Abstract
Gel polymer electrolytes are one of the candidates for solid electrolytes to solve safety issues and improve the energy density of solid-state lithium batteries. Gel polymer electrolyte has high ionic conductivity at room temperature (~ 10−3 S cm−1), but its mechanical properties are poor. Herein, we prepared a series of composite gel polymer electrolytes by N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide, poly(vinylidene fluoride-hexafluoropropylene), LiTFSI, and various inorganic fillers. The addition of 5 wt% TiO2 can not only increase the ionic conductivity of gel polymer electrolytes but also improve the mechanical properties of gel polymer electrolytes. Besides, as a semiconductor, the TiO2 has safety risk when uses in composite gel polymer electrolyte. TiO2-ILGPE safety is investigated by cyclic voltammetry and battery performance. Additionally, TiO2-ILGPE displays a perfect flame-retarding ability, and TiO2 would not be reduced by the lithium metal anode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries are widely used in electronic devices, hybrid vehicles, and vehicles [1,2,3]. However, lithium-ion batteries have reached their bottleneck, in which energy density and power density are limited by the electrode materials [4, 5]. Solid-state lithium metal batteries are one of the candidate’s electrolytes for achieving high energy density [6, 7]. Solid-state lithium metal batteries use lithium metal as the electrode, and its theoretical capacity is ten times than that of the graphite. Furthermore, solid-state lithium metal batteries use solid electrolytes to replace commercial organic liquid electrolytes, which can solve the safety issues.
Generally, solid-state electrolytes are classified into inorganic solid-state electrolytes, polymer electrolytes, and gel polymer electrolytes [8, 9]. Inorganic solid electrolytes are rigid, which causes interfacial contact issues and extremely high interfacial resistance [10,11,12]. Polymer electrolytes have low ionic conductivity at room temperature (< 10−4 S cm−1) [13, 14]. Gel polymer electrolytes are prepared by adding a plasticizer to polymer electrolytes to improve the ionic conductivity, where ionic conductivity is about 10−3 S cm−1 [15,16,17,18,19]. However, the mechanical properties of gel polymer electrolytes are poor, and filling with inorganic particles can improve that. It is a common method to improve the mechanical properties of polymer electrolytes by adding inorganic particles. Therefore, many inorganic particles have been composited into polymer electrolytes to improve mechanical properties and electrochemical performance [20,21,22,23,24]. Tambelli et al. [25] reported that the highest room temperature ionic conductivity of PEO-Al2O3 composite polymer electrolytes was less than 10−4 S cm−1. Xiao et al. [25] fabricated a high-performance composite polymer electrolyte doped with Li0.1Ca0.9TiO3 particles with a high room temperature ionic conductivity of 3.947 mS cm−1, where the as-prepared CPE membranes needed to be immersed into an organic electrolyte for 0.5 h before the test. Liu group [26] used Li0.33La0.557TiO3 nanowires as fillers to enhance the ionic conductivity of polymer electrolyte, and the ionic conductivity was 0.24 mS cm−1 at room temperature. Others [27,28,29,30,31,32] reported inorganic particle composite polymer electrolytes, where the room temperature ionic conductivity was around < 10−4 S cm−1.
In our previous work [33], we prepared a high-performance ionic liquid gel polymer electrolyte (ILGPE), which exhibited good battery performance. We utilized an ionic liquid as polymer electrolytes plasticizer to enhance their ionic conductivity, and the optimized ILGPE had a high ionic conductivity of 1.3 mS cm−1 at 23 °C. To improve the ionic conductivity and mechanical properties of the optimized ILGPE, we combined ILGPE and TiO2, MgO, SiO2, ZrO2, CeO2, NiO, Bi2O3, and Al2O3 inorganic electrolytes to fabricate composite gel polymer electrolytes, and the properties of these electrolytes were tested. The interaction of TiO2 in ILGPE was investigated, and the safety of TiO2-ILGPE was discussed.
Methods and materials
Fabrication of composite gel polymer electrolytes
The preparation of ionic liquid gel polymer electrolytes (ILGPE) and composite ionic liquid gel polymer electrolytes (CILGPE) filled with inorganic particles (TiO2, MgO, SiO2, ZrO2, CeO2, NiO, Bi2O3, and Al2O3, 99.8%, Aladdin) followed a previous report [33]. N-Methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide (PP13TFSI, Lanzhou Greenchem ILs) ionic liquid, LiTFSI (98+%, Alfa Aesar), and poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP, MW ~ 455,000, Sigma) were dissolved in N-methyl-2-pyrrolidone (NMP) with the weight ratio of 3:1:1, and then vigorously stirred to obtain a homogenous electrolyte slurry at room temperature. For xwt% CILGPE (x = 0~20), x g inorganic particles were dispersed in the NMP solvent by ultrasonic treatment for 15 min and then mixed with the 100 g ILGPE slurry. Finally, the resulting slurry was cast onto a glass plate and dried in a vacuum oven for 48 h at 80 °C. The dried polymer electrolytes were transferred to an Ar-filled glovebox and then punched into round disks with a diameter of 19 mm.
Ionic conductivity of electrolytes
Ionic conductivity of the polymer electrolytes was tested by potentiostat electrochemical impedance spectroscopy (EIS) on the Princeton Applied Research VestaSTAT4 by using a stainless steel/stainless steel (SS/SS) structure in a coin cell as previously reported [15, 33]. The frequency ranged from 100 kHz to 1 Hz with an AC amplitude of 5 mV. The bulk resistance (Rb) of the polymer electrolytes was obtained by the equivalent circuit of EIS Nyquist plots; the area of the polymer electrolyte was S (cm2); the thickness (L) of the polymer electrolyte was measured by a digital thickness gauge (Mitutoyo 547-526S 0–12 mm), and the ionic conductivity (σ) was calculated using the following equation:
Mechanical test
Tensile specimens of the polymer electrolytes were cut off in the form of “dogbone shaped” strips from the molded plate (the size of the specimens was a length of 9 mm, the width of 3.5 mm, and thickness of around 200~300 μm). The measurements were performed on a TA Instruments DMA Q800 V21.2 with a test speed of 1.5 N min−1.
Cell performance testing
Five wt% TiO2-ILGPE as the working electrode, Li-metal as the counter electrode, and the reference electrode, glass fiber as the separator, and 1 M LiPF6 EC/DMC (v/v = 1/1) as the electrolyte at C/10 and 23 °C. The mass loading of the active material (TiO2) of the working electrode is about 0.09 mg cm−2. The 1C is defined as 335 mAh g−1.
Results and discussions
Ionic conductivity of electrolytes
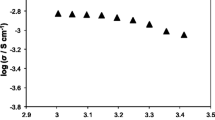
Table S1 shows the ionic conductivity of ILGPE with various inorganic particles with weight percentages of 2.5~20% at 30 °C. The highest ionic conductivity of CILGPE shows various weight percentage of inorganic particles. The highest conductivity with optimized composition is shown in Fig. 1.
Figure 1 displays the highest ionic conductivity of inorganic composite ionic liquid gel polymer electrolytes (CILGPE) with their optimized compositions at 30 °C. As can be observed in Fig. 1, the 2.5 wt% SiO2-ILGPE has the highest ionic conductivity of 2.5 mS cm−1 at 30 °C, while that of 15% Bi2O3-ILGPE and 5 wt% TiO2-ILGPE is 1.5 mS cm−1 at 30 °C. However, the 2.5 wt% NiO-ILGPE show the lowest ionic conductivity amount of these polymer electrolytes. α-Al2O3, selected as a filer in the work, is a lithium-ion nonconductor, and the ionic conductivity of 2.5 wt% α-Al2O3-ILGPE is relatively low. The ionic conductivity of 20 wt% ZrO2-ILGPE and 5 wt% MgO-ILGPE is acceptable. The ionic conductivity of these polymer electrolytes with inorganic particles is ~ 10−3 S cm−1.
Furthermore, there is a sudden increase in ionic conductivity with x% addition of inorganic fillers (Table S1). While higher or lower than that concentration of x, the ionic conductivity is obviously low. For instance, the ionic conductivity of 2.5 wt% TiO2-ILGPE and 10 wt% TiO2-ILGPE is 1.1 mS cm−1 and 0.98 mS cm−1, respectively. The ionic conductivity of polymer electrolytes could exhibit an optimized value with the addition of 5 wt% TiO2. The results are in agreement with those described in the literature, where a maximum in the ionic conductivity occurs as the increase of the filler concentration [25, 34], followed by a decrease in ionic conductivity values for higher filler weight percentage. Additionally, the decrease observed in high filler concentration could be associated with the blocking effect of the inorganic fillers in the conductivity pathway. The increase, for low filler concentration, could be, on the other hand, related to the reduction in the fusion enthalpy, which can be obtained by DSC measurement. Therefore, there is a maximum value of ionic conductivity with the optimized composition of composite ionic liquid gel polymer electrolytes.
Mechanical property of electrolytes
Figure 2 shows the mechanical properties of these CILGPE by tensile tests. As shown in Fig. 2, the mechanical properties of these composite ILGPE were affected by the addition of inorganic particles. The addition of TiO2 could increase their mechanical properties; the addition of Al2O3 and NiO would cause a poor mechanical property, while the concentration of CeO2, SiO2, Bi2O3, ZrO3, and MgO would show the various effects on their mechanical properties, such as 15 wt% Bi2O3-ILGPE and 2.5 wt% display a poor mechanical property. By the mechanical properties and ionic conductivity measurements, we selected TiO2 as a filler for the gel polymer electrolytes in this study.
Characterization of electrolytes
According to the results of ionic conductivity measurements, we observe that the concentration of TiO2 nanoparticles in the polymer matrix could affect ionic conductivity. As known to all, TiO2 nanoparticles cannot conduct lithium-ions. Thereby, the addition of TiO2 would sit in the lithium-ion pathway. The addition of high amounts of TiO2 filler can reduce the ionic conductivity. The mechanism scheme is shown in Figure S1, indicating that the concentration of non-conductive fillers cannot be over the optimizing ratio. When the 5 wt% TiO2 is added into ILGPE, the highest ionic conductivity is obtained and the value is 1.51 mS cm−1.
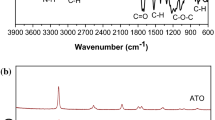
To investigate the effects of TiO2, the interaction between TiO2, PP13TFSI, LiTFSI, and PVDF-HFP in composite gel polymer electrolytes was investigated by FT-IR and Raman spectroscopy (Fig. 3). By the FT-IR spectroscopy, the TiO2 does not react with the PP13TFIS, LiTFSI, and PVDF-HFP during ILGPE preparation (Fig. 3a). In the ILGPE matrix, PP13TFSI, LiTFSI, and PVDF-HFP have “coordination,” such as Raman shift of 315 cm−1 and 740 cm−1 (Fig. 3b). After the addition of TiO2, however, these “coordination” disappeared or weakened, while the new “coordination” appeared around 1600–1800 cm−1. The results indicate that TiO2 could break the “original coordination” between PP13TFSI, LiTFSI, and PVDF-HFP, and then form a “new coordination” with PP13TFSI, LiTFSI, and PVDF-HFP. Thus, TiO2 particles sit in the lithium-ion pathway and decrease lithium-ion transport [35].
Besides, the XRD pattern of 5 wt% TiO2-ILGPE shows a new diffraction peak at 25° (2θ), indicating that the amorphous phase of PVDF-HFP increases by the 5 wt% TiO2 (Figure S2). There is literature reporting that the amorphous phase of the polymer can increase the ionic conductivity of polymer electrolytes. That is the reason that the ionic conductivity increases and reaches the highest value and then decreases after that (5 wt%) [36,37,38,39].
Figure 4 shows the SEM images of the CILGPE with various weight percentages of TiO2. ILGPE is a porous film and the pore size is less than 5 μm (as shown in Fig. 4a and a-magnified). With the addition of 5 wt% TiO2, the pores of ILGPE are filled and the surface of CILGPE is getting smooth and flat (Fig. 4b), while with the weight percentage of TiO2 increase, the surface of the CILGPE turns ununiform due to the TiO2 particle aggregation (Fig. 4e). The compact surface of 5 wt% TiO2 probably could inhibit lithium dendrites’ growth and solve the safety issue of lithium metal batteries.
Safety of TiO2-ILGPE
We assembled a Cu/ILGPE/Li cell and a Cu/5 wt% TiO2-ILGPE/Li cell, and then the lithium stripping/plating was carried out with a current density of 0.5 mA cm−2 for long-term cycles. After the Cu/Li cells shorted, we disassemble them and the digital photos of ILGPE and 5 wt% TiO2-ILGPE are shown in Figure S3. Figure S4 displays the pristine ILGPE and 5 wt% TiO2-ILGPE before cycling, and these electrolytes are free-standing membranes. The color of the polymer electrolytes surface becomes “black” in some regions after cycling, because the deposited lithium metal adheres to the surface of the polymer electrolytes. The ILGPE membrane is still transparent, and the 5 wt% TiO2-ILGPE membrane remains light yellow and nontransparent. The results indicate that TiO2 is not reduced by lithium metal during the charging/discharging. Meanwhile, we performed cycle voltammetry (CV) and battery measurements for the Li/5 wt% TiO2-ILGPE battery with 1 M LiPF6 EC/DMC (v/v = 1/1) as the electrolyte and glass fiber as the separator (Fig. 5). The CV curves do not show any redox peak, indicating that the Li/5 wt% TiO2-ILGPE battery does not have any redox reaction in the measured voltage range (Fig. 5a). The cells cannot deliver a noticeable capacity during the constant current charging/discharging, indicating that TiO2 particles in the ILGPE are not redox-active (Fig. 5b). In a word, TiO2 fillers in the CILGPE do not reduce against lithium metal anode and its safety to add to polymer electrolytes.
a CV curves for the cell with 5 wt% TiO2-ILGPE as the working electrode and Li-metal as the counter electrode (glass fiber as the separator, and 1 M LiPF6 EC/DMC (v/v = 1/1) as the electrolyte) in a voltage range of 0–3 V (vs. Li+/Li) at a scan rate of 1 mV s−1. b The voltage profile of Li/5 wt% TiO2-ILGPE battery (5 wt% TiO2-ILGPE as the working electrode, Li-metal as the counter electrode and the reference electrode, glass fiber as the separator, and 1 M LiPF6 EC/DMC (v/v = 1/1) as the electrolyte) at C/10 and 23 °C. The 1C is 335 mAh g−1. The mass loading of the active material (TiO2) of the working electrode is about 0.09 mg cm−2
The combustion test of the polymer electrolytes is performed, and the digital images are shown in Fig. 6. As shown in Fig. 6a, the PVDF-HFP membrane is flammable. When putting the PVDF-HFP membrane on fire, it shrinks immediately (~ 3 s) and gets on fire in a short time (~ 5 s). Finally, it turns “black” in color. The ILGPE is a non-flammable membrane, but it shrinks at ~ 5 s without blackening. While 5 wt% TiO2-ILGPE is a non-flammable membrane, it displays a perfect flame retarding ability. Furthermore, both ILGPE and TiO2-ILGPE are a promising electrolyte for safe lithium metal batteries.
Conclusion
In this study, the non-ionic conductive inorganic particles were used to improve the mechanical properties and ionic conductivity of gel polymer electrolytes. By the properties of these composite gel polymer electrolytes, TiO2 particles were selected as the filler of gel polymer electrolytes. The addition of TiO2 particles not only can fill the pores of gel polymer electrolytes to obtain dense electrolytes membrane but also can coordinate with the PP13TFSI or/and LiTFSI inside the composite gel polymer electrolytes. The safety of TiO2-ILGPE was evaluated by cyclic voltammetry, battery performance, and combustion test. With TiO2-ILGPE as an anode and Li-metal as a cathode, we assembled TiO2-ILGPE/Li cells to investigate the stability of TiO2-ILGPE against lithium metal. The results indicated that the TiO2 particles would not undergo a redox reaction during TiO2-ILGPE/Li cells cycling, and the cells delivered a charging specific capacity of 0.02 μAh g−1 at C/10, which could be neglected. Besides, the TiO2-ILGPE is a non-flammable membrane and a promising polymer electrolyte for the next generation of safe lithium metal batteries.
References
Yi TF, Pan JJ, Wei TT, Li Y, Cao G (2020) NiCo2S4-based nanocomposites for energy storage in supercapacitors and batteries. Nano Today 33:100894. https://doi.org/10.1016/j.nantod.2020.100894
Yi TF, Mei J, Peng PP, Luo S (2019) Facile synthesis of polypyrrole-modified Li5Cr7Ti6O25 with improved rate performance as negative electrode material for Li-ion batteries. Compos Part B Eng 167:566–572. https://doi.org/10.1016/j.compositesb.2019.03.032
Yi TF, Wei TT, Li Y, He YB, Wang ZB (2020) Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Storage Mater 26:165–197. https://doi.org/10.1016/j.ensm.2019.12.042
Rosero-Navarro NC, Kinoshita T, Miura A, Higuchi M, Tadanaga K (2017) Effect of the binder content on the electrochemical performance of composite cathode using Li6PS5Cl precursor solution in an all-solid-state lithium battery. Ionics (Kiel) 23:1619–1624. https://doi.org/10.1007/s11581-017-2106-x
Pan XN, Hou J, Liu L, Yang PX, Zhang JQ, An MZ, Li N (2017) A piperidinium-based ester-functionalized ionic liquid as electrolytes in Li/LiFePO4 batteries. Ionics (Kiel) 23:3151–3161. https://doi.org/10.1007/s11581-017-2104-z
Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R (2016) High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy 1:1–7. https://doi.org/10.1038/nenergy.2016.30
Navarra MA, Manzi J, Lombardo L, Panero S, Scrosati B (2011) Ionic liquid-based membranes as electrolytes for advanced lithium polymer batteries. ChemSusChem 4:125–130. https://doi.org/10.1002/cssc.201000254
Lian PJ, Zhao BS, Zhang LQ, Xu N, Wu MT, Gao XP (2019) Inorganic sulfide solid electrolytes for all-solid-state lithium secondary batteries. J Mater Chem A 7:20540–20557. https://doi.org/10.1039/c9ta04555d
Thangadurai V, Weppner W (2006) Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics (Kiel) 12:81–92. https://doi.org/10.1007/s11581-006-0013-7
Richards WD, Miara LJ, Wang Y, Kim JC, Ceder G (2016) Interface stability in solid-state batteries. Chem Mater 28:266–273. https://doi.org/10.1021/acs.chemmater.5b04082
Luo W, Gong Y, Zhu Y, Li Y, Yao Y, Zhang Y, Fu KK, Pastel G, Lin CF, Mo Y, Wachsman ED, Hu L (2017) Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv Mater 29:1–7. https://doi.org/10.1002/adma.201606042
Wang L, Liu D, Huang T, Geng Z, Yu A (2020) Reducing interfacial resistance of a Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte/electrode interface by polymer interlayer protection. RSC Adv 10:10038–10045. https://doi.org/10.1039/d0ra00829j
Weppner W (2003) Engineering of solid state ionic devices. Ionics (Kiel) 9:444–464. https://doi.org/10.1007/BF02376599
Ulaganathan M, Rajendran S (2010) Preparation and characterizations of PVAc/P(VdF-HFP)-based polymer blend electrolytes. Ionics (Kiel) 16:515–521. https://doi.org/10.1007/s11581-009-0415-4
Yang P, Liu L, Li L, Hou J, Xu YP, Ren X, An MZ, Li N (2014) Gel polymer electrolyte based on polyvinylidenefluoride-co-hexafluoropropylene and ionic liquid for lithium ion battery. Electrochim Acta 115:454–460. https://doi.org/10.1016/j.electacta.2013.10.202
Kim K, Park S, Choi S, Lee H (2006) Ionic liquid – polymer gel electrolytes based on morpholinium salt and PVdF ( HFP ) copolymer. J Power Sources 155:385–390. https://doi.org/10.1016/j.jpowsour.2005.05.018
Chaudoy V, Ghamouss F, Luais E, Tran-Van F (2016) Cross-linked polymer electrolytes for Li-based batteries: from solid to gel electrolytes. Ind Eng Chem Res 55:9925–9933. https://doi.org/10.1021/acs.iecr.6b02287
Zhang MY, Li MX, Chang Z, Wang YF, Gao J, Zhu YS, Wu YP, Huang W (2017) A Sandwich PVDF/HEC/PVDF gel polymer electrolyte for lithium ion battery. Electrochim Acta 245:752–759. https://doi.org/10.1016/j.electacta.2017.05.154
Jayathilaka PARD, Dissanayake MAKL, Albinsson I, Mellander BE (2003) Dielectric relaxation, ionic conductivity and thermal studies of the gel polymer electrolyte system PAN/EC/PC/LiTFSI. Solid State Ionics 156:179–195. https://doi.org/10.1016/S0167-2738(02)00616-1
Yang L, Wang Z, Feng Y, Tan R, Zuo Y, Gao R, Zhao Y, Han L, Wang Z, Pan F (2017) Flexible composite solid electrolyte facilitating highly stable “soft contacting” Li–electrolyte interface for solid state lithium-ion batteries. Adv Energy Mater 7:1–9. https://doi.org/10.1002/aenm.201701437
Choudhary S, Sengwa RJ (2017) Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochim Acta 247:924–941. https://doi.org/10.1016/j.electacta.2017.07.051
Quartarone E, Mustarelli P, Magistris A (1998) PEO-based composite polymer electrolytes. Solid State Ionics 110:1–14. https://doi.org/10.1016/s0167-2738(98)00114-3
Monroe C, Newman J (2005) The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J Electrochem Soc 152:A396. https://doi.org/10.1149/1.1850854
Lopez J, Mackanic DG, Cui Y, Bao Z (2019) Designing polymers for advanced battery chemistries. Nat Rev Mater 4:312–330. https://doi.org/10.1038/s41578-019-0103-6
Tambelli CC, Bloise AC, Rosário AV, Pereira EC, Magon CJ, Donoso JP (2007) Characterisation of PEO–Al2O3 composite polymer electrolytes. Electrochim Acta 47:1677–1682
Liu W, Liu N, Sun J, Hsu PC, Li Y, Lee HW, Cui Y (2015) Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett 15:2740–2745. https://doi.org/10.1021/acs.nanolett.5b00600
Chee SC, Heng YA, Lian TL, et al. (2018) Effect of Al2O3 in poly(methyl methacrylate) composite polymer electrolytes. 3rd International Conference on the Science and Engineering of Materials 2017.
Mohamad AA, Mohamed NS, Yahya MZA, et al. (2003) Ionic conductivity studies of poly(vinyl alcohol) alkaline solid polymer electrolyte and its use in nickel–zinc cells. Solid State Ionics 156(1-2):171–177
Yang Z, Peng H, Wang W, Liu T (2010) Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J Appl Polym Sci 116:2658–2667. https://doi.org/10.1002/app
Hu J, Wang W, Zhou B, Feng Y, Xie X, Xue Z (2019) Poly(ethylene oxide)-based composite polymer electrolytes embedding with ionic bond modified nanoparticles for all-solid-state lithium-ion battery. J Memb Sci 575:200–208. https://doi.org/10.1016/j.memsci.2019.01.025
Zhao B, Lu X, Wang Q, Yang J, Zhao J, Zhou H (2020) Enhancing the ionic conductivity in a composite polymer electrolyte with ceramic nanoparticles anchored to charged polymer brushes. Chinese Chem Lett 31:831–835. https://doi.org/10.1016/j.cclet.2019.06.009
Hu XL, Hou GM, Zhang MQ, Rong MZ, Ruan WH, Giannelis EP (2012) A new nanocomposite polymer electrolyte based on poly(vinyl alcohol) incorporating hypergrafted nano-silica. J Mater Chem 22:18961–18967. https://doi.org/10.1039/c2jm33156j
Pan X, Liu T, Kautz DJ, Mu L, Tian C, Long TE, Yang P, Lin F (2018) High-performance N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide/poly(vinylidene fluoride-hexafluoropropylene) gel polymer electrolytes for lithium metal batteries. J Power Sources 403:127–136. https://doi.org/10.1016/j.jpowsour.2018.09.080
Choi BK, Shin KH (1996) Effects of SiC fillers on the electrical and mechanical properties of (PEO)16LiClO4 electrolytes. Solid State Ionics 86–88:303–306. https://doi.org/10.1016/0167-2738(96)00134-8
Köster TKJ, van Wüllen L (2010) Cation-anion coordination, ion mobility and the effect of Al2O3 addition in PEO based polymer electrolytes. Solid State Ionics 181:489–495. https://doi.org/10.1016/j.ssi.2010.02.005
Gadjourova Z, Andreev YG, Tunstall DP, Bruce PG (2001) Ionic conductivity in crystalline polymer electrolytes. Nature 412:520–523. https://doi.org/10.1038/35087538
Angulakshmi N, Nahm KS, Swaminathan V et al (2012) Nanocomposite polymer electrolytes for lithium batteries. Polym Process Charact 394:55–65. https://doi.org/10.1201/b13105
Mohamad AA, Mohamed NS, Yahya MZA, et al (2003) Ionic conductivity studies of poly ( vinyl alcohol ) alkaline solid polymer electrolyte and its use in nickel – zinc cells. 156:171–177
Gorecki W, Donoso P, Berthier C et al (1988) NMR, DSC and conductivity study of the polymer solid electrolytes P(EO) (LiCp+1F2p+3SO3)x. Solid State Ionics 28–30:1018–1022. https://doi.org/10.1016/0167-2738(88)90323-2
Funding
The work was supported by the National Natural Science Foundation of China (Grant No. 21878061). X.P. and Q.H. acknowledge the support from China Scholarship Council.
Author information
Authors and Affiliations
Contributions
P.Y., X.P., and Q.H. designed the experiments. X.P. and Q.H. performed preparation, materials characterization, and electrochemical measurements. L.L, J.Z., and M.A. participated in the scientific discussion. X.P. and Q.H. analyzed the data and wrote the manuscript with assistance from coauthors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 815 kb)
Rights and permissions
About this article
Cite this article
Pan, X., Hou, Q., Liu, L. et al. Semiconductor TiO2 ceramic filler for safety-improved composite ionic liquid gel polymer electrolytes. Ionics 27, 2045–2051 (2021). https://doi.org/10.1007/s11581-020-03850-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03850-9