Abstract
Ln0.5Sr0.5Fe0.8Cu0.2O3-δ (LnSFC, Ln = Pr, Nd, Sm, Gd) perovskite oxide as a cobalt-free cathode was systematically evaluated for intermediate-temperature solid oxide fuel cell (IT-SOFC). XRD results show that PrSFC presents the cubic structure, while NdSFC, SmSFC, and GdSFC present an orthorhombic structure. The conductivity of the four samples is in accordance with the GdSFC < SmSFC < NdSFC < PrSFC relationship. AC impedance testing was performed using a symmetrical fuel cell of the structure LnSFC/Ce0.9Sm0.1O1.95(SDC)/LnSFC. The polarization resistance values of PrSFC, NdSFC, SmSFC, and GdSFC are 0.036, 0.089, 0.097, and 0.160 Ω cm2 at 800 °C, respectively. Then, SDC electrolyte-support single cell was fabricated and the power densities of PrSFC, NdSFC, SmSFC, and GdSFC cathodes were 364, 311, 254 and 104 mW cm−2, respectively, at 800 °C. Our preliminary experiment results show that LnSFC oxide meets the requirements of the electrode, and it can be a possible cathode for IT-SOFC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
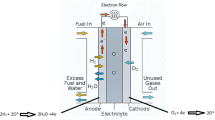
With the increase of global fossil energy consumption and environmental pollution, exploring new green energy is currently the hottest and most relevant research topic [1,2,3]. Solid oxide fuel cell (SOFC) is electrochemical device which convert the chemical energy of fuels directly into electrical energy in an efficient and clean way. It has the superiority of fuel efficiency and low environmental pollution [4, 5]. However, the conventional SOFC generally has a relatively high operating temperature (850~1000 °C), which cause various problems such as interfacial reaction, seal hard, and the high cost, resulting in a decrease in performance of SOFC. Therefore, lowering the operating temperature is particularly important. Unfortunately, the reduction in temperature will cause a decrease in cathode catalytic performance. Consequently, it is the primary research direction to find new cathodes with high catalytic performance and low polarization loss in intermediate temperatures (500~800 °C) [6, 7].
In the past, La1-xSrxMnO3-δ (LSM) [8, 9] cathode materials have been extensively studied. However, the ion conductivity of LSM cathode is lower at intermediate temperature. And thus, the polarization resistance becomes large, resulting in a decrease in the electrochemical performance of the cathode. Recently, many cobalt-based perovskite cathodes such as Ba0.5Sr0.5Co1-yFeyO3-δ and Ln1-xSrxCo1-yFeyO3-δ (Ln = La, Sm) [10,11,12] have been well studied. The researchers demonstrate that these cobalt-based cathodes exhibited high ionic and electronic conductivity and excellent catalytic properties. For example, Tu et al. [13] investigated the properties of Ln0.4Sr0.6Co0.8Fe0.2O3-δ (LnSCF, Ln = La, Pr, Nd, Sm, Gd) cathode materials. They demonstrated that the thermal expansion coefficient (TEC) and electrical conductivity decrease with the Ln3+ ion size decreasing from Ln = La to Gd. This indicates that LnSCF oxide such as Gd3+ with the smallest ion size can effectively reduce TEC. Unfortunately, the TEC of LnSCF for Ln = La to Gd is 21.1–17.6 × 10−6 K−1, which is much higher than the commonly used intermediate temperature electrolytes (~ 12 × 10−6 K−1). High TEC values limit the use of these Co-based oxides as SOFC cathodes. The instability and high cost of cobalt is another issue. Therefore, developing cobalt-free cathodes with sufficient catalytic activity is significant.
Recently, Fe-based perovskite oxides, Ln1-xSrxFeO3 (Ln = La, Pr, Nd, Sm, Eu, and Gd) [14, 15] and Ba1-xSrxFeO3 [16,17,18], have shown good catalytic activity as cathode material. For instance, the polarization resistance (Rp) of Pr0.8Sr0.2FeO3-δ cathode achieves 0.886 Ω cm2 at 750 °C. More recently, many researchers reported that partial substitution of Cu by Fe could further improve oxygen mobility for the oxygen reduction reaction (ORR). The Rp of Bi0.5Sr0.5Fe0.8Cu0.2O3-δ [19], Pr0.6Sr0.4Cu0.2Fe0.8O3-δ [20], and BaFe0.85Cu0.15O3-δ [21] cathode materials were 0.13, 0.07, and 0.35 Ω cm2 at 700 °C, respectively. Moreover, the average TEC of Pr0.6Sr0.4Cu0.2Fe0.8O3-δ at 50 to 800 °C is 16.4 × 10−6 K−1, which is smaller than the TEC of many cobalt-based cathodes [22]. However, there is no report on the systematic study for the Ln0.5Sr0.5Fe0.8Cu0.2O3-δ (LnSFC, Ln = Pr, Nd, Sm, Gd) oxides until now. In this paper, cobalt-free LnSFC was prepared and investigated as potential applications for the cathode material of IT-SOFCs. The Ln0.5Sr0.5Fe0.8Cu0.2O3-δ (Ln = Pr, Nd, Sm, and Gd) oxide was synthesized by a sol-gel method, and the effect of lanthanide on electrochemical performance was systematically investigated.
Experimental
Sample synthesis
The powder of Ln0.5Sr0.5Fe0.8Cu0.2O3-δ (LnSFC, Ln = Pr, Nd, Sm, and Gd) was synthesized by sol-gel method. Pr2O3, Nd2O3, Sm2O3, Gd2O3, Sr(NO3)2, Fe(NO3)3·9H2O, and Cu(NO3)2·3H2O were weighed according to the stoichiometric ratio. Nitrate solutions were prepared by dissolving Pr2O3, Nd2O3, Sm2O3, and Gd2O3 in nitric acid, respectively. Other nitrates were dissolved in deionized water. And citric acid (a complexing agent) was added in the mixed nitrate solutions. The ratio of cation to citric acid is 1:1.2. After that, the mixture was placed on a magnetic stirrer, heating and stirring continuously until the solution was viscous, put it in a drying oven at 150 °C for 3 h, removed organics and then grinded it to obtain the precursor powder, and then it was calcined at 950 °C for 10 h. The SDC electrolyte powder was prepared by a glycine combustion method (GNP) [23]. According to the stoichiometric ratio, the corresponding masses of CeO2 and Sm2O3 were weighed and dissolved in concentrated nitric acid to prepare a nitrate solution. The glycine as a complexant was added into the mixed nitrate solution. The mole ratio of total metal ions/glycine is 1:1.2. The above mixed glycine-nitrate solution was heated on a hot plate until the final combustion to obtain an ultrafine powder. The SDC powder was calcined at 600 °C for 6 h. The anode was NiO-SDC (65:35 by weight).
Cell fabrication
The impedance measurement is using a symmetrical cell of the LnSFC|SDC| LnSFC structure. The LnSFC powder was thoroughly mixed with an appropriate amount of organic matter to obtain cathode slurry. LnSFC slurry was screen-printed onto both sides of the SDC electrolyte disk (0.4 mm), and then sintered at 950 °C for 2 h. The power density of LnSFC cathode material was carried out using an electrolyte-supported single cell. First, the anode material was thoroughly mixed with a binder, coated on one side of an SDC pellet with 0.3 mm thickness, and sintered at 1250 °C for 4 h. After that, LnSFC slurry was applied to the other side of SDC pellet and sintered at 950 °C for 2 h in the muffle furnace. Finally, the single cell was sealed with silver paste at one end of the alumina tube. The final valid surface area of the cell was about 0.1 cm2.
Measurements
An X-ray diffractometer (Rigaku-D-Max γA, Japan) was used to measure the crystal structure of the LnSFC powder. The microstructure of cell cross-section was investigated by a scanning electron microscope (JEOL JSM-6480LV). The valence of the LnSFC material was analyzed using ESCALAB 250XI (Thermo Fisher, USA). The temperature function of the conductivity was measured using a standard dc four-terminal method. The electrochemical impedance spectrum was measured by a CHI660C workstation with 0.01~100 kHz frequency range under open-circuit conditions. The single cells with the NiO + SDC|SDC|LnSFC structure were tested at a temperature of 600~800 °C. The cathode gas is air and the anode gas is H2.
Results and discussion
Phase structures
The XRD patterns of LnSFC powders calcined at 950 °C for 10 h are displayed in Fig. 1a. These samples exhibited a single-phase structure consistent with previously reported results [24,25,26]. The PrSFC material presents a cubic perovskite structure and the space group is pm-3m. However, the NdSFC, SmSFC, and GdSFC materials exhibit an orthogonal structure with space group pnma. The average ion radius of A-site cation is reduced with the radius of the lanthanide cation decreasing from Pr to Gd. This presumably may be the reason why the crystal structure changes from a cubic structure to an orthorhombic structure [27, 28]. The structure parameter is shown in Table 1. The orthorhombic lattice constant decreases as the ionic radius decreases, and a similar phenomenon is observed in other perovskite oxides [29]. Figure 1b shows the XRD patterns of the mixtures of LnSFC cathode and SDC electrolyte. The LnSFC powder was sintered with SDC (50:50 wt.%) at 950 °C for 10 h. It can be seen that no additional peaks appear, indicating that SDC can act as an electrolyte material for the LnSFC cathode.
XPS analysis
The valence state of B-site metal cation for LnSFC sample was analyzed using XPS. Figures 2 and 3 show the XPS spectra of Fe 2p and Cu 2p. Table 2 lists the relative content and average valence of different valence elements. As shown in Fig. 2, the peaks of Fe 2p3/2 and 2p1/2 appeared at 709.7–710.2 eV and 722.9–723.6 eV, pointing the existence of Fe3+ species. The remaining peaks were observed near 711.6–713.0 eV and 725.3–725.8 eV, which were attributable to Fe4+ species. This value matches well with the data in some literature [30, 31]. This indicates that both Fe3+ and Fe4+ are present in the LnSFC sample. The atomic ratio of Fe3+/Fe4+ is listed in Table 2. The atomic ratio of Fe3+/Fe4+ in PrSFC, NdSFC, SmSFC, and GdSFC was calculated to be 68:32, 72:28, 61:39, and 89:11, respectively.
Figure 3 represents the Cu 2p XPS spectrum of the LnSFC sample. The Cu 2p3/2 and 2p1/2 peaks appeared at 932.1–933.7 eV and 952.5–952.9 eV, respectively, pointing the existence of Cu+ species. And the other two peaks were observed near 933.7–934.8 eV and 954.2–954.4 eV, respectively, pointing the existence of Cu2+ species. The peaks observed at 941.4–942.7 eV are satellite peak. This is in good agreement with some of the previous results [32, 33]. This indicates that there are two valence states of Cu+ and Cu2+ in the LnSFC sample. Table 2 shows the atomic ratio of Cu+/Cu2+. The atomic ratio of PrSFC, NdSFC, SmSFC, and GdSFC were calculated to be 42:58, 64:36, 46:54, and 38:62, respectively.
The average valence of the B-site metal ion is also shown in Table 2. It can be seen that the average valence of Cu and Fe cations in all samples are less than 3+. In order to maintain electrical neutrality, the oxygen vacancies can be produced with the reduction of positive charge [34]. Therefore, the Fe3+/Fe4+ and Cu+/Cu2+ mixed valence states of B-site cations has positive effect on the electrochemical performance of the LnSFC.
Electrical conductivity
The perovskite LnSFC cathode material is a mixed ion and electron (MIEC) conductor. The conductivity herein is mainly refers to the electronic conductivity because the electronic conductivity is several orders of magnitude higher than the ionic conductivity in MIEC oxides. Figure 4 shows the relationship between the conductivity of the LnSFC sample and the test temperature. As the temperature increases, the conductivity of the sample begins to increase, exhibiting a semiconductor-like behavior. The maximum values of conductivity appear at 300~450 °C. After the temperature exceeds 450 °C, the conductivity decreases and exhibits a similar metal conduction behavior. This phenomenon has also been found in previous studies [25, 35]. The reduction of the B-site cations (Fe and Cu) accompanied by the formation of oxygen vacancies results in a decrease in the conductivity of the LnSFC at elevated temperatures. As shown in Fig. 4, in LnSFC samples, the conductivity of PrSFC is the highest at all the temperatures tested. Over the entire temperature range, as the ionic radius decreases from Pr to Gd, the conductivity decreases monotonously. Lee et al. observed similar relationship for Ln0.6Sr0.4CoO3-δ (Ln = La, Pr, Nd, Sm, and Gd) [36]. They investigated that the reason of these observations may be due to an increase of oxygen vacancy concentration with the decrease of Ln3+ ionic radius from Ln = Pr to Gd. Currently, at operating temperatures, conductivity greater than 100 S cm−1 is a significant symbol for fuel cell cathode materials, ensuring that sufficient current is collected [26]. Nevertheless, it has been reported that several perovskite cathode materials have relatively low electrical conductivity and good electrochemical properties. For example, Zhao et al. reported that the maximum conductivity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ was 45 S cm−1 at 550 °C [37]. The conductivity of Cu1.4Mn1.6O4 cathode materials reached ~ 70 S cm−1 at 750 °C [38]. In the impedance section, we can see that LnSFC sample shows a better electrochemical property.
The Arrhenius plot of conductivity for LnSFC oxides is shown in Fig. 5. At lower temperatures (100−400 °C), the plots are nearly linear. The conductivity activation energy of the four samples can be accessed on the slope of the linear part. The calculated activation energies of the PrSFC, NdSFC, SmSFC, and GdSFC are 0.072, 0.081, 0.120, and 0.169 eV, respectively. Compared with NdSFC, SmSFC, and GdSFC, the transport of charge carriers for PrSFC is relatively easier. These values are similar in magnitude to previously reported activation energies for La0.6Sr0.4Fe0.8Cu0.2O3-δ oxide [39]. Moreover, the activation energies of LnSFC are lower than other cobalt-free cathode materials such as Sm1.875Ba3.125Fe5O15-δ (22.4 kJ/mol) [40] and SmBa0.5Sr0.5CuFeO5+δ (116.89 kJ/mol) [41]. This conductive behavior is consistent with small polaron hopping mechanism (i.e., localized electronic carriers having a thermally activated mobility), follows the relation:
where A is the pre-exponential factor, Ea is the activation energy for hopping conduction, k is the Boltzmann constant, and T is the absolute temperature. The charge compensation for LnSFC is primarily electrons, and the conductivity can be attributed to the migration of electron holes. At higher temperatures, the electrical conductivity decreases with the increase of testing temperature. It is obvious that there is a significant negative deviation from linearity in the plots, indicating that this conductive behavior is not consistent with low temperature assumption. At high temperature range (400−800 °C), ionic compensation becomes significant due to the loss of the lattice oxygen and can thus be related to a decreasing concentration of electron holes as the concentration of oxygen vacancies increases in the material, as expressed in Eq. (2) [42]:
where “x” refers to neutrality with respect to the lattice. Therefore, electrical conductivity decreases with increasing temperature due to the partial annihilation of electron holes. Similar results have been reported in the other perovskite materials [43].
Thermal expansion
Figure 6 shows the TEC of the LnSFC samples tested from room temperature to 800 °C. And the average TEC values of the LnSFC samples are shown in Table 3. The TEC values of the PrSFC, NdSFC, SmSFC, and GdSFC are 17.30, 17.19, 16.72, and 12.89×10−6 K−1, respectively. The averages TEC are comparable to previous reports [26, 44]. In LnSFC oxides, the TEC values decrease from Ln = Pr to Gd and the TEC value of GdSFC is very close to the extensively used SDC electrolyte (12.2 × 10−6 K−1). As the binding energy of ions in the crystal structure increases with the decrease of ion distance, the ionicity of Ln–O bond decreases, which leads to a decrease of TEC. Similar results can be seen in Ln0.4Sr0.6Co0.8Fe0.2O3-δ (Ln = La, Pr, Nd, Sm, Gd) [13] and Ln0.6Sr0.4CoO3−δ (Ln = La, Pr, Nd, Sm, and Gd) materials [36]. Figure 6 also shows that the slope of the TEC curve increases greatly when the temperature is higher than 450 ° C. It indicates that the lattice expansion accelerates with the temperature rising due to the reduction of Cu and Fe cations and consumption of the lattice oxygen. With temperature increasing, the oxygen vacancies are formed due to loss of lattice oxygen, which in turn leads to a reducing in the strength of the B–O bond intensity and an increase in the size of the BO6 octahedron, resulting in lattice expansion. Also, in order to examine the expansibility of the samples, high-temperature XRD (HT-XRD) measurement was conducted on the LnSFC powders after sintering at 950 °C for 10 h. The XRD patterns recorded every 100 °C are shown in Fig. 7. With increasing temperature, the main XRD peaks of the LnSFC sample shift toward the low-angle direction gradually, indicating the expansion of the perovskite lattice, which is similar with the reported B-site double perovskites [45].
Microstructure
Figure 8a–d show a cross-section microstructure of LnSFC cathodes coated on an SDC electrolyte. The sintering condition is 950 °C for 2 h. As shown in Fig. 8, the cathode structure has uniform particle and porosity, which can make the transmission of oxygen and oxygen ions more rapidly. Figure 8 illustrates that the SDC electrolyte is very dense, preventing direct contact of the fuel gas with the air, put an end to an internal short circuit. The cathode and electrolyte interface is also tightly bonded, and there is no phenomenon that the cathode layer breaks or falls off at high temperatures.
Impedance analysis
Figure 9a–d show the impedance measured for LnSFC samples in air at 750 °C and 800 °C. An equivalent circuit diagram after impedance fitting is given in Fig. 9e. R is an ohmic resistor caused by wires and electrolytes. R1 corresponds to the polarization resistance generated by the oxygen ion migration and diffusion process of LnSFC at high frequencies. R2 corresponds to adsorption/dissociation during molecular oxygen, surface oxygen, or bulk oxygen diffusion at low frequencies. The sum of R1 and R2 is the polarization resistance (Rp) [46,47,48]. QPE1 and QPE2 are the capacitances of the entire electrode. As the temperature increased, the polarization resistance is reduced. The results show that the test temperature has a great influence on the electrochemical catalytic activity. The catalytic performance of the cathode is dissatisfactory at low temperatures, because the low temperature is not conducive to the migration of oxygen ions. As the temperature rises, it is easier to overcome the corresponding activation energy barrier, and the catalytic activity of the cathode is significantly improved, which promotes the reaction of the electrode, so the polarization resistance decreases with increasing temperature.
Figure 10 shows the Rp values in the range of 600 to 800 °C. The Rp of PrSFC, NdSFC, SmSFC, and GdSFC is 0.036, 0.089, 0.097, and 0.160 Ω cm2 at 800 °C, respectively. At 750 °C, the Rp of PrSFC, NdSFC, SmSFC, and GdSFC are 0.068, 0.152, 0.190, and 0.280 Ω cm2, respectively. It is shown that Rp of LnSFC cathode decreases with the ionic radius of the lanthanide increases. The reason of this trend may be that the surface exchange and diffusion coefficient of oxygen in the perovskite materials decreases with the decrease of rare earth ion radius, leading to an increase in Rp [49]. In previous studies [50, 51], the perovskite cathodes containing Pr ions have unusually rapid oxygen transport kinetics. In our study, in LnSFC samples, the PrSFC cathode has a lower area-specific resistance, which may be due to the change in the Pr3+/Pr4+ valence state [32, 51]. Also, the electronic transmission capability of the PrSFC is also higher (see “XPS analysis”), which is another reason for the better electrochemical performance of the PrSFC. Moreover, the Rp of PrSFC is also related with the microstructure of electrodes. The porous microstructure will not only provide sufficient three-phase boundaries (TPB) for adsorption and diffusion of fuels, but also ensure efficient charge transfer from electrode to electrolyte or from electrolyte to electrode. In LnSFC, the GdSFC cathode has the highest polarization resistance, but the RP values are also smaller than other Co-free cathodes such as La0.7Sr0.3CuO3-δ (0.234 Ω cm2 at 800 °C) [52] and Nd1.6Sr0.4NiO4 (0.93 Ω cm2 at 700 °C) [53]. The Arrhenius plot of the Rp for the LnSFC is displayed in Fig. 11. The calculated activation energy was 1.20, 1.25, 1.20, and 1.15 eV for PrSFC, NdSFC, SmSFC, and GdSFC, respectively. It is much smaller than 1.26 eV of the cobalt-free Ba0.95Ca0.05FeO3-δ cathode [54].
Single-cell performance
Figure 12 shows I−V curves of an SDC electrolyte-supporting single cell using cobalt-free LnSFC as cathodes. As the temperature rises, power density of single cell gradually increases. However, the open-circuit voltage of cells is still lower than theoretical value, which is mainly caused by the reduction of Ce4+ to Ce3+ at the anode atmosphere. When the temperature is 800 °C, the peak power densities of PrSFC, NdSFC, SmSFC, and GdSFC cathodes are 364, 311, 254, and 103 mW cm−2, respectively. When the temperature is 700 °C, the maximum power densities of PrSFC, NdSFC, SmSFC, and GdSFC is 280, 212, 205, and 76 mW cm−2, respectively. Compared with other three samples, PrSFC obtained the best cell performance, which is consisted with the results of conductivity and polarization impedance. Moreover, the single cell with LnSFC cathodes get better power density compared with some cobalt-free cathode materials. For example, the peak power density of Pr1.95Ce0.05CuO4 cathode based on electrolyte-supported fuel cell is 150 mW cm−2 800 °C [55]. The initial finding suggests that Co-free LnSFC perovskite oxide is a potential cathode for IT-SOFC. In addition, a higher cell performance can be achieved through optimizing the cathode structure and using anode-supported single cells.
Conclusions
The cobalt-free LnSFC perovskite was successfully synthesized as a cathode for IT-SOFC. The LnSFC cathode materials exhibit structural change with increasing radius size of the lanthanide ions from cubic (Ln = PrSFC) to orthorhombic (Ln = NdSFC, SmSFC, and GdSFC) perovskite. The LnSFC material has good chemical compatibility with the SDC electrolyte. At 800 °C, the Rp values of PrSFC, NdSFC, SmSFC, and GdSFC are 0.036, 0.089, 0.097, and 0.160 Ω cm2, respectively. The peak power densities of an electrolyte-supported single cell with PrSFC, NdSFC, SmSFC, and GdSFC as cathodes are 363.8, 311.3, 253.6, and 103.2 mW cm−2, respectively. The results show that the fuel cell using LnSFC cathode has good electrochemical performance. Preliminary results indicate that the LnSFC material can be applied as a potential cathode for SOFC.
References
Baharuddin NA, Muchtar A, Somalu MR (2017) Short review on cobalt-free cathodes for solid oxide fuel cells. Int J Hydrog Energy 42:9149–9155. https://doi.org/10.1016/j.ijhydene.2016.04.097
Hossain S, Abdalla AM, Jamain SNB, Zaini JH, Azad AK (2017) A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew Sust Energ Rev 79:750–764. https://doi.org/10.1016/j.rser.2017.05.147
Ramadhani F, Hussain MA, Mokhlis H, Hajimolana S (2017) Optimization strategies for solid oxide fuel cell (SOFC) application: a literature survey. Renew Sust Energ Rev 76:460–484. https://doi.org/10.1016/j.rser.2017.03.052
Meng G, Ma G, Ma Q, Peng R, Liu X (2007) Ceramic membrane fuel cells based on solid proton electrolytes. Solid State Ionics 178:697–703. https://doi.org/10.1016/j.ssi.2007.02.018
Shin JF, Xu W, Zanella M, Dawson K, Savvin SN, Claridge JB, Rosseinsky MJ (2017) Self-assembled dynamic perovskite composite cathodes for intermediate temperature solid oxide fuel cells. Nat Energy 2:16214. https://doi.org/10.1038/nenergy.2016.214
Jiang S, Zhou W, Sunarso J, Ran R, Shao Z (2015) A cobalt-free layered oxide as an oxygen reduction catalyst for intermediate-temperature solid oxide fuel cells. Int J Hydrog Energy 40:15578–15584. https://doi.org/10.1016/j.ijhydene.2015.09.097
Brett DJ, Atkinson A, Brandon NP, Skinner SJ (2008) Intermediate temperature solid oxide fuel cells. Chem Soc Rev 37:1568–1578. https://doi.org/10.1039/B612060C
Heremans JJ, Carris M, Watts S, Yu X, Dahmen KH, von Molnár S (1997) Characterization of films of La1−xSrxMnO3−δ grown by means of metal organic chemical vapor deposition. J Appl Phys 81:4967–4969. https://doi.org/10.1063/1.365014
Co AC, Xia SJ, Birss VI (2005) A kinetic study of the oxygen reduction reaction at LaSrMnO3-YSZ composite electrodes. J Electrochem Soc 152:A570–A576. https://doi.org/10.1149/1.1859612
Kim C, Park H, Jang I, Kim S, Kim K, Yoon H, Paik U (2018) Morphologically well-defined Gd0.1Ce0.9O1.95 embedded Ba0.5Sr0.5Co0.8Fe0.2O3-δ nanofiber with an enhanced triple phase boundary as cathode for low-temperature solid oxide fuel cells. J Power Sources 378:404–411. https://doi.org/10.1016/j.jpowsour.2017.12.065
Zhou W, Ran R, Shao Z, Jin W, Xu N (2008) Evaluation of A-site cation-deficient (Ba0.5Sr0.5)1−xCo0.8Fe0.2O3−δ (x>0) perovskite as a solid-oxide fuel cell cathode. J Power Sources 182:24–31. https://doi.org/10.1016/j.jpowsour.2008.04.012
Świerczek K (2011) Physico-chemical properties of Ln0.5A0.5Co0.5Fe0.5O3−δ (Ln: La, Sm; A: Sr, Ba) cathode materials and their performance in electrolyte-supported intermediate temperature solid oxide fuel cell. J Power Sources 196:7110–7116. https://doi.org/10.1016/j.jpowsour.2010.08.063
Tu HY, Takeda Y, Imanishi N, Yamamoto O (1999) Ln0.4Sr0.6Co0.8Fe0.2O3-δ (Ln=La, Pr, Nd, Sm, Gd) for the electrode in solid oxide fuel cells. Solid State Ionics 117:277–281. https://doi.org/10.1016/S0167-2738(98)00428-7
Chaianansutcharit S, Hosoi K, Hyodo J, Ju YW, Ishihara T (2015) Ruddlesden popper oxides of LnSr3Fe3O10−δ (Ln = La, Pr, Nd, Sm, Eu, and Gd) as active cathodes for low temperature solid oxide fuel cells. J Mater Chem A 3:12357–12366. https://doi.org/10.1039/c5ta01273b
Piao J, Sun K, Zhang N, Chen X, Xu S, Zhou D (2007) Preparation and characterization of Pr1−xSrxFeO3 cathode material for intermediate temperature solid oxide fuel cells. J Power Sources 172:633–640. https://doi.org/10.1016/j.jpowsour.2007.05.023
Huang S, Wang G, Sun X et al (2012) Cobalt-free perovskite Ba0.5Sr0.5Fe0.9Nb0.1O3-δ as a cathode material for intermediate temperature solid oxide fuel cells[J]. J Alloys Compd 543:26–30. https://doi.org/10.1016/j.jallcom.2012.07.115
Vázquez S, Basbus J, Soldati AL, Napolitano F, Serquis A, Suescun L (2015) Effect of the symmetric cell preparation temperature on the activity of Ba0.5Sr0.5Fe0.8Cu0.2O3-δ as cathode for intermediate temperature Solid Oxide Fuel Cells. J Power Sources 274:318–323. https://doi.org/10.1016/j.jpowsour.2014.10.064
Long W, Xu H, He T (2014) Preparation and electrochemical performance of cobalt-free cathode material Ba0.5Sr0.5Fe0.9Nb0.1O3-δ for intermediate-temperature solid oxide fuel cells. Chem Res Chinese U 30:806–810. https://doi.org/10.1007/s40242-014-4130-y
Gao L, Zhu M, Li Q, Sun L, Zhao H, Grenier J-C (2017) Electrode properties of Cu-doped Bi0.5Sr0.5FeO3−δ cobalt-free perovskite as cathode for intermediate-temperature solid oxide fuel cells. J Alloys Compd 700:29–36. https://doi.org/10.1016/j.jallcom.2017.01.026
Gong Z, Hou J, Wang Z, Cao J, Zhang J, Liu W (2015) A new cobalt-free composite cathode Pr0.6Sr0.4Cu0.2Fe0.8O3−δ-Ce0.8Sm0.2O2−δ for proton-conducting solid oxide fuel cells. Electrochim Acta 178:60–64. https://doi.org/10.1016/j.electacta.2015.07.159
Zhu M, Cai Z, Xia T, Li Q, Huo L, Zhao H (2016) Cobalt-free perovskite BaFe0.85Cu0.15O3-δ cathode material for intermediate-temperature solid oxide fuel cells. Int J Hydrog Energy 41:4784–4791. https://doi.org/10.1016/j.ijhydene.2016.01.071
Lim YH, Lee J, Yoon JS, Kim CE, Hwang HJ (2007) Electrochemical performance of Ba0.5Sr0.5CoxFe1−xO3−δ (x=0.2–0.8) cathode on a ScSZ electrolyte for intermediate temperature SOFCs. J Power Sources 171:79–85. https://doi.org/10.1016/j.jpowsour.2007.05.050
Cong L, He T, Ji Y, Guan P, Huang Y, Su W (2003) Synthesis and characterization of IT-electrolyte with perovskite structure La0.8Sr0.2Ga0.85Mg0.15O3−δ by glycine–nitrate combustion method. J Alloys Compd 348:325–331. https://doi.org/10.1016/S0925-8388(02)00859-9
Ling Y, Yu J, Lin B, Zhang X, Zhao L, Liu X (2011) A cobalt-free Sm0.5Sr0.5Fe0.8Cu0.2O3−δ–Ce0.8Sm0.2O2−δ composite cathode for proton-conducting solid oxide fuel cells. J Power Sources 196:2631–2634. https://doi.org/10.1016/j.jpowsour.2010.11.017
Shahgaldi S, Yaakob Z, Khadem DJ, Ahmadrezaei M, Daud WRW (2011) Synthesis and characterization of cobalt-free Ba0.5Sr0.5Fe0.8Cu0.2O3−δ perovskite oxide cathode nanofibers. J Alloys Compd 509:9005–9009. https://doi.org/10.1016/j.jallcom.2011.06.117
Lu J, Yin Y-M, Ma Z-F (2013) Preparation and characterization of new cobalt-free cathode Pr0.5Sr0.5Fe0.8Cu0.2O3−δ for IT-SOFC. Int J Hydrog Energy 38:10527–10533. https://doi.org/10.1016/j.ijhydene.2013.05.164
Wang J, Saccoccio M, Chen D, Gao Y, Chen C, Ciucci F (2015) The effect of A-site and B-site substitution on BaFeO3−δ : an investigation as a cathode material for intermediate-temperature solid oxide fuel cells. J Power Sources 297:511–518. https://doi.org/10.1016/j.jpowsour.2015.08.016
Zheng K, Klimkowicz A, Świerczek K, Malik A, Ariga Y, Tominaga T, Takasaki A (2015) Chemical diffusion and surface exchange in selected Ln–Ba–Sr–Co–Fe perovskite-type oxides. J Alloys Compd 645:S357–S360. https://doi.org/10.1016/j.jallcom.2014.12.110
Xu Q, D-p H, Chen W, Zhang F, Wang B-t (2007) Structure, electrical conducting and thermal expansion properties of Ln0.6Sr0.4Co0.2Fe0.8O3 (Ln=La, Pr, Nd, Sm) perovskite-type complex oxides. J Alloys Compd 429:34–39. https://doi.org/10.1016/j.jallcom.2006.04.005
Jin F, Shen Y, Wang R, He T (2013) Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate-temperature solid-oxide fuel cells. J Power Sources 234:244–251. https://doi.org/10.1016/j.jpowsour.2013.01.172
Ghaffari M, Shannon M, Hui H, Tan OK, Irannejad A (2012) Preparation, surface state and band structure studies of SrTi(1−x)Fe(x)O(3−δ) (x=0–1) perovskite-type nano structure by X-ray and ultraviolet photoelectron spectroscopy. Surf Sci 606:670–677. https://doi.org/10.1016/j.susc.2011.12.013
Meng X, Lü S, Yu WW, Ji Y, Sui Y, Wei M (2018) Layered perovskite LnBa0.5Sr0.5Cu2O5+δ (Ln = Pr and Nd) as cobalt-free cathode materials for solid oxide fuel cells. Int J Hydrog Energy 43:4458–4470. https://doi.org/10.1016/j.ijhydene.2018.01.033
Liu Y-X, Wang S-F, Hsu Y-F, Kai H-W, Jasinski P (2018) Characteristics of LaCo0.4Ni0.6-xCuxO3-δ ceramics as a cathode material for intermediate-temperature solid oxide fuel cells. J Eur Ceram Soc 38:1654–1662. https://doi.org/10.1016/j.jeurceramsoc.2017.11.019
Xing GZ, Yi JB, Wang DD, Liao L, Yu T, Shen ZX, Huan CHA, Sum TC, Ding J, Wu T (2009) Strong correlation between ferromagnetism and oxygen deficiency in Cr-doped In2O3−δ nanostructures. Phys Rev B 79:174406. https://doi.org/10.1103/PhysRevB.79.174406
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Structure and electrical-properties of La1−xSrxCo1−yFeyO3. Part 1.The system La0.8Sr0.2Co1−yFeyO3. Solid State Ionics 76:259–271. https://doi.org/10.1016/0167-2738(94)00244-M
Lee KT, Manthiram A (2006) Comparison of Ln0.6Sr0.4CoO3−δ (Ln=La, Pr, Nd, Sm, and Gd) as cathode materials for intermediate temperature solid oxide fuel cells. J Electrochem Soc 153:A794–A798. https://doi.org/10.1149/1.2172572
Zhao H, Shen W, Zhu Z, Li X, Wang Z (2008) Preparation and properties of BaxSr1−xCoyFe1−yO3−δ cathode material for intermediate temperature solid oxide fuel cells. J Power Sources 182:503–509. https://doi.org/10.1016/j.jpowsour.2008.04.046
Zhen S, Sun W, Li P, Tang G, Rooney D, Sun K, Ma X (2016) High performance cobalt-free Cu1.4Mn1.6O4 spinel oxide as an intermediate temperature solid oxide fuel cell cathode. J Power Sources 315:140–144. https://doi.org/10.1016/j.jpowsour.2016.03.046
Zhou QJ, Xu L, Guo YJ, Jia D, Li Y, Wei WCJ (2012) La0.6Sr0.4Fe0.8Cu0.2O3-δ perovskite oxide as cathode for IT-SOFC. Int J Hydrogen Energy 37:11963–11968. https://doi.org/10.1016/j.ijhydene.2012.05.114
Zhou Q, Chen L, Cheng Y, Xie Y (2016) Cobalt-free quintuple perovskite Sm1.875Ba3.125Fe5O15−δ as a novel cathode for intermediate temperature solid oxide fuel cells. Ceram Int 42:10469–10471. https://doi.org/10.1016/j.ceramint.2016.03.174
Ding X, Kong X, Wu H, Zhu Y, Tang J, Zhong Y (2012) SmBa0.5Sr0.5Cu2O5+δ and SmBa0.5Sr0.5CuFeO5+δ layered perovskite oxides as cathodes for IT-SOFCs. Int J Hydrog Energy 37:2546–2551. https://doi.org/10.1016/j.ijhydene.2011.10.080
Stevenson JW, Armstrong TR, Pederson LR et al (1996) Electrochemical properties of mixed conducting La1-xMxCo1-yFeyO3-δ (M = Sr, Ca, Ba) perovskites. J Electrochem Soc 9:2722–2729. https://doi.org/10.1149/1.1837098
Zhao C, Zhou QJ, Zhang T, Qu LW, Yang X, Wei T (2019) Preparation and electrochemical properties of La1.5Pr0.5NiO4 and La1.5Pr0.5Ni0.9Cu0.1O4 cathode materials for intermediate-temperature solid oxide fuel cells. Mater Res Bull 113:25–30. https://doi.org/10.1016/j.materresbull.2019.01.016
Lu J, Yin Y-M, Yin J, Li J, Zhao J, Ma Z-F (2015) Role of Cu and Sr in improving the electrochemical performance of cobalt-free Pr1-xSrxFe1-yCuyO3-δ cathode for intermediate temperature solid oxide fuel cells. J Electrochem Soc 163:F44–F53. https://doi.org/10.1149/2.0181602jes
Du Z, Zhao H, Li S et al (2018) Exceptionally high performance anode material based on lattice structure decorated double perovskite Sr2FeMo2/3Mg1/3O6−δ for solid oxide fuel cells. Adv Energy Mater 2018:1800062. https://doi.org/10.1002/aenm.201800062
Adler S, Chen X, Wilson J (2007) Mechanisms and rate laws for oxygen exchange on mixed-conducting oxide surfaces. J Catal 245:91–109. https://doi.org/10.1016/j.jcat.2006.09.019
Fu C, Sun K, Zhang N, Chen X, Zhou D (2007) Electrochemical characteristics of LSCF–SDC composite cathode for intermediate temperature SOFC. Electrochim Acta 52:4589–4594. https://doi.org/10.1016/j.electacta.2007.01.001
Wang Y, Zhao X, Lü S, Meng X, Zhang Y, Yu B, Li X, Sui Y, Yang J, Fu C, Ji Y (2014) Synthesis and characterization of SmSrCo2−xMnxO5+δ (x=0.0, 0.2, 0.4, 0.6, 0.8, 1.0) cathode materials for intermediate-temperature solid-oxide fuel cells. Ceram Int 40:11343–11350. https://doi.org/10.1016/j.ceramint.2014.03.113
Kim JH, Manthiram A (2008) LnBaCo2O5+δ oxides as cathodes for intermediate-temperature solid oxide fuel cells. J Electrochem Soc 155:B385–B390. https://doi.org/10.1149/1.2839028
Jin F, Xu H, Long W, Shen Y, He T (2013) Characterization and evaluation of double perovskites LnBaCoFeO5+δ (Ln = Pr and Nd) as intermediate-temperature solid oxide fuel cell cathodes. J Power Sources 243:10–18. https://doi.org/10.1016/j.jpowsour.2013.05.187
Ishihara T, Kudo T, Matsuda H, Takita Y (1995) Doped PrMnO3 perovskite oxide as a new cathode of solid oxide fuel cells for low temperature operation. J Electrochem Soc 142:1519–1524. https://doi.org/10.1149/1.2048606
Ding X, Kong X, Jiang J, Cui C, Guo L (2010) Electrochemical performance of La0.7Sr0.3CuO3-δ-Sm0.2Ce0.8O2-δ functional graded composite cathode for intermediate temperature solid oxide fuel cells. Int J Hydrog Energy 35:1742–1748. https://doi.org/10.1016/j.ijhydene.2009.12.025
Sun L-P, Li Q, Zhao H, Huo L-H, Grenier J-C (2008) Preparation and electrochemical properties of Sr-doped Nd2NiO4 cathode materials for intermediate-temperature solid oxide fuel cells. J Power Sources 183:43–48. https://doi.org/10.1016/j.jpowsour.2008.05.001
Wang J, Lam KY, Saccoccio M, Gao Y, Chen D, Ciucci F (2016) Ca and In Co-doped BaFeO3−δ as a cobalt-free cathode material for intermediate-temperature solid oxide fuel cells. J Power Sources 324:224–232. https://doi.org/10.1016/j.jpowsour.2016.05.089
Kolchina LM, Lyskov NV, Kuznetsov AN, Kazakov SM, Galin MZ, Meledin A, Abakumov AM, Bredikhin SI, Mazo GN, Antipov EV (2016) Evaluation of Ce-doped Pr2CuO4 for potential application as a cathode material for solid oxide fuel cells. RSC Adv 6:101029–101037. https://doi.org/10.1039/C6RA21970E
Funding
This work was supported by the Natural Science Foundation of China (nos. 51902126, 61605059 and 21701047), Program for the development of Science and Technology of Jilin province (nos. 20180414008GH and 20180520191JH), the 13th Five-Year Program for Science and Technology of Education Department of Jilin Province (nos. JJKH20180784KJ and JJKH20191024KJ), Open Project of Key Laboratory of Functional Materials Physics and Chemistry of the Ministry of Education, Jilin Normal University (no. 2016002) and Program for the development of Science and Technology of Siping City (no. 2017055).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, X., Liu, M., Meng, X. et al. Cobalt-free perovskite Ln0.5Sr0.5Fe0.8Cu0.2O3-δ (Ln = Pr, Nd, Sm, and Gd) as cathode for intermediate-temperature solid oxide fuel cell. Ionics 26, 1285–1295 (2020). https://doi.org/10.1007/s11581-019-03314-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03314-9