Abstract
Polymer electrolyte systems composed of poly(vinyl alcohol) (PVA) and lithium trifluoromethanesulfonate (LiTf, also known as LiCF3SO3) at 60:40 weight ratios with varying weight percentages (wt.%) of titanium (IV) oxide (TiO2) nanofiller were prepared by solution casting technique. The most conductive nanocomposite polymer electrolyte which was added with 10 wt.% of TiO2 nanofiller has an ionic conductivity of (1.08 ± 0.002) × 10−4 S cm−1 at room temperature. The ionic transport mechanism in the nanocomposite polymer electrolyte was found to obey Arrhenius relationship which is related to thermally activated principle. Fourier-transform infrared (FTIR) study confirmed complexation between the PVA, LiTf, and TiO2. TGA thermogram of the most conductive polymer electrolyte shows good thermal stability which can withstand high temperature. The increase in glass transition temperature (Tg) of PVA upon addition of 10 wt.% TiO2 nanofiller is attributed to the interaction between TiO2 nanofiller and polymer matrix that causes the polymeric backbone to become more rigid. Linear sweep voltammogram of the sample shows a wide potential window of 4.95 V. The ionic transference number was found to be close to unity which suggests that the charge carriers are mostly ionic species. A simple electrical double layer capacitor (EDLC) was fabricated using nanocomposite polymer electrolyte with 10 wt.% of TiO2 nanofiller and its performance was evaluated. The EDLC was found to have specific capacitance of 444.06 mF g−1 (or 3.87 mF cm−2) with good electrochemical stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, issues pertaining to high demand and usage of fossil fuel and their detrimental impacts on the environment has pushed for the development of eco-friendly and efficient energy resources and energy storage devices. Supercapacitors exhibit higher power density than conventional batteries and higher energy density than dielectric capacitors. Electrical double layer capacitor (EDLC) is one of a type of supercapacitors where the ability to store electrical energy arises from the formation of an electrical double layer of charge at the electrode–electrolyte interface [1]. Conventional EDLCs contain two carbon-based electrodes, an aqueous electrolyte, and an electrolyte separator which can be made from cellulose or other materials such as polymers, glass wool, fiberglass, and lignin [2,3,4]. Nonetheless, the polymer electrolyte can simultaneously act as the separator when a solid-state polymer electrolyte is employed [5]. EDLC is a useful device which has the potential to be applied in memory backup systems, energy device in electric or hybrid vehicles and the potential as a substituent for common batteries and fuel cells [6,7,8]. However, liquid electrolytes in the EDLC are often linked to problems such as leakage and poor compatibility with electrodes, as well as fire hazards and risk of explosion [9, 10]. The damage caused by an explosion of such electrochemical storage device would be catastrophic. Therefore, safety performance of the electrochemical storage device would be a concern to the consumers. One method to overcome the safety issues is to replace the liquid electrolyte with a solid-state electrolyte. However, the main issue which thwarts the application of solid-state electrolytes in commercial products is their low ambient temperature ionic conductivities [11]. Various plasticizers are commonly added to improve the ionic conductivities of polymer electrolytes, but this usually comes at the expense of mechanical strength and electrochemical stability [12, 13]. Therefore, composite polymer electrolytes (CPEs) are developed to replace the plasticized-polymer electrolytes. The CPEs are formed by dispersing ceramic fillers into a polymer electrolyte system. Addition of ceramic fillers enhances the ionic conductivity without compromising the mechanical stability. Ceramic fillers can be further classified into active and passive fillers in which the former is actively involved in the ionic conduction process while the latter does not take part in the conduction process [14]. Although polymer electrolytes with active ceramic fillers are able to achieve considerably high ionic conductivities in the orders of 10−4 S cm−1 at moderate temperatures, they have some disadvantages such as high cost and being prone to oxidation [11, 15,16,17]. Hence, passive ceramic fillers such as SiO2, Al2O3, TiO2, and zeolite are often added to enhance ionic conductivity and mechanical properties [18]. Based on the results of several studies, enhancement of ionic conductivity by incorporation of passive ceramic filler is comparable to that of active ceramic filler which gives conductivity in the order of 10−5 to 10−4 S cm−1 at moderate temperature [19,20,21]. Remarkably, Premila and co-workers (2018) reported ionic conductivity of their polymer electrolyte systems comprising of poly(ethylene methacrylate)/poly(vinyl acetate)/lithium perchlorate/ethylene carbonate doped with 10 wt.% TiO2 nanofiller achieved 2.75 × 10−3 S cm−1 at room temperature [11].
In the present work, the structural, thermal, and electrochemical properties of a nanocomposite polymer electrolyte (NCPE) based on PVA and LiTf salt at 60:40 weight ratios with varying mass loadings of titanium (IV) oxide (TiO2) nanofiller were studied. The PVA:LiTf ratio of 60:40 chosen for this work is based on the study conducted and optimized by our peers which corresponds to the SPE with the highest ionic conductivity at room temperature [22]. PVA was selected as the polymer host due to its fascinating properties such as high tensile strength, biodegradability, ionic conduction ability, excellent film-forming ability, non-toxicity, and high hydrophilicity [23, 24]. In addition, the polymeric backbone of PVA is attached with -OH functional groups which permits the formation of a polymer-salt complexes [25, 26]. Estimation of degree of crystallinity of the PVA-based polymer electrolyte using deconvolution with ATR-FTIR is also reported in this present work which is rarely reported in other literatures. Finally, the most suitable polymer electrolyte system was chosen to be fabricated into a simple EDLC cell and the performances in terms of specific capacitance, power density, energy density, and cyclability were evaluated accordingly. To the best of our knowledge, there has yet to be literature reporting on the solid polymer electrolyte based on PVA/LiTf/nano-sized TiO2. Even though extensive research has been done on PVA-based polymer electrolytes, we employ different salt or filler in this work. To the best of our knowledge, there is no work reported on the preparation of nanofiller-doped ion conductors using PVA, LiTf, and nano-sized TiO2. It leads to a great exposure to nanotechnology field in renewable energy area. In addition, the quantitative analysis of Fourier-transform infrared studies is another highlight of this work. The quantitative analysis of Fourier-transform infrared studies is rarely reported in literatures. Quantitative analysis is essential in this research to obtain the information of a material in microscopic view. The percentage of mobile ions, ion pairs, and ion aggregates are estimated using the deconvolution technique in the quantitative analysis of FTIR studies. Apart from that, the mobility (μ) and diffusion coefficient (D) of both cations and anions (also known as charge carriers) are determined in the transference number studies. The degree of crystallinity of PVA in polymer electrolyte is also determined using FTIR spectroscopy which is rarely reported in literatures.
Experimental
Materials
Poly(vinyl alcohol) (PVA, 99% hydrolyzed) purchased from Chemiz (Malaysia) was used as the polymer host. Lithium trifluoromethanesulfonate (LiTf, purity > 99.5%) and titanium (IV) oxide (TiO2) nano-powder (particle size 21 nm, purity ≥ 99.5%) were used as the salt and the nanofiller, respectively. Both salt and fillers were obtained from Sigma-Aldrich, USA. Activated carbon (AC) (particle size of 5.0–20.0 µm and surface area of 1800–2000 m2 g−1), multiwalled carbon nanotubes (MWCNT) (outer diameter between 7 and 15 nm and length ranging from 0.5 to 10 µm), and polyvinylidene fluoride (PVdF) with molecular weight of 534,000 g mol−1 were obtained from Aldrich, USA, whereas carbon black (Super P ®) (surface area of 62 m2 g−1) was obtained from TIMCAL Belgium S.A., Belgium. N-Methyl-2-pyrrolidone (NMP, purity ≥ 99.5%) was purchased from Merck, Germany. The AC, MWCNT, carbon black, PVdF, and NMP were used for preparation of the carbon-based electrode for EDLC fabrication.
Preparation of polymer electrolyte
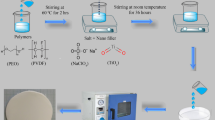
Appropriate amount of PVA was dissolved in 30 mL of distilled water followed by the addition of LiTf with a fixed ratio of 60wt.% PVA to 40 wt.% of LiTf [22]. TiO2 nanofillers were thus added into the solution. The mixture was stirred at room temperature for 24 h. In order to ensure homogeneity, the stirred solution was subjected to ultra-sonication for 45 min prior to casting and dried in an oven at 60 ℃ for 24 h. A thin film of polymer electrolyte was obtained from this step. The compositions by percentage of weight (wt. %) of PVA, LiTf, and TiO2 nanofillers used in the preparation of the polymer electrolyte samples are shown in Table 1.
Characterization of polymer electrolytes
Electrochemical impedance spectroscopy (EIS)
EIS was used to measure bulk resistance of the NCPE. The average thickness of the polymer electrolyte film was firstly measured using a digital micrometer screw gauge. The electrical impedances of the prepared samples were determined using an LCR HiTESTER impedance analyzer (model 3532–50, HIOKI, Japan). The AC voltage used for this research work was 10 mV at frequencies ranging from 50 Hz to 8 MHz. The measurement was carried out at room temperature. The temperature-dependent ionic conductivity study was also conducted by heating the sample from ambient temperature to 120 ℃. The ionic conductivities of CPEs were calculated from Eq. 1.
where σ is the ionic conductivity of the samples (S cm−1), l is the thickness of the polymer electrolyte, Rb is the bulk resistance obtained from the Nyquist impedance plot, and A is the surface area of the electrodes.
Attenuated Fourier-transform infrared spectroscopy (ATR-FTIR)
The polymer electrolyte samples were subjected to FTIR analysis using a Perkin Elmer FTIR Spectrum 100 equipped with an ATR internal reflection system to investigate the complexation between the PVA, LiTf, and TiO2 nanofillers. The samples were scanned with wavenumber ranging from 4000 to 650 cm−1 with a resolution of 4 cm−1 for a total of 64 scans at room temperature. The FTIR spectra were analyzed and deconvolution on the peaks was carried out using OMNIC 8 software. The deconvolution of FTIR spectra was done by fitting with Gaussian–Lorentzian mixed mode.
Thermogravimetry analysis
Thermogravimetry analysis (TGA) of the polymer electrolyte samples was carried out using TA instrument, Q50, starting from 25 to 800 ℃ at a heating rate of 20 ℃ min−1 under nitrogen atmosphere. Nitrogen gas flow rate was set at 60 mL min−1 with approximately 10 mg of the sample being placed on a platinum sample pan. The purpose of carrying out this analysis was to get an insight on the thermal stability and thermal degradation of polymer electrolyte sample as a function of change in temperature under an inert gas atmosphere.
Differential scanning calorimetry (DSC)
Approximately 5 mg of the polymer electrolyte sample was weighed and enclosed in an aluminum Tzero sample pan. The sample was then analyzed using a TA Instrument Universal Analyzer Q200 at nitrogen gas flow rate of 60 mL min−1 under nitrogen atmosphere, with an empty aluminum Tzero pan as the reference cell. The sample was firstly heated at a rate of 10 ℃ min−1, from 25 to 105 ℃ for dehydration purpose. The sample was then kept at 105 ℃ for 5 min to remove any residual moisture. The sample was subsequently cooled to 25 ℃, followed by a heating process at a rate of 10 ℃ min−1 from 25 to 200 ℃. The sample was cooled to − 100 ℃ and re-heated to 200 ℃ at the same cooling and heating rate. The sample was eventually cooled down to 25 ℃ before it could be removed from the system. The data obtained from the last heating process in DSC analysis was used to determine the glass transition temperature (Tg) of the CPEs.
Linear sweep voltammetry
Linear sweep voltammetry (LSV) was performed on the CPEs using an AUTOLAB Potentiostat/Galvanostat (PGSTAT204) equipped with NOVA 2.1 software. The LSV analysis was conducted at a scan rate of 0.01 V s−1 in the potential window range of ± 3 V. The polymer electrolyte sample was sandwiched between two stainless-steel electrodes throughout the analysis.
Transference number measurement
The ionic transference number (tion) of the most conductive polymer electrolyte film was measured by chronoamperometric method using an AUTOLAB Potentiostat/Galvanostat (PGSTAT204) equipped with NOVA 2.1 software. A steady supply of DC potential of 1.0 V was applied to the polymer electrolyte. The polymer electrolyte sample was sandwiched between two stainless-steel (SS) ion-blocking electrodes throughout the procedure.
Preparation of carbon-based electrodes
AC, MWCNT, carbon black, PVdF, and NMP were used to prepare the carbon slurry. PVdF served as the binder whereas NMP was used as the solvent for the carbon slurry. AC, MWCNT, carbon black, and PVdF each made up of 80, 5, 5, and 10 wt.% for preparation of carbon slurry. Appropriate amount of PVdF was dissolved in NMP solvent. Appropriate amounts of AC, MWCNT, and carbon black were added to the resulting solution to form carbon slurry. The carbon slurry was coated onto aluminum foil current collector and dried at 65 ℃ in an oven. The masses of electrodes were weighed and recorded.
Fabrication of electrochemical double layer capacitor
Electrochemical double layer capacitors (EDLCs) were fabricated by sandwiching the polymer electrolyte film between two identical electrodes, followed by pressing with a hydraulic press at 3 US tons to ensure good interfacial contact between electrolyte and electrodes. The cell was placed in a test jig for further characterization.
Characterization of the fabricated EDLC
Cyclic voltammetry
Cyclic voltammetry (CV) was carried out using AUTOLAB Potentiostat/Galvanostat (PGSTAT204) equipped with NOVA 2.1 software for analysis. The EDLC cell was subjected to the CV analysis at a scan rate of 0.01 V s−1 within the potential range between 0 and 1 V. The specific capacitance (Csp) of the EDLC was calculated using equations as follows Eq. 2 and 3:
where i is the average anodic–cathodic current (A), s is the potential scan rate (V s−1), m is the mass of active electrode materials, and A is the surface area of the electrodes.
Galvanostatic charge–discharge
Similarly, galvanostatic charge–discharge (GCD) analysis was done using AUTOLAB Potentiostat/Galvanostat (PGSTAT204) equipped with NOVA 2.1 software. The specific discharge capacitance Csp (F g−1), energy density E (W h kg−1), and power density P (kW kg−1) were determined using Eqs. 4, 5, and 6 respectively.
where i is the current applied (A), m is the average mass of active electrode materials, and \(\frac{dV}{dt}\) was obtained from the gradient of the discharge curve of the GCD response. The equivalent series resistance (ESR, Ω) of EDLC was calculated from the ohmic drop on the GCD curves using the following equation Eq. 7 [27, 28]:
where Vd is the ohmic drop before the discharge process initiates.
Results and discussions
Ionic conductivity studies
Table 2 shows the ionic conductivity of the CPE samples at ambient temperature. The ionic conductivity of polymer electrolytes shows an increasing trend up to a maximum value of (1.08 ± 0.002) × 10−4 S cm−1 at 10 wt.% of TiO2 nanofiller content. As comparison, the nanofiller-free polymer electrolyte (CPE 0) has an ionic conductivity value of (4.57 ± 0.12) × 10−8 S cm−1 at room temperature. In other words, addition of 10 wt.% of TiO2 nanofiller enhances the ionic conductivity by four orders of magnitude. Nonetheless, further addition of TiO2 nanofiller (beyond the optimum mass loading) causes an abrupt decline in ionic conductivity. The enhancement of ionic conductivity could be well-explained by the Lewis acid–base interaction between surface of TiO2 nanofiller and the polymer host which forms a new conductive pathway for ionic transport. Besides, the Lewis acid–base interaction also aids in dissociation of the LiTf salt by weakening interaction between Li+ and CF3SO3− ions, thus leading to increased amounts of free ions [11, 19]. Besides, as suggested by Li et al. (2019), the increase in ionic conductivity at high mass loadings of TiO2 fillers could be related to the space charge regions which are formed at the interface between the TiO2 nanoparticles and the matrix of PVA [29]. Space charge regions are formed by the migration of lithium ions toward the surface of the TiO2 lattice sites which cause accumulation of high concentration of positive charges at the surface sites while leaving the “negatively charged” lithium vacancies behind [29]. Upon exceeding the percolation limit, the space charge regions serve as the fast conduction pathway which facilitates the migration of lithium ions, as illustrated in Fig. 1. The decrease in conductivity as observed upon addition of 12 wt.% TiO2 loading is due to agglomeration of the nanoparticles which forms clusters that could obstruct the ionic transports, leading to decrease in ionic conductivity [30].
The TiO2 nanofiller-doped polymer electrolyte (or designated as CPE 5) has demonstrated relatively high ionic conductivity at room temperature. As comparisons, Vignarooban and co-workers (2014) reported a maximum ionic conductivity of 4.9 × 10−5 S cm−1 at 30 ℃ upon adding 10 wt.% of TiO2 nanofiller to poly(ethylene oxide) (PEO)/LiTf-based polymer electrolyte [31]. On the other hand, Polu and Rhee (2016) fabricated a nanocomposite polymer electrolyte based on PEO/lithium 2-trifluoromethyl-4,5-dicyanoimidazole (LiTDI) with incorporation of 8 wt.% of TiO2 nanofiller [19]. They reported a maximum ionic conductivity of 2.11 × 10−5 S cm−1 at room temperature for the polymer electrolyte. Arya et al. (2018) prepared polymer electrolytes based on PEO and poly(vinyl chloride) (PVC) with lithium hexafluorophosphate (LiPF6) salt with the addition of different types of ceramic nanofillers. The group found that the polymer electrolyte which consisted of 10 wt.% of TiO2 nanofiller had an ionic conductivity at the range of 10−5 S cm−1 at room temperature [32]. Hence, the synthesized CPE 5 in this present work has a great potential to be further developed and to be applied in electrochemical devices such as EDLCs.
Temperature-dependent ionic conductivity study was performed from room temperature to 120 ℃ to investigate the effect of temperature on ionic conductivity on the selected CPE samples and verify the mechanism pertaining ionic conduction within the polymer electrolytes. The temperature-dependence plots of the polymer electrolyte samples were fitted with Arrhenius equation as illustrated in Fig. 2. The activation energy (Ea) for the CPE samples was calculated and summarized in Table 2. Since the R2 values for the samples approach unity, it can therefore be concluded that the CPE samples in this present work obey Arrhenius type conductivity. The Arrhenius equation is expressed as shown in Eq. 8 [20, 33]:
where A is a constant value, Ea is the activation energy, and kB refers to the Boltzmann constant. In Arrhenius type conductivity, the transport of Li+ ions arises from ion-hopping mechanism where the cation would jump to another coordination sites in the polymer matrix [34]. From Table 2, the most conductive CPE 5 has the lowest Ea of 88.8 meV whereas the least conductive CPE 0 has the highest Ea of 446.2 meV. Lower Ea indicates that the energy barrier which has to be overcome for ionic transport is also lower [35]. As a result, ionic conductivity increases as more Li+ would be excited to a free-ion-like state and hop from one coordination site to another [36].
Attenuated total reflectance–Fourier-transform infrared spectroscopy (ATR-FTIR)
Interactions between the polymer host, lithium salt, and TiO2 nanofiller can be observed by FTIR analysis as the appearance or disappearance of peaks, the changes in peak shape and intensity as well as shifting of the position of the FTIR bands [37].
Figure 3 shows the FTIR spectra of PVA, LiTf, TiO2 nanofiller, CPE 0, and CPE 5 while Table 3 shows the vibration modes and frequency of vibration for the FTIR spectral lines. Several new peaks which were initially absent in the pristine PVA, have been observed upon incorporation of LiTf salt. The appearance of the characteristic peak of LiTf at 1645 cm−1 in addition to other peaks at 765, 1029, 1175, and 1228 cm−1 which are assigned as CF3 deformation, symmetric SO3 stretching, asymmetric and symmetric CF3 stretching modes, respectively, signifies the successful complexation between PVA and LiTf [38]. Conversely, the disappearance of the peak at 1292 cm−1 which is assigned for asymmetric SO3 stretching of LiTf also suggests complexation between the PVA matrix and LiTf salt.
As aforementioned, upward and downward shifts in the position of the FTIR peaks are also expected. When LiTf is added, several peaks originating from PVA shift to higher wavenumbers, such as peaks assigned for C-O stretching and CH2 wagging of PVA both shift from 1088 to 1093 cm−1 and from 1374 to 1384 cm−1, respectively. The doublet as observed in the FTIR spectrum of pristine PVA which initially exist at wavenumbers of 2913 and 2940 cm−1 and are assigned for symmetric and asymmetric CH stretching both shift to higher wavenumbers of 2920 and 2950 cm−1, respectively. Moreover, the peak which is formed from the overlapping of -CHOH- bending, CH3 deformation, and CH wagging of PVA also shifts from 1327 to 1337 cm−1. On the other hand, downward shifts are more prevalent in bands which originate from the LiTf salt. For example, all peaks assigned to CF3 in-plane deformation mode, symmetric SO3 stretching mode, asymmetric CF3 stretching mode, and the characteristic peak of LiTf exhibit downward shifts from their initial wavenumbers at 776 cm-1, 1045 cm-1, 1184 cm-1, and 1662 cm-1 to lower frequencies at 765 cm-1, 1029 cm-1, 1175 cm-1, and 1645 cm-1, respectively. These shifting in positions are suggestive of an interaction between the PVA polymer and LiTf salt.
As shown in Fig. 3, only two peaks are observed in the case of the pristine TiO2 nanofiller. The two peaks located at 3379 cm−1 and 1638 cm−1 are assigned for OH stretching of surface functional group of TiO2 and OH bending of water adsorbed on the TiO2 surface, respectively [39, 40]. Interactions between the PVA polymer, LiTf salt, and TiO2 nanofiller can be confirmed by comparing the deconvoluted bands from 3000 to 3700 cm−1 for both CPE 0 and CPE 5. The results of deconvolution for the said bands are displayed in Fig. 4. An additional component peak is detected in the band of CPE 5 whereas only two component peaks are observed in the band of CPE 0 at the same frequency range. The new band at 3528 cm−1 which arises from the hydrogen bonding between -OH of PVA backbone and the titanium ions while the upward shift from the initial position at 3406 cm−1 suggests the complexation between TiO2 nanofiller and the polymer matrix [41]. Furthermore, the FTIR spectrum of CPE 5 shows a slump at region below 1000 cm−1 which is characteristic of TiO2 nanofiller further proving the interaction between TiO2 nanofiller and the polymer electrolyte.
Furthermore, using deconvolution technique, the degree of crystallinity (α) of the polymer electrolytes could be estimated based on the method as ascribed by Tretinnikov and Zagorskaya (2012) using the equation as shown below Eq. 9 [42]:
where A1094 represents the intensity of absorbance of the C-O stretching band while A1144 corresponds to the C-O stretching band in the crystalline region. Fig. 5 shows deconvolution of C-O stretching band for (a) PVA, (b) CPE 0, (c) CPE 3, and (d) CPE 5.
Based on the deconvoluted curves, the degrees of crystallinity for PVA, CPE 0, CPE 3, and CPE 5 are determined and displayed in Table 4. From Table 4, the degrees of crystallinity of PVA, CPE 0, CPE 3, and GPE 5 are determined to be 23.56%, 20.76%, 14.33%, and 5.72%, respectively. This finding suggests that both LiTf and TiO2 nanofiller can enhance the amorphous nature of the PVA matrix by disrupting the orderly arrangement of the polymer chains in the crystalline region, thereby lowering the degree of crystallinity.
Apart from that, deconvolution is used to probe the dissociation of LiTf salt [43]. It is vital to verify the presence of mobile ions, ion pairs, and ion aggregates in the polymer electrolytes as the conduction in the electrolytes arises from the transportation of free ions. On the contrary, the ionic conduction would be impeded if the ion pairs and ion aggregates are formed in the electrolytes. As a result, the percentages of free ions, ion pairs, and ion aggregates are determined via deconvolution technique using the following equations Eqs. 10, 11, and 12 [44]:
where AF, AP, and AG represent area under the peaks of free ions, ion pairs, and ion aggregates, respectively. We postulate that the maximum ionic conductivity of CPE 5 is due to the formation of high amounts of free ions as a result of the Lewis acid–base interaction which helps in dissociating the LiTf salt by weakening interaction between Li+ and CF3SO3− ions, as aforementioned in “Ionic conductivity studies.” The result is in good agreement with the ambient temperature-ionic conductivity analysis as CPE 0 shows the lowest percentage of free ions and the highest percentage of ion pairs, whereas CPE 5 illustrates the highest percentage of free ions and the lowest percentage of ion pairs, as shown in Fig. 6. Similarly, the percentage of free ions decreases to 77.64% upon addition of 12 wt.% of TiO2 nanofiller to the electrolyte. It is mainly due to formation of TiO2 agglomerates that reduces the total surface area for Lewis acid–base interaction to occur. The percentages of free ions, ion pairs, and ion aggregates for all polymer electrolyte films were determined accordingly and summarized in Table 5.
Thermogravimetric analysis
Thermogravimetric analysis (TGA) is used to assess the thermal stability of the polymer electrolyte samples. Figure 7 shows the thermogravimetric curves of pristine PVA, filler-free CPE 0, CPE 3 and CPE 5. As per the TGA curves, a small step can be observed in all samples from temperature ranging from 55 to 140 ℃, where PVA, CPE 0, CPE 3, and CPE 5 show a weight loss of 2.50%, 7.94%, 8.32%, and 7.21%, respectively. Weight loss at this temperature range is because of the evaporation of water, moisture, and solvent trapped in the polymer matrix. CPE 0, CPE 3, and CPE 5 experience higher weight losses at this temperature regime as compared to the pristine PVA since all of the polymer electrolyte samples contain LiTf salt which is highly hygroscopic and thus more moisture is absorbed by the CPE samples. A major weight loss can be observed at temperatures between 300–355℃, 279–303℃, 277–300℃, and 274–303℃ for PVA, CPE 0, CPE 3, and CPE 5, respectively, due to elimination of OH groups of the PVA backbone. Several degradation pathways may occur in this segment. A rapid chain-stripping process which involves OH functional groups and β-hydrogens on the PVA main chain could result in liberation of water from PVA backbone and formation of conjugated and non-conjugated polyenes as shown below [45,46,47].

In contrast, conjugated polyenes are rather stable and will not degrade further at this temperature regime; non-conjugated polyenes, on the other hand, still contain OH groups that are attached onto the polyene backbone which destabilize the regular structure. Hence, non-conjugated polyenes may undergo further degradation to form smaller compounds such as acetaldehyde, acetone, furan and polyenes with low molecular weight [47]. Alternatively, this elimination of water may form ether cross-linkages between two PVA polymer chains, thus joining them together.
The subsequent degradation step occurs at temperatures above 400 ℃. Pristine PVA, CPE 0, CPE 3, and CPE 5 experience weight losses of 27.05%, 41.90%, 35.79%, and 27.94%, at temperature ranges of 411–434℃, 426–470℃, 426–463℃, and 425–463℃, respectively. The increase in temperature range for thermal degradation to happen indicates improved thermal stability. Weight loss at this temperature regime is attributed to chemical decomposition processes such as degradation of ether cross-linkages and random chain scission of carbon–carbon bond of the PVA main chain [48]. High temperature at this degradation step provides sufficient energy to further break down the stable conjugated polyenes formed in the previous degradation step into low molecular weight polyenes through a random chain-scission mechanism as illustrated below [47]:

Additionally, some other products such as benzenoid derivative are also formed from Diels–Alder reaction and intramolecular cyclization reaction of conjugated polyenes as illustrated as follows [47]:

Addition of LiTf into PVA results in sudden increase in weight loss from 27.05% to 41.90% due to decomposition of LiTf which occurs simultaneously at this temperature regime [49].
Mass loadings of TiO2 nanofiller do not show significant effect to the thermal stability of the PVA-based polymer electrolytes in this present work since the TGA curves of CPE 0, CPE 3, and CPE 5 show similar degradation temperatures. On the overall, CPE 5 was found to have the highest amount of residue as compared to pristine PVA and CPE 0. The residue could be constituted of the TiO2 nanofiller that can withstand degradation even at high temperatures.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) analysis was carried out to study the changes in Tg of PVA polymer upon addition of LiTf salt as well as TiO2 nanofiller. The DSC thermograms of PVA, CPE 0, CPE 3, and CPE 5 are depicted in Fig. 8. From the DSC thermogram, the Tg of PVA is found to be 78.51 ℃. Inclusion of LiTf lowers the Tg due to plasticizing effect of the LiTf salt [50]. However, Tg has shifted to higher temperatures of 81.71 ℃ and 83.82 ℃ as can be observed for CPE 3 and CPE 5, respectively. This increase in Tg is caused by interaction between TiO2 nanoparticles and PVA polymer matrix which leads to the increased rigidity and restriction of thermal actions of the polymeric backbone, as illustrated below 51.

Despite the increment of Tg, ionic conductivity of TiO2 nanofiller-added CPE was found to be improved with increasing amount of TiO2 nanofiller. One possible explanation for this observation is that TiO2 enhances the ionic conductivity via a mechanism that is not dependent on the Tg [52]. This additional conduction mechanism could counteract the detrimental effect caused by increased Tg; therefore, the overall ionic conductivity was improved.
Linear sweep voltammetry (LSV)
Fig. 9 shows the linear sweep voltammogram of (a) CPE 0 and (b) CPE 5. From Fig. 9, the cathodic and anodic potentials of the filler-free CPE 0 are found to be − 2.4 V and 2.6 V, respectively, whereas the cathodic and anodic potentials of filler-added CPE 5 are − 2.4 V and 2.55 V, respectively. Both CPE 0 and CPE 5 exhibit very comparable potential window range of 5 V and 4.95 V, respectively. In this work, no significant change in the potential window was observed after addition of TiO2 nanofiller. This could be due to the amount of TiO2 nanofiller added being too little, so it has insignificant effect on the potential window range. Nonetheless, both polymer electrolytes in this present work have wider potential windows as compared to other polymer electrolytes [53,54,55].
Transference number
Transference number measurement was performed on the most conductive CPE 5 in this work and the chronoamperometry curve obtained is illustrated in Fig. 10. As can be seen in Fig. 10, the initial total current (It) is the combination of the ionic current (Iion) and electronic current (Ielectron). Since SS electrode is blocking to ions, at steady state, electrons are the only charge carrier contributing to charge transfer process. In other words, the steady state current (Iss) only consists of the electronic current (Ielectron) [56]. The drastic decline in current as polarization goes on is thus due to lesser mobile charge carrier which is able to transfer the charge. The ionic transference number can therefore be calculated using Eqs. 13 and 14 as follows [57].
where telectron is the electronic transference number and tion is the ionic transference number. As seen in Fig. 10, It and Iss were found to be 8.8532 × 10−5 A and 5.4352 × 10−8 A, respectively. The tion value of CPE 5 is therefore determined to be 0.9994, which is very close to unity. This infers that charge carriers in CPE 5 are primly constituted of ions. Based on the result, we conclude that CPE 5 is an ion conductor. Subsequently, the ionic transport parameters such as mobility of cations (μ+) and anions (μ−) are calculated using Eqs. 15 and 16 while coefficients of diffusion of cations (D+) and anions (D−) are calculated using Eqs. 17 and 18 [58, 59]:
where μ+ and μ− are cationic and anionic mobility, respectively; n is the charge carrier number based on the composition of salt; e is the charge of electron; D+ and D− are coefficients of diffusion of cation and anions, respectively; k is the Boltzmann constant; and T is the absolute temperature. Based on the calculations, the μ+ and μ− are found to be 1.7292 × 10−6 cm2 V−1 S−1 and 1.0 × 10−9 cm2 V−1 S−1, respectively. On the contrary, D+ and D− are 1.2450 × 10−8 cm2 s−1 and 7.0 × 10−12 cm2 s−1, respectively. Both of the ionic mobility and coefficient of diffusion of the cations are relatively higher than those of the anions. Therefore, it can be concluded that the major contributors of the ionic conductivity of CPE 5 are the Li+ cations.
Cyclic voltammetry (CV)
Figure 11 shows the cyclic voltammograms of EDLC fabricated using CPE 5 at scan rates of 0.01, 0.03, 0.06, and 0.1 V s−1, whereas Fig. 12 depicts the comparison of CV curves of EDLCs fabricated using CPE 0 and CPE 5 at fixed scan rate of 0.01 V s−1. The specific capacitance of EDLC fabricated using CPE5 at different scan rates is summarized in Table 6. From both figures, redox peaks are clearly absent from the CV curves of the EDLCs fabricated in this work. Absence of redox peaks signifies that charge storage ability of the EDLC cells arises from the adsorption–desorption of ions at the electrode–electrolyte interface which forms an electric double layer (also called Helmholtz layer) without any redox reaction taking place in the cell. Non-Faradaic reaction is responsible for the charge storage in the EDLC cell [60]. From Fig. 11, it appears that at the low scan rate of 0.01 V s−1, the CV curve is closer to being a rectangular shape while at higher scan rates, CV curve deviates toward a leaf-like shape. The deviation from ideal rectangular shape in the CV response is mainly attributed to the porosity of the carbon-based electrode and internal resistance of the EDLC causing a current–voltage dependency [61, 62]. Furthermore, the CV curve at low scan rate of 0.01 V s−1 shows a flat plateau region, which signifies that ion migration occurs at a slow and steady rate. As a result, ions can accumulate properly at the electrode–electrolyte interface with low ohmic resistance, leading to higher specific capacitance. As shown in the table below, the specific capacitance of the EDLC fabricated with CPE 5 has a value of 444.06 mF g−1 (or equivalent to 3.87 mF cm−2) at scan rate of 0.01 V s−1. The value decreases gradually to 99.51 mF g−1 (or equivalent to 0.67 mF cm−2) as the scan rate was increased to 0.1 V s−1.
In order to illustrate the effect of addition of TiO2 nanofillers, cyclic voltammetry was also performed on an EDLC fabricated with the TiO2 nanofiller-free polymer electrolyte (CPE 0) at a single scan rate of 0.01 V s−1 as shown in Fig. 12. The specific capacitance of the TiO2 nanofiller-free CPE 0 was found to be 96.62 mF g−1 (or equivalent of 0.39 mF cm−2). Nonetheless, upon incorporation of TiO2 nanofiller, the specific capacitance was found to improve significantly by ~ 360% (in terms of mF g−1) and by ~ 892% (in terms of mF cm−2) to 444.06 mF g−1 (or equivalent of 3.87 mF cm−2), respectively. The specific capacitance obtained in this work is found to be higher than several literatures. For example, Senokos et al. (2018) fabricated an EDLC by sandwiching poly(vinylidene fluoride-co-hexafluoropropylene) (PVdF-HFP)-based solid polymer electrolyte added with 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquid between of two carbon nanotube fiber sheets. The specific capacitance was reported to be 88 mF g−1 [63]. Besides, Snyder et al. (2006) fabricated a cell which consisted of polymer electrolyte formed with crosslinked tetraethylene glycol dimethacrylate and methoxy poly(ethylene glycol)-acrylate added with lithium imide salt and the specific capacitance of the cell was found to be 35 mF g−1 [64]. On the other hand, Xu et al. (2021) formed EDLCs using solid polymer electrolyte based on poly(vinylidene fluoride), lithium triflate, and epoxy resin. Their EDLC was reported to have a specific capacitance of 11.62 mF g−1 (equivalent to 0.128 mF cm−2) [65].
The tremendous improvement of the specific capacitance is mainly attributed to the enhanced ionic conductivity of the CPE upon inclusion of TiO2 nanofiller. As aforementioned, TiO2 nanofiller can promote dissociation of LiTf salt via Lewis acid–base interactions. This contributes to a higher density of mobile Li+ ions in the electrolyte which in turn results in more Li+ ions being able to be adsorbed and desorbed on the electrode surface. Furthermore, the additional conductive pathway formed by TiO2 nanofiller enables a more rapid Li+ transport across the polymer matrix, allowing for a rapid formation of the Helmholtz double layer. All these factors thus lead to an overall increase in the specific capacitance of the EDLC.
Galvanostatic charge–discharge
Galvanostatic charge–discharge (GCD) is used to assess the electrochemical stability and specific discharge capacitance of the EDLC cell fabricated with the most conductive polymer electrolyte (CPE 5). The GCD curves of the fabricated EDLC over the first five cycles are illustrated in Fig. 13. The linear discharge curves further confirm that the charge storage mechanism in the fabricated cell follows those of typical EDLC which involves non-Faradaic reactions instead of redox reactions [60, 66]. A small voltage drop can be observed as a gap at the beginning of each discharge process. This voltage drop is caused by ohmic loss due to the existence of equivalent series resistance (ESR), which can be thought as the internal resistance of the EDLC. ESR is caused by several factors such as the resistance of the current collector, interfacial resistances at the electrode–electrolyte interface and electrode-current collector interface, as well as resistances of the polymer electrolyte, electrode active material, and the circuit itself. The values of ESR were calculated using Eq. 7 and summarized in Table 7.
As can be seen from Table 7, the value of ESR generally shows an increasing trend with number of cycles albeit with some fluctuation. From the first to the 1000th cycle, ESR has increased by 62.79% from the initial value of 729 Ω to 1187 Ω, respectively. Azli et al. (2020) explained that this phenomenon can be attributed to the irreversible formation of ion pairs due to rapid desorption of ions on the cathode and anode during charge–discharge cycles. Formation of ion pairs leads to depletion of ions in the electrolyte which ultimately increases the ESR [67]. Moreover, ion pairs could hinder the ionic transport within the electrode which further increases the ESR. We noticed that the ESR values of the EDLC in this present work are relatively high as compared to other literatures [60, 66]. We speculate that the high ESR as observed in this present work is due to poor interfacial contact between the electrode and the electrolyte which causes high interfacial resistance.
Furthermore, specific discharge capacitance (Csp) from GCD profile of the fabricated EDLC over 1000 cycles is also summarized in Table 7. The initial increase in Csp at charge–discharge cycles below 400 cycles could arise from electrical conditioning of the carbon electrodes’ surface which makes them to become more receptive for adsorption and desorption of ions [68]. Csp of the fabricated EDLC becomes stable beyond the 400th cycle with Csp values within the range of 20 mF g−1 to 90 mF g−1. Overall, from the first to the 1000th cycle, capacitance of the EDLC declines by approximately 44% from 99.56 mF g−1 to 56.11 mF g−1. As mentioned earlier, ion pairs can form during charge–discharge cycles. These ion pairs can obstruct the adsorption of ions into the porous structure of the carbon electrode, thereby reducing the accumulation of ions at the electrode–electrolyte interface, leading to decline in specific discharge capacitance [28].
Finally, energy density and power density of the fabricated EDLC are found to have average values of 0.01 W h kg−1 and 1.04 kW kg−1, respectively, over 1000 cycles. From the first to the last cycle, energy density and power density are found to be decreased by 56.94% and 12.43%, respectively. This deterioration of energy and power densities can be attributed to the increased ESR as aforementioned [69]. Formation of ion pairs could make the ions being unable to be efficiently adsorbed onto the porous surface of the carbon-based electrodes, resulting in decreased energy density. The energy density of the EDLC in this present work is comparable to those reported by Shirshova et al. (2013) where their study on different prototypes of supercapacitors shows a maximum energy density and power density of ~ 0.01 W h kg−1 and ~ 0.09 kW kg−1 [70]. Nevertheless, the electrochemical stability of the fabricated EDLC in this present work is still considered stable over the course of 1000 cycles of charge–discharge process. Thus, CPE 5 is still a potential candidate to be further improved and used for EDLC applications.
Conclusion
TiO2 nanofiller-added CPEs have been formed by solution casting technique in this present work. Ionic conductivity of the CPE was found to be increased by four orders of magnitude from (4.57 ± 0.12) × 10−8 S cm−1 to (1.08 ± 0.002) × 10−4 S cm−1 at room temperature upon addition of 10 wt.% of TiO2 nanofiller. This enhancement of ionic conductivity is due to the Lewis acid–base interaction between the surface of TiO2 nanofiller and the PVA polymer that forms an alternative conduction pathway for ionic transport. The interaction can also promote dissociation of LiTf salt which helps to produce more mobile ions that increases the ionic conductivity. Temperature-dependent ionic conductivity study shows that the CPE samples obey Arrhenius type conductivity which is related to thermally activated ion hopping mechanism. Complexation of PVA, LiTf, and TiO2 nanofiller has been proven by studying the FTIR spectra. TGA study of the CPE samples shows that TiO2 nanofiller does not have a significant role in terms of improving the thermal stability of the CPE. DSC analysis shows increasing glass transition temperature (Tg) of the PVA polymer upon inclusion of TiO2 nanofiller. This suggests an interaction between the TiO2 nanofiller and the PVA polymer which causes increased rigidity in the polymer backbone. The most conductive polymer electrolyte, CPE 5, was found to be stable up to 4.95 V. Besides, the ionic transference number measurement of CPE 5 confirms that ions are responsible for the conductivity across the polymer electrolyte. Moreover, comparison between the specific capacitance of the EDLCs fabricated with filler-free CPE 0 and the most conductive CPE 5 shows an improvement by ~ 360% (in terms of mF g−1) or ~ 892% (in terms of mF cm−2) which is from 96.62 mF g−1 (or 0.39 mF cm−2) to 444.06 mF g−1 (or 3.87 mF cm−2) when 10 wt.% of TiO2 nanofiller is added. The fabricated EDLC displayed electrochemical stability up to 1000 cycles of charge–discharge process. TiO2 nanofiller-added CPE is a potential candidate to be applied in EDLC since it has good electrochemical properties which can also be maintained over 1000 charge–discharge cycles.
References
Guan L, Yu L, Chen GZ (2016) Capacitive and non-capacitive faradaic charge storage. Electrochim Acta 206:464–478
Noorden ZA, Sugawara S, Matsumoto S (2014) Noncorrosive separator materials for electric double layer capacitor. IEEJ Trans Electr Electron Eng 9:235–240
Koda K, Taira S, Kubota A, Isozaki T, You X, Uraki Y, Sugimura K, Nishio Y (2019) Development of lignin-based terpolyester film and its application to separator material for electric double-layer capacitor. J Wood Chem Technol 39:198–213
Vargun E, Ozaltin K, Fei H, Harea E, Vilčáková J, Kazantseva N, Saha P (2020) Biodegradable porous polylactic acid film as a separator for supercapacitors. J Appl Polym Sci 137:1–15
Arya A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23:497–540
You X, Misra M, Gregori S, Mohanty AK (2018) Preparation of an electric double layer capacitor (EDLC) using Miscanthus-derived biocarbon. ACS Sustain Chem Eng 6:318–324
Zainuddin NK, Rasali NMJ, Mazuki NF, Saadiah MA, Samsudin AS (2020) Investigation on favourable ionic conduction based on CMC-K carrageenan proton conducting hybrid solid bio-polymer electrolytes for applications in EDLC. Int J Hydrogen Energy 45:8727–8741
Asnawi ASFM, Aziz SB, Nofal MM, Hamsan MH, Brza MA, Yusof YM, Abdilwahid RT, Muzakir SK, Kadir MFZ (2020) Glycerolized Li+ ion conducting chitosan-based polymer electrolyte for energy storage EDLC device applications with relatively high energy density. Polymers (Basel) 12:1–19
Ye L, Feng Z (2010) Polymer electrolytes as solid solvents and their applications. In: Sequeira C, Santos D (eds) Polym Electrolytes Fundam Appl, 1st edn. Woodhead Publishing Limited, Cambridge, pp 550–582
Mauger J, Paolella A, Zaghib, (2019) Building better batteries in the solid state: a review. Materials (Basel) 12:3892
Premila R, Subbu C, Rajendran S, Selva kumar K (2018) Experimental investigation of nano filler TiO2 doped composite polymerelectrolytes for lithium ion batteries. Appl Surf Sci 449:426–434
Prabakaran P, Manimuthu RP, Gurusamy S, Sebasthiyan E (2017) Plasticized polymer electrolyte membranes based on PEO/PVdF-HFP for use as an effective electrolyte in lithium-ion batteries. Chinese J Polym Sci 35:407–421
Peng J, Wu LN, Lin JX, Shi CG, Fan JJ, Bin CL, Dai P, Huang L, Li JT, Sun SG (2019) A solid-state dendrite-free lithium-metal battery with improved electrode interphase and ion conductivity enhanced by a bifunctional solid plasticizer. J Mater Chem A 7:19565–19572
Malhotra S, Varshney PK (2015) Effect of fillers on composite polymer electrolytes- a study. Int J Appl Eng Res 10:108–112
Chen L, Li Y, Li SP, Fan LZ, Nan CW, Goodenough JB (2018) PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic.” Nano Energy 46:176–184
Huo H, Chen Y, Luo J, Yang X, Guo X, Sun X (2019) Rational design of hierarchical “ceramic-in-polymer” and “polymer-in-ceramic” electrolytes for dendrite-free solid-state batteries. Adv Energy Mater 9:1–8
Zhang X, Liu T, Zhang S, Huang X, Xu B, Lin Y, Xu B, Li L, Nan CW, Shen Y (2017) Synergistic coupling between Li6.75La3Zr175Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J Am Chem Soc 139:13779–13785
Yao P, Yu H, Ding Z, Liu Y, Lu J, Lavorgna M, Wu J, Liu X (2019) Review on polymer-based composite electrolytes for lithium batteries. Front Chem 7:1–17
Polu AR, Rhee HW (2016) Effect of TiO2 nanoparticles on structural, thermal, mechanical and ionic conductivity studies of PEO12-LiTDI solid polymer electrolyte. J Ind Eng Chem 37:347–353
Ganesan SV, Mothilal KK, Selvasekarapandian S, Ganesan TK (2018) The effect of titanium dioxide nano-filler on the conductivity, morphology and thermal stability of poly(methyl methacrylate)—poly(styrene-co-acrylonitrile) based composite solid polymer electrolytes. J Mater Sci Mater Electron 29:8089–8099
Tan X, Wu Y, Tang W, Song S, Yao J, Wen Z, Lu L, Savilov SV, Hu N, Molenda J (2020) Preparation of nanocomposite polymer electrolyte via in situ synthesis of SiO2 nanoparticles in PEO. Nanomaterials 10:1–12
Tan HW, Ramesh S, Liew CW (2019) Electrical, thermal, and structural studies on highly conducting additive-free biopolymer electrolytes for electric double-layer capacitor application. Ionics (Kiel) 25:4861–4874
Albu AM, Maior I, Nicolae CA, Bocăneală FL (2016) Novel Pva proton conducting membranes doped with polyaniline generated by in-situ polymerization. Electrochim Acta 211:911–917
Abdullah OG, Aziz SB, Rasheed MA (2018) Incorporation of NH4NO3 into MC-PVA blend-based polymer to prepare proton-conducting polymer electrolyte films. Ionics (Kiel) 24:777–785
Yusof YM, Illias HA, Kadir MFZ (2014) Incorporation of NH4Br in PVA-chitosan blend-based polymer electrolyte and its effect on the conductivity and other electrical properties. Ionics (Kiel) 20:1235–1245
Badr S, Sheha E, Bayomi RM, El-Shaarawy MG (2009) Structural and electrical properties of pure and H2SO4 doped (PVA)0.7(NaI)0.3 solid polymer electrolyte. Ionics 16:269–275
Muchakayala R, Song S, Wang J, Fan Y, Bengeppagari M, Chen J, Tan M (2018) Development and supercapacitor application of ionic liquid-incorporated gel polymer electrolyte films. J Ind Eng Chem 59:79–89
Das S, Ghosh A (2017) Solid polymer electrolyte based on PVDF-HFP and ionic liquid embedded with TiO2 nanoparticle for electric double layer capacitor (EDLC) application. J Electrochem Soc 164:F1348–F1353
Li Z, Huang HM, Zhu JK, Wu JF, Yang H, Wei L, Guo X (2019) Ionic conduction in composite polymer electrolytes: case of PEO:Ga-LLZO composites. ACS Appl Mater Interfaces 11:784–791
Banitaba SN, Semnani D, Rezaei B, Ensafi AA (2019) Evaluating the electrochemical properties of PEO-based nanofibrous electrolytes incorporated with TiO2 nanofiller applicable in lithium-ion batteries. Polym Adv Technol 30:1234–1242
Vignarooban K, Dissanayake MAKL, Albinsson I, Mellander BE (2014) Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ionics 266:25–28
Arya A, Saykar NG, Sharma AL (2018) Impact of shape (nanofiller vs. nanorod) of TiO2 nanoparticle on free-standing solid polymeric separator for energy storage/conversion devices. J Appl Polym Sci 136:1–15
Bandara TMWJ, Karunathilaka DGN, Ratnasekera JL, Ajith De Silva L, Herath AC, Mellander BE (2017) Electrical and complex dielectric behaviour of composite polymer electrolyte based on PEO, alumina and tetrapropylammonium iodide. Ionics (Kiel) 23:1711–1719
Aziz SB, Woo TJ, Kadir MFZ, Ahmed HM (2018) A conceptual review on polymer electrolytes and ion transport models. J Sci Adv Mater Devices 3:1–17
Aziz SB, Abdullah RM, Rasheed MA (2017) Role of Ion Dissociation on DC Conductivity and Silver Nanoparticle Formation in PVA:AgNt Based Polymer Electrolytes: Deep Insights to Ion Transport Mechanism. Polymers 9:338
Ahmed HT, Abdullah OG (2020) Structural and ionic conductivity characterization of PEO:MC-NH4I proton- conducting polymer blend electrolytes based film. Results Phys 16:102861
Mohamed AS, Shukur MF, Kadir MFZ, Yusof YM (2020) Ion conduction in chitosan-starch blend based polymer electrolyte with ammonium thiocyanate as charge provider. J Polym Research
Ali AMM, Subban RHY, Bahron H, Winie T, Latif F, Yahya MZA (2008) Grafted natural rubber-based polymer electrolytes: ATR-FTIR and conductivity studies. Ionics (Kiel) 14:491–500
León A, Reuquen P, Garín C, Segura R, Vargas P, Zapata P, Orihuela PA (2017) FTIR and raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl Sci 7:1–9
Haghighat N, Vatanpour V, Sheydaei M, Nikjavan Z (2020) Preparation of a novel polyvinyl chloride (PVC) ultrafiltration membrane modified with Ag/TiO2 nanoparticle with enhanced hydrophilicity and antibacterial activities. Sep Purif Technol 237:116374
Abdel-Hady EE, Mohamed HFM, Abdel-Hamed MO, Gomaa MM (2019) Physical and electrochemical properties of PVA/TiO2 nanocomposite membrane. Adv Polym Technol 37:3842–3853
Tretinnikov ON, Zagorskaya SA (2012) Determination of the degree of crystallinity of poly(Vinyl alcohol) by ftir spectroscopy. J Appl Spectrosc 79:521–526
Huang W, Frech R (1992) Raman spectra of PPO-salt complexes: mixed cations and mixed anions. Solid State Ionics 53–56:1095–1101
Aziz SB, Asnawi ASFM, Kadir MFZ et al (2001) Structural, Electrical and Electrochemical Properties of Glycerolized Biopolymers Based on Chitosan (CS): Methylcellulose (MC) for Energy Storage Application. Polymers 13:1183
Alexy P, Káchová D, Kršiak M, Bakoš D, Šimková B (2002) Poly(vinyl alcohol) stabilisation in thermoplastic processing. Polym Degrad Stab 78:413–421
Stoševski I, Krstić J, Vokić N, Radosavljević M, Popović ZK, Miljanić Š (2015) Improved poly(vinyl alcohol) (PVA) based matrix as a potential solid electrolyte for electrochemical energy conversion devices, obtained by gamma irradiation. Energy 90:595–604
Peng Z, Kong LX (2007) A thermal degradation mechanism of polyvinyl alcohol/silica nanocomposites. Polym Degrad Stab 92:1061–1071
Gilman, JW, VanderHart DL, Kashiwagi T (1995) Thermal Decomposition Chemistry of Poly(vinyl alcohol): Char Characterization and Reactions with Bismaleimides. In: Nelson GL (ed) Fire and Polymer II: Materials and Tests for Hazard Prevention, 1st edn. Washington: American Chemical Society, pp 161–185. https://doi.org/10.1021/bk-1995-0599.ch011
Ganesan SV, Mothilal KK, Ganesan TK (2018) The role of zirconium oxide as nano-filler on the conductivity, morphology, and thermal stability of poly(methyl methacrylate)–poly(styrene-co-acrylonitrile)-based plasticized composite solid polymer electrolytes. Ionics 24:3845–3860
Kingslin Mary Genova F, Selvasekarapandian S, Vijaya N, Sivadevi S, Premalatha M, Karthikeyan S (2017) Lithium ion-conducting polymer electrolytes based on PVA–PAN doped with lithium triflate. Ionics (Kiel) 23:2727–2734
Ahmad J, Deshmukh K, Habib M, Hägg MB (2014) Influence of TiO2 nanoparticles on the morphological, thermal and solution properties of PVA/TiO2 nanocomposite membranes. Arab J Sci Eng 39:6805–6814
Eriksson T, Mindemark J, Yue M, Brandell D (2019) Effects of nanoparticle addition to poly(ε-caprolactone) electrolytes: crystallinity, conductivity and ambient temperature battery cycling. Electrochim Acta 300:489–496
Noor NAM, Isa MIN (2019) Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int J Hydrogen Energy 44:8298–8306
Wang J, Chen G, Song S (2019) Na-ion conducting gel polymer membrane for flexible supercapacitor application. Electrochimica Acta 330:135322
Abdulkadir BA, Dennis JO, Al-Hadeethi Y, Shukur MFBA, Mkawi EM, Al-Harbi N, Ibnaouf KH, Aldaghri O, Usman F, Abbas Adam A (2021) Optimization of the electrochemical performance of a composite polymer electrolyte based on PVA-K2 CO3-SiO2 composite. Polymers (Basel) 13:1–24
Isa KBM, Othman L, Hambali D, Osman Z (2017) Electrical and Electrochemical Studies on Sodium Ion-based Gel Polymer Electrolytes. AIP Conf Proc 1877:040001
Shukur MF, Ithnin R, Kadir MFZ (2016) Ionic conductivity and dielectric properties of potato starch-magnesium acetate biopolymer electrolytes: the effect of glycerol and 1-butyl-3-methylimidazolium chloride. Ionics (Kiel) 22:1113–1123
Radha KP, Selvasekarapandian S, Karthikeyan S, Hema M, Sanjeeviraja C, Pandi DV (2014) Characterization of composite proton conducting polymer electrolyte based on poly(vinyl alcohol). Int J Electroact Mater 2:28–33
Winie T, Arof AK (2006) Transport properties of hexanoyl chitosan-based gel electrolyte. Ionics (Kiel) 12:149–152
Dannoun EMA, Aziz SB, Brza MA, Nofal MM, Asnawi ASFM, Yusof YM, Al-Zangana S, Hamsan MH, Kadir MFZ, Woo HJ (2020) The study of plasticized solid polymer blend electrolytes based on natural polymers and their application for energy storage EDLC devices. Polymers (Basel) 12:1–19
Hamsan MH, Aziz SB, Kadir MFZ, Brza MA, Karim WO (2020) The study of EDLC device fabricated from plasticized magnesium ion conducting chitosan based polymer electrolyte. Polym Test 90:106714
Aziz SB, Nofal MM, Abdulwahid RT, Ghareeb HO, Dannoun EMA, Abdullah RM, Hamsan MH, Kadir MFZ (2021) Plasticized sodium-ion conducting pva based polymer electrolyte for electrochemical energy storage—eec modeling, transport properties, and charge-discharge characteristics. Polymers (Basel) 13:1–19
Senokos E, Ou Y, Torres JJ, Sket F, González C, Marcilla R, Vilatela JJ (2018) Energy storage in structural composites by introducing CNT fiber/polymer electrolyte interleaves. Sci Rep 8:1–10
Snyder JF, Carter RH, Wong EL, Nguyen PA, Xu K, Ngo EH, Wetzel ED (2006) Multifunctional structural composite batteries. Int. SAMPE Tech. Conf
Xu Y, Pei S, Yan Y, Wang L, Xu G, Yarlagadda S, Chou TW (2021) High-performance structural supercapacitors based on aligned discontinuous carbon fiber electrodes and solid polymer electrolytes. ACS Appl Mater Interfaces 13:11774–11782
Aziz SB, Hamsan MH, Brza MA, Kadir MFZ, Muzakir SK, Abdulwahid RT (2020) Effect of glycerol on EDLC characteristics of chitosan:methylcellulose polymer blend electrolytes. J Mater Res Technol 9:8355–8366
Azli AA, Manan NSA, Aziz SB, Kadir MFZ (2020) Structural, impedance and electrochemical double-layer capacitor characteristics of improved number density of charge carrier electrolytes employing potato starch blend polymers. Ionics 26:5773–5804
Adams K, Mallows J, Li T, Kampouris D, Thijssen JHJ, Robertson N (2019) Cs3Bi2I9 as high-performance electrode material achieving high capacitance and stability in an economical supercapacitor. J Phys Energy 1:034001
Aziz SB, Hamsan MH, Karim WO et al (2019) High Proton Conducting Polymer Blend Electrolytes Based on Chitosan: Dextran with Constant Specific Capacitance and Energy Density. Biomolecules 9:267
Shirshova N, Qian H, Shaffer MSP, Steinke JHG, Greenhalgh ES, Curtis PT, Kucernak A, Bismarck A (2013) Structural composite supercapacitors. Compos Part A Appl Sci Manuf 46:96–107
Funding
This work was supported by the Fundamental Research Grant Scheme (FRGS/1/2019/STG07/TARUC/02/1) from the Ministry of Higher Education of Malaysia.
Author information
Authors and Affiliations
Contributions
Austin Yuhang Yap was involved in formal analysis, investigation, and writing of the original manuscript, and Chiam-Wen Liew was involved in review and editing of the manuscript, resources, funding acquisition, project administration, and supervision.
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yap, A.Y., Liew, CW. Structural, thermal, and electrochemical studies on PVA-LiTf-TiO2 nanocomposite polymer electrolyte and the performance on electric double layer capacitor application. Ionics 28, 4111–4128 (2022). https://doi.org/10.1007/s11581-022-04659-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04659-4