected area electron diffraction (SAED) pattern of NiFeBx samples (inset of Fig. 1b) was not distinct rings or dots, exhibiting an amorphous structure [40]. This is in accordance with the XRD results (Fig. 2a). The energy dispersive X-ray spectrum (EDS) elemental distributions (Fig. 1c, d, e, and f) revealed the even distribution of Ni, Fe, and B species over the surface of NiFeBx/NF, indicating the uniform growth of NiFeBx. In addition, amorphous NiFe-OH nanostructures were grown vertically upon Ni foam with a smooth surface after redox-corroding and hydrolysis co-precipitation as shown in Figure S3. Meanwhile, deep etch marks of microcracks or holes were distinctly seen in the exposed areas of the Ni foam, while barely observed in the original area (Figure S2). They were derived from redox corroding of the added iron precursor on a Ni foam substrate. The unique architecture of NiFeBx/NF and NiFe-OH/NF electrodes is expected to add contact area with the electrolyte and specific surface area, which contributes to improving catalytic activity. To investigate the structure of the as-synthesized self-supported electrode, XRD was further researched.
X-ray photoelectron spectroscopy (XPS) was utilized to deep analyze the surface chemistry of the samples [41]. The XPS spectrums of the as-obtained NiFeBx/NF and NiFe-OH/NF were compared in detail (Fig. 2b, c, and d). For NiFe-OH/NF, two main peaks of Ni located at 855.8 and 874.1 eV are assigned to Ni 2p3/2 and Ni 2p1/2, respectively. In addition, the binding energy of Ni centered at 861.8 and 879.8 eV, corresponding to two satellite peaks (Fig. 2b). In the Fe 2p spectrum of NiFe-OH (Fig. 2c), the Fe 2p3/2 and Fe 2p1/2 peaks were located at binding energies of 711.8 and 724.8 eV, respectively, indicating the presence of Fe3+ [42]. After one-pot NaBH4 reduction, boron not only formed the amorphous NiFeBx but also transferred certain electrons to transition metal atoms Ni and Fe, which led to the peak of Ni and Fe shifting to lower binding energy, providing active sites of transition metal with enriched d population, leading to excellent activity of amorphous metal borides. The B 1 s spectrum of NiFeBx was deconvoluted into two main species located at 187.8 and 191.7 eV, respectively (Fig. 2d). The former peak was ascribed to the interaction of boron with metals (Ni, Fe), while the latter was due to oxidized borate species, caused by prevalence surface oxidation of metal borides in the synthesis [43].
In addition to Ni foam, several other transition metal element substrates ( such as Cu foam) can also be used effectively. Accordingly, this modulation results in the formation of CuFeBx and CuFe-OH on the surface of Cu foam (Figure S4). These results demonstrate the extensibility of redox-corroding and boronizing engineering strategies. Additionally, this redox-corroding and boronizing engineering strategy is easily scaled up. A large-area electrode (20 × 25 cm) comprising uniform distribution active materials has been prepared (Figure S1).
The surface hydrophilicity/hydrophobicity properties of the bare Ni foam and the as-obtained NiFe-OH/NF and NiFeBx/NF were also studied. As depicted in Figure S5, it can be demonstrated that the NiFe-OH/NF and NiFeBx/NF electrodes show good hydrophilicity, which contributes to better contact between electrolytes and active material. The flexibility of the NiFe-OH/NF and NiFeBx/NF was further investigated by undergoing deformation and then their corresponding performances were measured (Figure S6-8). No change in catalytic activity indicated that these electrode has good mechanical flexibility.
We also studied the effects of soaking time on the resulting self-supported electrodes. Raman spectra results (Figure S9) show that well-resolved peaks appeared at 455 and 557 cm−1, which might be attributed to vibrations of Ni2+-OH and Ni2+-O bonds in Ni2+(OH)2 [44, 45]. The Raman peaks were also enhanced with increasing time, indicating that the metal hydroxides can generate gradually on Ni foam as the soaking time increases [46]. However, with the instillation of NaBH4, the Raman peaks weakened with increasing time (Figure S10). This indicated that under the reduction of NaBH4, the hydroxide of Ni and Fe were substantially changed to amorphous NiFeBx.
Combined with the above characterization consequence, it would successfully infer a rational growth mechanism of NiFeBx and NiFe-OH on Ni foam. A redox reaction between Ni foam and Fe3+ reacts and a large amount of Fe2+ and Ni2+ are synchronously generated near the surface of Ni foam (Eq. 1); multi-metal NiFeBx borides are successfully fabricated via a facile one-pot NaBH4 reduction method [47] (Eqs. 2 and 3); (III) NiFe-OH nanostructure growth directly on Ni foam can be finally realized by the coprecipitation of Ni2+, Fe2+, and Fe3+ (Eq. 4).
The advantages of Ni–Fe-based self-supported electrodes (well conductivity, 3D porous structure, presence of large exposed active sites, etc.) make them promising robust electrocatalysts. A typical three-electrode apparatus was used to evaluate and compare the HER performance of the Ni–Fe-based self-supported electrode in a 1 M KOH solution [48]. Figure 3a shows the linear sweep voltammetry (LSV) curves of all as-prepared electrodes. Figure s11 shows the LSV curves of the samples with different redox-corroding times in the FeCl3 solution. Among the soaking durations, 1, 3, 5, and 7 h, 7 h was found to impart better activity. Notably, bare Ni foam exhibited poor HER activity, while the HER performance of the as-obtained series of NiFeBx/NF and NiFe-OH/NF acquire much smaller overpotentials and larger current densities. Conversely, bare Ni foam exhibited poor HER activity. These results demonstrated that the coupling of Ni and Fe could observably improve the electrocatalytic HER performance. Among these catalysts, the NiFeBx/NF self-supported electrode showed the highest catalytic activity. The overpotential of the NiFeBx/NF self-supported electrode was 191 mV to render a current density of 10 mA cm−2 for HER, which was obviously lower than that of the NiFe-OH/NF (220 mV) and pure Ni foam (296 mV). This result confirmed that the incorporation of the B component could significantly increase the catalytic HER reactivity of NiFe-based materials. Furthermore, the corresponding Tafel slopes of the NiFeBx/NF are also smaller than those of the NiFe–OH/NF electrode and bare Ni foam, demonstrating a more rapid reaction kinetics of the NiFeBx/NF (Figure S13). In addition, a small Tafel slope is preferred for large-scale applications, and a significant increase in HER rate will be obtained as the overpotential increases [49]. The electrochemical stability of the NiFeBx/NF electrode was measured by continuous cyclic voltammetry (CV) scanning of 2000 cycles (Fig. 3b). The decay of the polarization curve after 2000 cycles is negligible, thus demonstrating the excellent durability of the NiFeBx/NF electrode. The durability is further verified with a long-term current-dependent time relation test. The current density of the NiFeBx/NF electrode remained unchanged over a 20-h test, confirming its excellent stability.
a Polarization curves of NF, NiFe-OH/NF, NiFeBx/NF, and 20% Pt-C/NF in 1 M KOH for the HER. b Polarization curves for NiFeBx/NF before and after 2000 cycles. Inset, I–t curve for NiFeBx/NF under static overpotential for 20 h. c Polarization curves of NF, NiFe-OH/NF, NiFeBx/NF, and RuO2/NF in 1 M KOH for the OER. d Polarization curves for NiFe-OH/NF before and after 2000 cycles. Inset, I–t curve for NiFe-OH/NF under static overpotential for 20 h. e Electrochemical impedance spectroscopy (EIS) spectra of as-prepared eletrodes. f Plots showing the extraction of the Cdl
In regard to OER, catalytic activities of the NiFeBx/NF, NiFe-OH/NF, bare Ni foam, and RuO2/NF are also assessed in the identical electrolyte to HER. Figure 3c and Figure S12 show the linear sweep voltammetry (LSV) curves of all as-prepared electrodes and the samples with different redox-corroding times in the FeCl3 solution, respectively. In contrast to HER, the NiFe-OH/NF electrode possesses the optimal OER performance, with an overpotential of 160 mV to achieve a current density of 10 mA cm−2, significantly lower than bare Ni foam (450 mV), NiFeBx/NF (242 mV), and RuO2/NF (298 mV). The corresponding Tafel plots in Figure S14 show that the NiFe-OH/NF catalyst owned the lowest Tafel value, further indicating its remarkable OER performance. Furthermore, both the current-dependent time relation and LSV curves after 2000 cycles showed no obvious decay (Fig. 3d), indicating the excellent long-term OER durability of the NiFe-OH/NF electrode.
To better elucidate the origins of improved activity for NiFeBx/NF and NiFe-OH/NF, electrochemical impedance spectroscopy (EIS) was performed to evaluate the electron transport ability [50]. The EIS spectra (Fig. 3e) reveal the reduced Rct values of the NiFeBx/NF and NiFe-OH/NF electrodes compared to Ni foam. This trend expresses that Fe incorporation can also efficiently reduce the impedance during the charge transfer, causing a faster charge transfer rate of the NiFeBx/NF and NiFe-OH/NF in the strong alkaline solution. The electrochemical active surface area (ECSA) was also calculated to measure the active area of the catalyst [51], for instance, the electrochemical double-layer capacitance (Cdl). The value of the NiFeBx/NF (3.89 mF cm−2) is similar to the NiFe-OH/NF catalyst (3.80 mF cm−2), yet much higher than the bare Ni foam (1.64 mF cm−2) electrode (Fig. 3f). The large ECSA of NiFeBx/NF and NiFe-OH/NF can be derived from the active site NiFe-based nanostructure uniformly decorated on the surface of Ni foam and the increased number of active sites by Fe incorporation. These properties further explain the prominent electrocatalytic performance of NiFeBx/NF and NiFe-OH/NF.
Encouraged by the excellent activity of both HER and OER, the overall alkaline water-splitting electrolyzer was fabricated using NiFe-OH /NF and NiFeBx/NF as the anode and cathode electrode, respectively ( NiFeBx/NF ( −)||NiFe-OH/NF ( +)). A current density of 10 mA cm−2 can be achieved at a small cell voltage of 1.63 V (Fig. 4a), which is similar to that of NiFeBx/NF||NiFeBx/NF (1.628 V) and superior to those for NiFe-OH /NF||NiFe-OH/NF (1.728 V) and NF||NF (1.838 V) (Figure S15). Long-term current-dependent time relation tests for NiFeBx/NF||NiFe-OH/NF achieved a constant 20 mA cm−2 in 1.0 M KOH, demonstrating that this electrode couple could well maintain full water splitting over 25 h (Fig. 4b). Furthermore, during the overall alkaline water splitting process, when the current density reaches 200 mA cm−2, it can be clearly seen (Fig. 4a) that the NiFeBx/NF//NiFe-OH/NF electrodes are able to achieve higher current densities than the Pt-C/NF//RuO2/NF electrodes at the same voltage. And most commercial overall water-splitting processes are accomplished under high current density conditions. This indicates that the NiFeBx/NF//NiFe-OH/NF electrode is able to exhibit higher catalytic activity than the commercial electrocatalysts at high current densities, which provides the potential for its practical application.
The morphological and structural stability after electrochemical durability tests of the NiFeBx/NF and NiFe-OH/NF electrodes were further explored. Figure S16 shows the largely unchanged XRD pattern of the NiFeBx/NF and NiFe-OH/NF electrode before and after electrochemical durability tests, indicating the stable amorphous structures of NiFeBx and NiFe-OH. Furthermore, it is worth noting that the morphology of NiFeBx/NF and NiFe-OH/NF nanostructures are well-preserved with negligible changes (Figures S17 and S18). The excellent morphology durability is attributed to the support of Ni foam with a 3D porous structure, restraining the shedding of active material and accelerating ion transport during HER/OER processes. These results suggest that the NiFeBx/NF and NiFe-OH/NF electrodes have excellent stability during the HER/OER process. The mechanical flexibility of the NiFe-OH/NF and NiFeBx/NF was further investigated by undergoing deformation and then their corresponding performances were measured (Figure S6-8). No change in catalytic activity indicated that this electrode has good mechanical flexibility.
To better understand the mutual effect between nickel and iron in NiFeBx for HER performance, DFT calculations were performed to focus on the changes in HER Gibbs free energy and electronic structure [52, 53]. NiFeBx is amorphous, and the amorphous structure is very complex. In order to explore the effect of iron introduction on the activity of hydrogen evolution, we took Ni2B as a model in this experiment.
Firstly, the free energies of adsorbed H (ΔGH) at the Ni site are presented in Fig. 5a. Obviously, the Gibbs free energy of all the H adsorption sites in the Ni site of Fe-doped Ni2B is close to zero compared to the pristine Ni site. Furthermore, the Gibbs free energy of the H adsorption in the Ni site is closer to zero with the further introduction of iron atoms. Therefore, the presence of Fe elements promotes the activity of active sites Ni as expected.
As we know that chemical properties are intensively determined by their electronic structure, a calculation of the density of states (DOS) could provide us insight into the different HER activity [54]. As can be seen from Fig. 5b, a large number of electronic states occur near the Fermi level, suggesting that more charge carriers can be introduced by Fe atom doping compared to that of the pristine Ni2B, thereby increasing electrical conductivity, which heavily accelerates the efficiency of NiFeBx HER catalysts. Through theoretical calculations, we found that the doping of Fe can effectively improve the activity of the catalyst, which has a certain reference value.
Conclusion
In summary, a simple room-temperature redox-corroding and boronizing engineering method was proposed for the in situ growth of NiFeBx and NiFe-OH on Ni foam (NiFeBx/NF and NiFe-OH/NF). Through rational control of the preparation environment such as reaction time, it can be partially etched and converted to the Ni–Fe-based catalysts with regulated physical forms and chemical compositions at the same time. This low-cost and facile controllable strategy affords Ni foam-substrate-derived electrodes owning remarkable catalytic activities and were used as asymmetric electrodes for overall water splitting. The NiFeBx/NF//NiFe-OH /NF electrode pair showed ultralow cell voltages of 1.63 V@10 mA cm−2 in 1 M KOH. More importantly, the NiFeBx/NF//NiFe-OH/NF electrode exhibited ultrahigh catalytic activities at high current densities, outperforming the benchmark electrode pair of Pt-C/NF//RuO2/NF. This inexpensive and simple controllable preparation strategy at room temperature provides Ni foam-substrate-derived electrodes with excellent catalytic activities and opens up new avenues for the rapid and simple synthesis of efficient electrocatalysts for overall water splitting with large-scale production.
Data Availability
Data will be made available on request.
References
Bonaccorso F, Colombo L, Yu G, Stoller M, Tozzini V, Ferrari AC, Ruoff RS, Pellegrini V (2015) Science 347:1246501
Shindell D, Smith CJ (2019) Nature 573:408–411
Dey RS, Purkait T, Kamboj N, Das M (2019) Carbonaceous Materials and Future Energy: Clean and Renewable Energy Sources, 1st edn. CRC Press. https://doi.org/10.1201/9781351120784
Chatenet M, Pollet BG, Dekel DR, Dionigi F, Deseure J, Millet P, Braatz RD, Bazant MZ, Eikerling M, Staffell I, Balcombe P, Shao-Horn Y, Schafer H (2022) Chem Soc Rev 51:4583–4762
Wang YZ, Liu SS, Hao XF, Luan SR, You HH, Zhou JS, Song DD, Wang D, Li H, Gao FM (2019) J Mater Chem A 7:10572–10580
Zhang A, Liang Y, Zhang H, Geng Z, Zeng J (2021) Chem Soc Rev 50:9817–9844
Chen S, Wang YH, Wang ZJ, Zhang K (2023) Ionics 29:9–32
Li C, Baek JB (2020) ACS Omega 5:31–40
Jiao Y, Zheng Y, Jaroniec M, Qiao SZ (2015) Chem Soc Rev 44:2060–2086
Liu HH, Liu SY, Cao SF, Zhang JR, Ni H, Lin XJ, Chen XD, Wei SX, Hou Q, Wang ZJ, Lu XQ (2022) ACS Sustain Chem Eng 10(21):7100–7107
Peng CW, Huang R, Pan GX, Liu WB, Wang L (2020) Ionics 26:301–309
Li XG, Liu C, Fang Z, Xu L, Lu CL, Hou WH (2022) J Mater Chem A 10:20626–20634
Yixin H, Zhenxiang Z, Ting L, Ping Y (2022) Int J Hydrogen Energy 47:12539–12546
Lewandowski M, Bartoszewicz M, Jaroszewska K, Mariadassou GD (2022) J Ind Eng Chem 116:75–98
Dong Y, Fang Z, Yang WY, Tang B, Liu Q, Appl ACS (2022) Mater Interfaces 14(8):10277–10287
Yang XN, Sui GZ, Guo DX, Chu DW, Li JL, Na SN, Yu MR, Li DQ (2023) Ionics 29:4115–4123
Tong Y, Chen PZ (2021) Dalton Trans 50:7776–7782
Wang J, Zhu R, Cheng J, Song Y, Mao M, Chen F, Cheng Y (2020) Chem Eng J 397:125481
Ge R, Zhao J, Huo J, Qu J, Yang J, Li Y et al (2022) Materials Today Nano 20:100248
Chen PZ, Feng DM, Li KX, Tong Y (2022) Dalton Trans 51:16990–16999
Li KX, Tong Y, Feng DM, Chen PZ (2022) J Colloid Interface Sci 622:410–418
Zhang L, Li Z, Lu YY, Yang S, Zhang HH, Tang JJ, Yu FL, Liu YB, Chen GJ, Zhou Y (2023) Ionics 28:1323–1335
Shen S, Wang Z, Lin Z, Song K, Zhang Q, Meng F, Gu L, Zhong W (2022) Adv Mater 34:2110631
Das M, Kumar G, Dey RS, Appl ACS (2022) Energy Mater 5:3915–3925
Lv XW, Xu WS, Tian WW, Wang HY, Yuan ZY (2021) Small 17:e2101856
Jin M, Zhang X, Shi R, Lian Q, Niu S, Peng O, Wang Q, Cheng C (2021) Appl Catal B 296:120350
Li KX, He JF, Guan XZ, Tong Y, Ye YT, Chen L, Chen PZ (2023) Small 19:2302130
Tong Y, Chen PZ (2021) Dalton Trans 50:7364–7371
Li KX, Tong Y, He JF, Liu XY, Chen PZ (2023) Anion-modulated CoP electrode as bifunctional electrocatalyst for anion-exchange membrane hydrazine-assisted water electrolyser. Mater Horiz 10:5277–5287. https://doi.org/10.1039/D3MH00872J
Xu X, Tian XM, Zhong Z, Kang LT, Yao JN (2019) J Power sources 424:42–51
Das M, Biswas A, Purkait T, Boruah T, Bhardwaj S, Das SK, Dey RS (2022) J Mater Chem A 10:13589–13624
Zhong B, Kuang P, Wang L, Yu J (2021) Appl Catal B-Environ 299:120668
Liu YP, Liang X, Gu L, Zhang Y, Li GD, Zou XX, Chen JS (2018) Nat Commun 9:2609
Pei Y, Ge YC, Chu H, Smith W, Dong P, Ajayan PM, Ye MX, Shen JF (2019) Appl Catal B Environ 244:583–593
Ning M, Wu L, Zhang F, Wang D, Song S, Tong T, Bao J, Chen S, Yu L, Ren ZF (2021) Mater Today Phys 19:100419
Das M, Kamboj N, Purkait T, Sarkar S, Dey RS (2020) J Phys Chem C 124:13525–13534
Sun H, Yan Z, Liu F, Xu W, Cheng F, Chen J (2020) Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv Mater 32:1806326. https://doi.org/10.1002/adma.201806326
Sun CY, Song QT, Lei JL, Li D, Li LJ, Pan FS, Appl ACS (2021) Energy Mater 4:8791–8800
Wang YZ, Zhou YY, Han MZ, Xi YK, You HH, Hao XF, Li ZP, Zhou JS, Song DD, Wang D, Gao FM (2019) Small 15:1805435
Lu X, Li YS, Tao L, Song DD, Wang YZ, Li Y, Gao FM (2019) Nanotechnology 30:055501
Gayathri S, Arunkumar P, Kim J, Han JH (2022) ACS Sustain Chem Eng 10(4):1689–1701
Liang HF, Gandi AN, Xia C, Hedhili MN, Anjum DH (2017) U Schwingenschlögl, HN Alshareef. ACS Energy Lett. 2:1035–1042
Li H, Wen P, Li Q, Dun C, Xing J, Lu C, Adhikari S, Jiang L, Carroll DL, Geyer SM (2017) Adv Energy Mater 7:1700513. https://doi.org/10.1002/aenm.201700513
Zhu KY, Zhu XF, Yang WS (2019) Angew Chem Int Ed 58:1252–1265
Wu YY, Li Y, Yuan MK, Hao HR, San XJ, Lv Z, Xu LL, Wei B (2022) Chem Eng J 427:131944
Niu SQ, Sun YC, Sun GJ, Rakov D, Li YZ, Ma Y, Chu JY, Xu P, Appl ACS (2019) Energy Mater 2:3927–3935
Yao R, Wu Y, Zhao Q, Li J, Liu G (2022) Int J Hydrogen Energy 47(13):8303–8313
Dionigi F, Zeng ZH, Sinev I, Merzdorf TS, Deshpande S, Lopez MB, Kunze S, Zegkinoglou I, Sarodnik H, Fan DX, Bergmann A, Drnec J, Araujo JF, Gliech M, Teschner D, Zhu J, Li W-X, Greeley J, Cuenya BR, Strasser P (2020) Nat Commun 11:2522
Yan H, Xie Y, Wu A, Cai Z, Wang L, Tian C, Zhang X, Fu H (2019) Anion-modulated HER and OER activities of 3D Ni–V-based interstitial compound heterojunctions for high-efficiency and stable overall water splitting. Adv Mater 31:1901174. https://doi.org/10.1002/adma.201901174
Lyu S, Guo CX, Wang JN, Li ZJ, Yang B, Lei LC, Wang LP, Xiao JP, Zhang T, Hou Y (2022) Nat Commun 13:6171
Huang XK, Xu XP, Li C, Wu DF, Cheng DJ, Cao DP (2019) Vertical CoP nanoarray wrapped by N, PDoped carbon for hydrogen evolution reaction in both acidic and alkaline conditions. Adv Energy Mater 9:1803970. https://doi.org/10.1002/aenm.201803970
Li PS, Wang MY, Duan XX, Zheng LR, Cheng XP, Zhang YF, Kuang Y, Li YP, Ma Q, Feng ZX, Liu W, Sun XM (2019) Nat Commun 10:1711
Yan JQ, Kong LQ, Ji YJ, White J, Li YY, Zhang J, An PF, Liu SZ, Lee ST, Ma TY (2019) Nat Commun 10:2149
Ouyang YX, Ling CY, Chen Q, Wang ZL, Shi L, Wang JL (2016) Chem Mater 28(12):4390–4396
Funding
This work was supported by the Hebei Key Laboratory of Applied Chemistry after Operation Performance (grant no. 22567616H).
Author information
Authors and Affiliations
Contributions
F.G. , Y.W. and G.S. wrote the main manuscript text and F.C. prepared figures 1. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, G., Wang, Y., Chen, F. et al. emaZero-additional-energy-consumption fabrication of a self-supported electrode: synergistic redox-corroding and boronizing engineering for overall water splitting. Ionics 30, 971–978 (2024). https://doi.org/10.1007/s11581-023-05330-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05330-2