Abstract
Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 (x = 0, 0.005, 0.01, 0.02, 0.03) cathode material was synthesized by co-precipitation. X-ray diffraction spectroscopy (XRD) and scanning electron microscopy (SEM) were used to analyze the crystal structure characteristics and morphology of the powder. The charge and discharge test, electrochemical impedance spectroscopy (EIS), and cyclic voltammetry (CV) were used to study the electrochemical properties of the battery in detail. XRD results show that Nb5+ substitution does not destroy the crystal structure, but it can enlarge the interplanar spacing, which is beneficial to the diffusion of lithium ions. The electrochemical properties of the material Li (Ni0.5Co0.25Mn0.25)0.99Nb0.01O2 are the best. The discharge specific capacity is 204.6, 186.0, 163.5, 141.6 mAh/g at 0.1C, 0.2C, 0.5C, and 1.0C, respectively. And the discharge specific capacity is as high as 174.1 mAh/g when returning to 0.1C again. After circulating 45 cycles at 0.1C, the capacity retention rate was 89.08%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of electric vehicles, large-scale electronic equipment, and hybrid vehicles, lithium-ion batteries have received extensive attention [1]. Lithium-ion batteries have enormous global potential for achieving energy sustainability and significantly reducing carbon emissions [2]. The requirements for lithium-ion batteries are increasing, such as high energy density, excellent cycle stability, and reliable safety [3, 4]. Lithium-ion batteries of widely used LiCoO2 materials are hexagonal α-NaFeO2-type belonging to the R-3m space group. This layered structure allows LiCoO2 to have good lithium-ion intercalation/deintercalation capabilities. However, the structural stability of LiCoO2 deteriorates drastically when lithium ions are half-extracted from the structure during charging. Therefore, in order to ensure its cycle performance limits its cut-off voltage, it can only provide half of the theoretical capacity of about 140 mAh/g capacity [5, 6]. The ternary cathode material LiNi1−x−yCoxMnyO2 has a structure similar to LiCoO2, and because it has the advantages of relatively low cost, high capacity, and better thermal stability, it can be used instead of LiCoO2 [7, 8]. However, studies have shown that LiNi1−x−yCoxMnyO2 (0 < x < 1, 0 < y < 1 x + y = 1) has a faster capacity loss. It is because of stone and rock salt phase transition due to Ni2+ being more likely to occupy the Li+ position [9,10,11,12], oxygen in the lattice released from the surface [3, 13, 14], and other shortcomings resulting in poor crystal structure [15].
In order to improve the performance of lithium-ion batteries, people mainly study cathode materials and electrolytes [16, 17]. The main modification methods for lithium-ion ternary cathode materials are doping [18,19,20], coating [21, 22], and core-shell structure [23].
The method of element doping refers to doping with other small amounts of metal or non-metal elements inside the crystal lattice, and it is desirable to improve certain electrochemical properties of the original material. The radius of Nb5+ is 0.064 nm, and the radius of Mn4+ is 0.053 nm. Since the radius of Nb5+ is close to the radius of the transition element and Nb5+ has a larger diameter than Mn4+ [24], Nb5+ can enhance the lattice parameters. It is important that it has a higher metal-oxygen bond energy. Hu et al. studied the effect of Nb doping on the positive electrode material 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2, and found that Nb is most effective in improving cycle performance and reducing voltage attenuation [25]. Yi et al. performed Nb doping on LiMn2O4. The results show that Nb doping can reduce electrode polarization and increase lithium-ion diffusion coefficient [26].
Considering the substitution of Nb5+ may improve the electrochemical properties of the material LiNi0.5Co0.25Mn0.25O2 in some extent. In this paper, we prepared Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 (x = 0, 0.005, 0.01, 0.02, 0.03) by co-precipitation. The effects of Nb5+ doping on the structure, morphology, and electrochemical properties of the cathode material LiNi0.5Co0.25Mn0.25O2 were investigated in detail.
Experiment
Synthesis
The precursor Ni0.5Co0.25Mn0.25(OH)2 was first prepared by hydroxide coprecipitation, and then Nb5+ was introduced in the ball milling stage. The mixture which was composed of NiSO4·6H2O, CoSO4·7H2O, and MnSO4·H2O in a molar ratio of 5:2.5:2.5 was dissolved in a small amount of deionized water and then configured as a solution having a transition metal ion concentration of 1 mol/L. The precipitant NaOH solution was prepared to be 2 mol/L, and the complexing agent NH3·H2O was set to 1 mol/L. The three solutions were simultaneously pumped into the reactor while maintaining a reaction temperature of 55 °C and a pH of 11. After the reaction was completed, the mixture was stirred at a constant temperature for 2 h, and finally it was aged overnight. The suspension was thoroughly washed and filtered to remove impurity ions (Na+, SO42+, NH3+, etc.). After the end of the suction filtration, the filter cake was placed in a blast drying oven at 102 °C to remove moisture.
According to the stoichiometric molar ratio of the chemical formula Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 (x = 0, 0.005, 0.01, 0.02, 0.03), the prepared powder was ball milled with Li2CO3 and Nb2O5, and the amount of Li2CO3 added was 5% higher than the calculated value, and Nb2O5 was used as the niobium source. The milling time is 5 h to make them mix uniformly.
The resulting mixture was placed in a muffle furnace and heated to 450 °C at a heating rate of 5 °C/min in an oxygen atmosphere, and then preheated for 5 h. After cooling to room temperature in a furnace, it was taken out and carefully ground. Finally, the temperature was raised to 850 °C at a heating rate of 5 °C/min, and the ground powder was calcined at 850 °C for 12 h to obtain a final product.
Electrode film and battery assembly preparation
First, the active material, acetylene black, and PVDF (polyvinylidene fluoride) were in an amount of 8:1:1, and the NMP (N-methyl-pyrrolidone) was taken in an appropriate amount. Acetylene black was added as a conductive agent, and PVDF was used as a binder. The active material and acetylene black were thoroughly ground in an agate mortar, and then PVDF was dissolved in NMP. The ground mixture was added to the solution. Stirring was carried out to obtain a uniformly dispersed slurry, which was then uniformly coated on an aluminum foil. The coated aluminum foil was dried at 110 °C for 10 h. After drying, it was compacted by a tableting machine and then punched into circular electrode sheets having a diameter of 10 mm.
Second, the preparation of the battery assembly was carried out in a dry inert gas glove box. The operation sequence was first placed with a negative electrode (lithium plate), a separator (Celgard 2400 porous polypropylene film), an appropriate amount of electrolyte 1 mol/L LiPF6 (EC+EMC+DMC volume ratio 1:1:1), and a positive electrode sheet. It was sealed and subjected to electrochemical test after standing for 24 h.
Physical characterization and electrochemical performance of materials
The crystal structure was analyzed using an X-ray diffraction analyzer ((XRD), Bruke D8-Fouse, Germany) which uses Cu-Kα as a radiation source with a scanning range of 2θ = 10–80° and a scanning speed of 12°/min. The morphology of the synthesized powder was analyzed by scanning electron microscopy ((SEM), Nova Nano SEM450 FEI), and energy dispersive spectrometer ((EDS), manufactured by AMETEK, model OCTANE PLUS) was used to test the element content in the material. The surface chemical compositions of the samples were measured by X-ray photoelectron spectroscopy ((XPS), ESCALAB 250Xi).
Charge and discharge were tested by the battery test system Land CT2001A with a test voltage range of 2.7–4.3 V. Both cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were tested on PARSTAT 4000 electrochemical analyzer. The test parameters for CV were a scan rate of 0.1 mV/s and a voltage range of 2.7–4.5 V. The EIS test amplitude is 5 mV in the frequency range of 100 kHz to 0.01 Hz. The EIS data was analyzed using the ZsimpWin 3.10 software. All the characterizations and measurements were taken at room temperature.
Results and discussion
Physical characterization
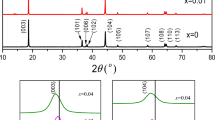
Figure 1 shows the XRD pattern of Li (Ni0.5Co0.25Mn0.25)1−xNbxO2, all of which are consistent with the layered features of α-NaFeO2. Compared with the undoped material, it is found that the position of the Nb5+ doped material peak does not change. Each peak has a characteristic peak of a typical α-NaFeO2 structure, and the shape of the peak is clear, and the intensity is large. This indicates that Nb5+ doping does not change the crystal structure of the original material. By carefully comparing the samples x = 0, x = 0.005, x = 0.01, x = 0.02, x = 0.03, it is found that the (006)/(102) peak is equally sharp except for sample x = 0.03. The (108)/(110) peaks all have good splitting, indicating a layered structure. Table 1 summarizes the unit cell parameters after refining of all samples. As the Nb5+ doping content increases, the lattice parameters a and c show a slight increase, indicating that the modification has induced the structure [27]. It can be seen from the volume parameter that the lattice volume after doping is larger than the original one. This may be due to the ionic radius of Nb5+ (0.64 Å) being larger than that of Mn4+ (0.53 Å) [24]. After doping, the intensity ratio of I(003)/I(104) are all larger than the original intensity ratio, which indicates that Li+/Ni2+ mixing is reduced [25].
In order to observe the surface topography of the material, Fig. 2 was obtained by scanning electron microscope. It can be seen that the Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 particles consist of densely packed particles. The micro-spherical particles can effectively shorten the Li+ diffusion path, reduce volume expansion/compression during charging/discharging, and promote diffusion of electrolyte into the electrode material [28]. The particle surface of Li (Ni0.5Co0.25Mn0.25)0.99Nb0.01O2 material is very smooth and clean. Its particle size is the most uniform, so its electrochemical performance should be the best.
In order to determine the elemental composition and distribution of the sample, and further prove the uniformity of Nb5+ doping, EDS spectrum testing was performed on this sample in Fig. 3. According to the test results, each region is found to contain nickel, cobalt, manganese, and niobium. The relative content of the elements in these two regions is approximately the same, and the atomic percentage ratio is very close to the target chemical formula. It is also proved that Nb5+ was successfully doped.
XPS was used to detect the chemical state of various elements on the surface. All spectra were calibrated by assigning the C 1s peak at 284.6 eV. Background type was Shirley. Figure 4a is the binding energy spectrum of Li (Ni0.5Co0.25Mn0.25) 0.99Nb0.01O2, and Ni 2p, Co 2p, Mn 2p, O 1s, C 1s, Nb 3d, and Li 1s peaks can be observed from it. The C 1s are mainly due to adventitious carbon formed during the atmospheric exposure [29]. The XPS spectrum of Ni 2p has two main peaks and two satellite peaks. The two main peaks are at the peaks of 854.9 eV and 872.4 eV, which are attributed to 2p3/2 and 2p1/2. According to the fitted XPS data, Ni 2p1/2 and Ni 2p3/2 both contain two splitting peaks corresponding to two different oxidation states of + 2 and + 3 [30, 31], respectively. In Fig. 4d, the two main peaks of the Co 2p spectrum are Co 2p3/2 at 780.0 eV and Co 2p1/2 at 794.9 eV, indicating that Co exists mainly in the oxidation state of Co3+ [32, 33]. There are two main peaks in the Mn XPS spectrum as shown in Fig. 4e. They are Mn 2p3/2 at 642.4 eV and Mn 2p1/2 at 653.9 eV, which are related to Mn4+ for the sample. In Fig. 4f, the satellite peaks of Nb appear at 206.4 eV and 209.2 eV corresponding to 3d5/2 and 3d3/2, respectively. Consistent with previous literature reports [34, 35], it was proved that Nb5+ was successfully doped.
Electrochemical performance
In order to evaluate the electrochemical properties of the material, Fig. 5 shows the initial charge-discharge curve of Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 (x = 0, 0.005, 0.01, 0.02, 0.03) for each material in the range of 2.7–4.3 V at a rate of 0.1C. All the curves in the figure have a charging or discharging platform, and the charging and discharging curves reflect the reversibility of Li insertion/extraction in Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 crystals during electrochemical process [36]. The generation of the charging or discharging platform in the curve is due to the phase change. If the platform lasts longer, we will find that the voltage changes more slowly. This results in a higher charge/discharge specific capacity. Due to the precipitation/dissolution of lithium on the electrode surface during charge and discharge, the surface state of the electrode changes and the polarization changes at the same time. This result may make the battery’s first charge-discharge curve not smooth [37]. It can be seen from Fig. 5 that when x = 0, 0.005, 0.01, 0.02, 0.03, the first discharge specific capacities are 199.0, 135.1, 204.6, 164.7, 188.7 mAh/g, respectively. When x = 0.01, the material has the largest initial discharge specific capacity. This indicates that an appropriate amount of Nb5+ doping can increase the discharge specific capacity of the original material. This may be due to the fact that an appropriate amount of Nb5+ doping can increase the layer spacing and increase the diffusion capacity of Li+. However, as the amount of Nb5+ doping continues to increase, this may result in a decrease in the first discharge specific capacity due to a decrease in the content of the active material Ni2+/Ni3+.
Figure 6 shows the rate performance of materials, and Fig. 7 shows the average specific discharge capacity of the materials at different discharge rates. Each battery is charged to 4.3 V at 0.1C each time, and then discharged to 2.7 V at 0.1C, 0.2C, 0.5C, 1.0C. It can be seen from Figs. 6 and 7 that the discharge specific capacities of different samples at the same discharge rate and the same sample at different discharge rates are significantly different. The former is due to the difference in the electrochemical properties of the material due to the different content of doped Nb5+. In the latter case, the polarization increases as the discharge rate increases. For detailed analysis, the specific discharge capacities of the original materials at 0.1C, 0.2C, 0.5C, 1.0C, and 0.1C are 199.0, 183.3, 154.0, 113.7, and 155.4 mAh/g, respectively. And its average discharge capacities are 196.72, 177.84, 142.16, 105.48, and 155.4 mAh/g, respectively. However, when x = 0.01, the discharge specific capacities of the material are 204.6, 186.0, 163.5, 141.6, and 174.1 mAh/g, respectively. And the corresponding average discharge specific capacities are 201.62, 181.34, 161.43, 138.36, and 169.2 mAh/g, respectively. Therefore, the sample Li (Ni0.5Co0.25Mn0.25)0.99Nb0.01O2 has the best rate performance. This result may be due to the proper amount of Nb5+ doped material, the better insertion/extraction reversibility of Li+ [25], thereby increasing the reversibility of lithium-ion transfer between the two electrodes [38].
Figure 8 shows the cycle performance of materials for charging and discharging at 2.7–4.3 V at a rate of 0.1C. The cycle performance analysis of the material is shown in Table 2. The capacity retention rate of the original material is 72.95%. When x = 0.005, x = 0.01, x = 0.02, x = 0.03 capacity retention rates were 75.93%, 89.08%, 87.17%, and 66.16%, respectively. It is apparent that the capacity retention of the original material is low due to surface structure changes of the active material and decomposition of the electrolyte, which is induced by highly reactive Ni4+ and/or some Li residues [5, 39]. The capacity retention of the material is improved when the proper amount of Nb5+ is doped, and the cycle stability of the material is best when x = 0.01. This may be because the bond energy of Nb-O is stronger than that of MO (M = Ni, Co, Mn), and an appropriate amount of Nb doping can stabilize the bulk structure of the cathode material during Li+-ion intercalation/deintercalation [40].
Figure 9 is a cyclic Nyquist diagram of the electrode, and Fig. 10 is an equivalent circuit diagram. It is clear that the Nyquist plots have similar shapes. The pattern consists of a small intercept, a semicircle in the high frequency region, and a straight line in the low frequency region. They are attributed to the ohmic resistance (Rs) determined by the resistance of the electrolyte and the electrode, the resistance (Rf) of the solid electrolyte interface ((SEI) film) layer, the charge transfer resistance (Rct) at the electrode/electrolyte interface, and Warburg impedance of Li+ diffusion, respectively [41,42,43].
The values of the impedance parameters of Table 3 were obtained by fitting the ZSimpWin software, in which the diffusion coefficient (DLi+) of lithium ions in the cathode can be calculated by the following formula [44]:
In Eq. 2, k represents a constant, and σ represents the Warburg factor opposite to Z'. Therefore, to obtain the slope σ, only a function graph of Z' and ω−1/2 is drawn (Fig. 11). In Eq. 1, n is the number of electrons required per unit reaction, R is the gas constant, T is the absolute temperature, n is the number of electrons required to participate in the unit reaction, F is the Faraday constant, A is the area of the cathode/electrolyte interface, and C is the concentration of lithium ions [45, 46].
By comparing the diffusion coefficients (DLi+) of lithium ions of various samples by Table 3, it was found that the original material is lower than Li (Ni0.5Co0.25Mn0.25)0.99Nb0.01O2, which were 2.3301 × 10−14 and 2.9515 × 10−14, respectively. The results show that doping Nb5+ can enlarge the interplanar spacing and promote the diffusion of lithium ions, which improves the rate performance and cycle stability of the electrode materials.
Figure 12 is a cyclic voltammogram of the material with a scan voltage of 2.7–4.5 V and a scan rate of 0.1 mV/s. As shown, all materials have only a pair of redox peaks, and no phase transition from the hexagonal phase to the spinel phase occurs during Li-ion intercalation/deintercalation [47]. The oxidation peak on the corresponding curve during charging is related to the oxidation which is related to the oxidation of Ni2+/Ni3+ to Ni4+ and the oxidation of Co3+ to Co4+, indicating that Li+ is detached from the compound Li (Ni0.5Co0.25Mn0.25)1−xNbxO2. Upon discharge, a similar reduction peak which corresponds to a decrease in Ni-ions (Ni4+→Ni2+/Ni3+) and Co-ions (Co4+→Co3+) indicated in the reverse lithium intercalation layered structure was found. Mn4+ is inactive in the structure of Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 because the valence is 4 [34]. When x = 0, 0.005, 0.01, 0.02, 0.03, the oxidation potential peaks were 3.9427 V, 3.9903 V, 3.8281 V, 3.8613 V, 3.9771 V, respectively; the reduction potential peaks were 3.6223 V, 3.6256 V, 3.6410 V, 3.6072 V, 3.6062 V, respectively; the peak difference between the two peaks are 0.3204 V, 0.3647 V, 0.1871 V, 0.2541 V, 0.3709 V, respectively. The peak difference is smaller, which indicates that the polarization of the material is smaller. Therefore, the reversibility of the material during charging and discharging is higher, and the electrochemical performance is better [48]. When the Nb5+ doping amount is x = 0.01, the minimum potential difference is 0.1871 V, which is smaller than the potential difference of the original material. Thus, an appropriate amount of Nb5+ doping reduces the electrochemical polarization of the original material [49]. Therefore, the material Li (Ni0.5Co0.25Mn0.25)0.99Nb0.01O2 has better electrochemical performance, which is consistent with the cycle performance and EIS analysis results.
Conclusion
In this paper, the material Li (Ni0.5Co0.25Mn0.25)1−xNbxO2 (x = 0, 0.005, 0.01, 0.02, 0.03) was synthesized by co-precipitation method, and the materials were structurally analyzed and electrochemically tested. Electrochemical data show that the material Li (Ni0.5Co0.25Mn0.25)0.99Nb0.01O2 has the best rate performance, and the specific discharge capacities at 0.1C, 0.2C, 0.5C, and 1C are 204.6, 186.0, 163.5, 141.6 mAh/g, respectively. From 1C to 0.1C, a discharge specific capacity of 174.1 mAh/g is still obtained. And after circulating 45 times at 0.1C, the capacity retention rate is the highest, which is 89.08%. The results show that an appropriate amount of Nb5+ doping can reduce the mixing of Li+/Ni2+ and increase the interlayer spacing. Furthermore, the cycle performance and rate performance of the material LiNi0.5Co0.25Mn0.25O2 are improved.
Change history
17 January 2020
In the originally published article, the name of the first author “Lina Li” was inadvertently removed from the list during typesetting and the second author’s name was presented twice. The correct and complete list of authors is presented above.
References
Li Y-C, Zhao W-M, Xiang W et al (2018) Promoting the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via LaAlO3 coating. J Alloys Compd 766:546–555
Zubi G, Dufo-López R, Carvalho M et al (2018) The lithium-ion battery: state of the art and future perspectives. Renew Sust Energ Rev 89:292–308
Liang C, Longo RC, Kong F et al (2017) Obstacles toward unity efficiency of LiNi1-2xCoxMnxO2 (x = 0 ∼ 1/3) (NCM) cathode materials: insights from ab initio calculations. J Power Sources 340:217–228
Da Silva SP, Sita LE, Dos Santos CS et al (2018) Physical and chemical characterization of LiCoO2 cathode material extracted from commercial cell phone batteries with low and high states of health. Mater Chem Phys 213:198–207
Ding Y, Mu D, Wu B et al (2017) Recent progresses on nickel-rich layered oxide positive electrode materials used in lithium-ion batteries for electric vehicles. Appl Energy 195:586–599
Oz E, Altin S, Demirel S et al (2016) Electrochemical effects and magnetic properties of B substituted LiCoO2: improving Li-battery performance. J Alloys Compd 657:835–847
Evertz M, Horsthemke F, Kasnatscheew J et al (2016) Unraveling transition metal dissolution of Li1.04Ni1/3Co1/3Mn1/3O2 (NCM 111) in lithium ion full cells by using the total reflection X-ray fluorescence technique. J Power Sources 329:364–371
Lars R, Jinlong L, Björn S et al (2016) Effect of pristine nanostructure on first cycle electrochemical characteristics of lithium-rich lithium–nickel–cobalt–manganese-oxide cathode ceramics for lithium ion batteries. J Power Sources 306:135–147
Liang C, Kong F, Longo RC et al (2016) Unraveling the origin of instability in Ni-rich LiNi1-2xCoxMnxO2 (NCM) cathode materials. Am Chem Soc 120:6383–6393
Zhang Q, Su Y, Chen L et al (2018) Pre-oxidizing the precursors of nickel-rich cathode materials to regulate their Li+/Ni2+ cation ordering towards cyclability improvements. J Power Sources 396:734–741
Yan P, Zheng J, Lv D et al (2015) Atomic-resolution visualization of distinctive chemical mixing behavior of Ni, Co, and Mn with Li in layered lithium transition-metal oxide cathode materials. Am Chem Soc 27:5393–5401
Xu C, Xiang W, Wu Z, Xu Y, Li Y, Wang Y, Xiao Y, Guo X, Zhong B (2019) Highly stabilized Ni-rich cathode material with Mo induced epitaxially grown nanostructured hybrid surface for high-performance lithium-ion batteries. ACS Appl Mater Interfaces 11:16629–16638
Zhang G, Qiu B, Xia Y et al (2019) Double-helix-superstructure aqueous binder to boost excellent electrochemical performance in Li-rich layered oxide cathode. J Power Sources 420:29–37
Ahmed S, Pokle A, Schweidler S et al (2019) The role of intragranular nanopores in capacity fade of nickel-rich layered Li (Ni1–x–yCoxMny)O2 cathode materials. ACS Nano:10694–10704
Sim S-J, Lee S-H, Jin B-S et al (2019) Improving the electrochemical performances using a V-doped Ni-rich NCM cathode. Sci Rep 9:8952
Ke X, Wang Y, Ren G et al. (2019) Towards rationally mechanical design of inorganic solid electrolytes for all-solid-state lithium ion batteries. Energy Storage Materials
Qiu L, Xiang W, Tian W et al (2019) Polyanion and cation co-doping stabilized Ni-rich Ni–Co–Al material as cathode with enhanced electrochemical performance for Li-ion battery. Nano Energy 63:103818
Sun L, Zhu L, Mi C et al (2019) The effects of Cr substitution on LiNi0.65Co0.1Mn0.25O2 for lithium-ion batteries. Ionics 25:3021–3030
Mi C, Han E, Li L et al (2018) Effect of iron doping on LiNi0.35Co0.30Mn0.35O2. Solid State Ionics 325:24–29
Lu M, Han E, Zhu L et al (2016) The effects of Ti4+-Fe3+ co-doping on Li [Ni1/3Co1/3Mn1/3]O2. Solid State Ionics 298:9–14
Liu X-H, Kou L-Q, Shi T et al (2014) Excellent high rate capability and high voltage cycling stability of Y2O3-coated LiNi0.5Co0.2Mn0.3O2. J Power Sources 267:874–880
Li L, Zhang Z, Fu S et al (2018) Co-modification by LiAlO2-coating and Al-doping for LiNi0.5Co0.2Mn0.3O2 as a high-performance cathode material for lithium-ion batteries with a high cutoff voltage. J Alloys Compd 768:582–590
Yoo G-W, Jang B-C, Son J-T (2015) Novel design of core shell structure by NCA modification on NCM cathode material to enhance capacity and cycle life for lithium secondary battery. Ceram Int 41:1913–1916
Wua J, Liu H, Ye X et al (2015) Effect of Nb doping on electrochemical properties of LiNi1/3Co1/3Mn1/3O2 at high cutoff voltage for lithium-ion battery. J Alloys Compd 644:223–227
Hu X, Guo H, Peng W et al (2018) Effects of Nb doping on the performance of 0.5Li2MnO3 ·0.5LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion batteries. J Electroanal Chem 822:75–65
Yi T-F, Yin L-C, Ma Y-Q et al (2013) Lithium-ion insertion kinetics of Nb-doped LiMn2O4 positive-electrode material. Ceram Int 39:4673–4678
Lei T, Li Y, Su Q et al (2018) High-voltage electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode materials via Al concentration gradient modification. Ceram Int 44:8809–8817
Liu H, Han E, Li L et al (2019) Effect of Cu2+ on Li [Li0.2Ni0.2Co0.08Mn0.52]O2 at different stages. Ionics 25:3009–3020
Xu Y-D, Xiang W, Wu Z-G et al (2018) Improving cycling performance and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by Li4Ti5O12 coating. Electrochim Acta 268:358–365
Huang D, Shi Y, Tornheim AP et al (2019) Nanoscale LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries via a polymer-assisted chemical solution method. Appl Mater Today 16:342–350
Park S, Kim D, Ku H et al (2019) The effect of Fe as an impurity element for sustainable resynthesis of Li [Ni1/3Co1/3Mn1/3]O2 cathode material from spent lithium-ion batteries. Electrochim Acta 296:814–822
Jo M, Park S, Song J et al (2018) Incorporation of Cu into Li [Ni1/3Co1/3Mn1/3]O2 cathode: elucidating its electrochemical properties and stability. J Alloys Compd 764:112–121
Zhang J, Cao Y, Ou X et al (2019) Constituting the NASICON type solid electrolyte coated material forming anti-high voltage system to enhance the high cut-off voltage performance of LiNi0.6Co0.2Mn0.2O2 via charge attracts electrostatic assembly. J Power Sources 436:226722
Yang Z, Xiang W, Wu Z et al (2017) Effect of niobium doping on the structure and electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Ceram Int 43:3866–3872
Lv C, Yang J, Peng Y et al (2019) 1D Nb-doped LiNi1/3Co1/3Mn1/3O2 nanostructures as excellent cathodes for Li-ion battery. Electrochim Acta 297:258–266
Tsai H-L, Hsieh C-T, Li J et al (2018) Enabling high rate charge and discharge capability, low internal resistance, and excellent cycleability for Li-ion batteries utilizing graphene additives. Electrochim Acta 273:200–207
Ivanishchev AV, Bobrikov IA, Ivanishcheva IA et al (2018) Study of structural and electrochemical characteristics of LiNi0.33Mn0.33Co0.33O2 electrode at lithium content variation. J Electroanal Chem 821:140–151
Huang Z, Wang Z, Jing Q et al (2016) Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material. Electrochim Acta 192:120–126
Yao X, Xu Z, Yao Z et al (2019) Oxalate co-precipitation synthesis of LiNi0.6Co0.2Mn0.2O2 for low-cost and high-energy lithium-ion batteries. Mater Today Commun 19:262–270
Lv C, Peng Y, Yang J et al (2018) Electrospun Nb-doped LiNi0.4Co0.2Mn0.4O2 nanobelts for lithium ion battery. Inorg Chem Front
Ding X, Li Y-X, Deng M-M et al (2019) Cesium doping to improve the electrochemical performance of layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material. J Alloys Compd 791:100–108
Mi C, Han E, Sun L et al (2019) Effect of Ti4+ doping on LiNi0.35Co0.27Mn0.35Fe0.03O2. Solid State Ionics 340:114976
Huang J, Fang X, Wu Y et al (2018) Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 by surface modification with lithium-active MoO3. J Electroanal Chem 823:359–367
Xu C-L, Xiang W, Wu Z-G et al (2018) A comparative study of crystalline and amorphous Li0.5La0.5TiO3 as surface coating layers to enhance the electrochemical performance of LiNi0.815Co0.15Al0.035O2 cathode. J Alloys Compd 740:428–435
Dong T, Peng P, Jiang F (2018) Numerical modeling and analysis of the thermal behavior of NCM lithium-ion batteries subjected to very high C-rate discharge/charge operations. Int J Heat Mass Transf 117:261–272
Zhao W, Luo G, Wang C-Y (2015) Modeling internal shorting process in large-format Li-ion cells. J Electrochem Soc 162:A1352–A1364
Ryu W-H, Lim S-J, Kim W-K et al (2014) 3-D dumbbell-like LiNi1/3Mn1/3Co1/3O2 cathode materials assembled with nano-building blocks for lithium-ion batteries. J Power Sources 257:186–191
Han E, Du X, Yang P et al (2017) The effects of copper and titanium co-substitution on LiNi0.6Co0.15Mn0.25O2 for lithium ion batteries. Ionics 24:393–401
Jia X, Yan M, Zhou Z et al (2017) Nd-doped LiNi0.5Co0.2Mn0.3O2 as a cathode material for better rate capability in high voltage cycling of Li-ion batteries. Electrochim Acta 254:50–58
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the originally published article, the name of the first author “Lina Li” was inadvertently removed from the list during typesetting and the second author’s name was presented twice. The correct and complete list of authors is presented above.
Rights and permissions
About this article
Cite this article
Li, L., Han, E., Zhu, L. et al. Effect of Nb5+ doping on LiNi0.5Co0.25Mn0.25O2 cathode material. Ionics 26, 2655–2664 (2020). https://doi.org/10.1007/s11581-019-03403-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03403-9