Abstract
A unique hierarchical hollow-nanostructure consists of ultrathin MoS2 nanosheets and hollow MoO2 nanospheres has been designed as anode material for lithium batteries. And a simple process for producing ultrathin MoS2 nanosheets in-situ grown on hollow MoO2 nanospheres is reported. Such a hierarchical nanostructure has four advantages: Firstly, the high electric conductivity of the MoO2 core can effectively increase the performance of the composite. Secondly, the shell of MoS2 nanosheets with highly exposed active sites can improve the electrochemical reaction activity of this heterostructure. Thirdly, the reciprocal hybridization between the MoO2 core and MoS2 shell can availably prevent the aggregation of MoS2 nanosheets. Owing to the unique hierarchical MoO2@MoS2 hollow-nanostructure, it exhibits great electrochemical performance and can deliver reversible capacity as high as 820.7 mA h g−1 at a current density of 0.5 A g−1 after 100 cycles, while it is used as a new anode material for lithium-ion batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rechargeable lithium-ion batteries have been applied widely in portable electronics and electric vehicles for their advantages of high energy density, high voltage, and environmental friendliness. Whereas, graphite used as commercial lithium-ion batteries, anode material has only a theoretical capacity of 372 mA h g−1 and cannot meet people’s ever-increasing demanding of higher energy-density and power-density energy storage system [1,2,3,4]. Compared to graphite, transitional metal oxides and chalcogenides have advantages of low-cost facile fabrication and much higher specific capacity [5,6,7,8,9]. Among them, MoO2 has a theoretical capacity of 838 mA h g−1 and high electric conductivity near to metal, but on account of internal sluggish kinetics reaction rates and accumulated volume variation in bulk MoO2, it needs a relatively long period to activate MoO2 and results in reversible capacity decay in a short time [10,11,12,13]. MoS2 has a two-dimension structure like graphene with high reactivity and delivers a capacity of 800–1100 mA h g−1 but fades rapidly due to volume expansion and lamella staking produced in the process of charge/discharge [14,15,16,17].
To overcome the above obstacles of MoS2 and MoO2, many strategies have been reported. One effective strategy is to assemble MoS2 nanosheets into the three-dimensional hierarchical structure to maintain high contact areas [18,19,20]. The nanosheet subunits could provide plenty of active edge sites, and the primary structure could avoid the aggregation of MoS2 nanosheets [21, 22]. Another is to synthesize MoS2/C composite to reduce aggregation and pulverization of MoS2 and improve its electric conductivity [23, 24].
MoO2 has high electric conductivity and high volumetric capacity density (6.4 g cm−3) compared with that of carbon. As a result, MoO2@MoS2 composite also has been considered as an ideal composite for Li storage. Recently, a few studies demonstrated that the MoO2/MoS2 composites could deliver higher specific capacity and better cycle performance compared with that of pure MoO2 or MoS2. Such as, Deng and co-worker synthesized ultrafine MoO2 particles with few-layer MoS2 delivering a high specific capacity of 787 mA h g−1 at 0.8 A g−1 [25]. Xu and co-worker fabricated MoO2@MoS2 nanoarchitectures by growing MoS2 nanosheets vertically on the surface of MoO2 particle. And its specific capacity remained 1019 mA h g−1 at 0.1 A g−1 after 200 cycles [26]. Nanocarved MoS2-MoO2 nanobelt hybrid was synthesized by Xiao and co-worker exhibiting impressive electrochemical performance [27]. However, the electrochemical performance of the MoO2/MoS2 composites in these reports has not yet gelled into a satisfying result, so additional improvement is still needed. It is highly expected that the rational design of MoO2/MoS2 nanostructure can effectively integrate the advantages of MoO2 and MoS2. Considering that, both MoO2 and MoS2 would have volume variation during charge/discharge process [13, 28], leading to pulverization and poor cyclability. So, the employment of hollow nanostructure would provide interior space to alleviate the strain and accommodate the volume change [29,30,31,32,33]. In addition, the permeable shell could also shorten the diffusion distance [19]. Therefore, synthesizing MoO2/MoS2 heterostructure by assembling MoS2 subunits into three-dimensional hollow MoO2 would be an effective way to enhance the electrochemical performance of the MoO2/MoS2 composites.
In this work, a unique hierarchical MoO2@MoS2 nanostructure consisting ultrathin MoS2 nanosheets assembled on hollow MoO2 nanospheres was prepared as an anode material for Li storage. Moreover, the MoO2@MoS2 heterostructure is synthesized via the reaction between thiourea and a solid MoO2 nanospheres. In the reaction process, thiourea decomposes to sulfuretted hydrogen and reacts with the MoO2 forming MoS2 nanosheets on the surface simultaneously. Thereafter, due to the Ostwald ripening mechanism, MoO2 core gradually dissolved and MoS2 shell recrystallized, which finally gives rise to a hollow hierarchical MoO2@MoS2 nanostructure [34,35,36]. In this strategy, the ultrathin MoS2 nanosheets are in-situ grown on hollow MoO2 nanospheres. In consideration of the unique structure and the reciprocal hybridization between MoO2 and MoS2, it is rational to expect that MoO2@MoS2 heterostructure would show excellent electrochemical performance in lithium-ion batteries.
Experimental section
Material preparation
Synthesis of MoO2: uniform MoO2 nanospheres was firstly obtained according to our previous literature method [37]. In a typical procedure, 0.2 g MoO3 was firstly dispersed in the mixed solution of 15 ml ethanol and 15 ml ethylene glycol, followed with stirring around 6 h. Subsequently, the milk-white solution was put into a 50-ml Teflon-lined autoclave and heated to 200 °C for 6 h. After cooling down to room temperature, the brown products were obtained by centrifugation and washed several times alternatively with distilled water and absolute ethyl alcohol then dried for 24 h at 60 °C. The dried precursors were loaded in the heating zone of quartz tube in the furnace and were calcined at 600 °C for 5 h in the atmosphere of Ar with the heating rate of 5 °C min−1. After cooling down to ambient temperature, the samples were passivated in the atmosphere of 1% O2/Ar(v/v) for 4 h.
Synthesis of MoO2@MoS2: the above obtained 0.16 g MoO2 products were dispersed in 30 ml distilled water by ultrasonication for 15 min. After that, 0.32 g thiourea was added into the solution and stirred for 20 min. Then the solution was transferred into a 50-ml Teflon-lined autoclave and kept in an oven at 200 °C for 24 h. After the autoclave cooling down to ambient temperature in the oven, the black products were collected and washed with distilled water and absolute ethanol via centrifugation and dried at 60 °C for 24 h.
Material characterization
X-ray powder diffraction (XRD) to analyze the crystal structure was performed on a Rigaku D/max 2500 XRD diffractometer (Cu-Kα radiation, l 1/4 1.54178 Å). The morphology and microstructure of the products were characterized by field emission scanning electron microscope (FESEM, FEI Nova NanoSEM 230) and field emission transmission electron microscope (FETEM, Tecnai G2 F20 S-TWIN TMP). The chemical composition was evaluated by energy dispersive X-ray spectroscope (EDX). N2 adsorption/desorption measurement was performed on Micromeritics ASPA 2460 Surface Area and Porosity Analyzer. The specific area was calculated by Brunauer-Emmett-Teller (BET) method.
Electrochemical characterization
To prepare the testing electrode, the active materials, a conductive agent (super P) and binder (polyacrylic acid) were mixed with a weight ratio of 7:2:1. Then, the mixture was dissolved in an appropriate amount of N-methyl-2-pyrrolidinone (NMP) and stirred for 24 h to obtain a homogeneous slurry. Afterward, the resulting slurry was coated on Cu foil and dried at 100 °C for 12 h under vacuum. The mass loading of each electrode is about 1.4–1.7 mg (equals to 1.24–1.51 mg cm−2). The 2016-type coin cells were then assembled in an argon-filled glove box by using lithium disc as a counter electrode, 1 M LiPF6 in ethylene carbonate/dimethyl carbonate with fluoroethylene carbonate (EC/DMC, 1:1 v/v + 2%FEC) as an electrolyte, and polyethylene membrane as a separator. Galvanostatically charge/discharge (GCD) measurement was performed on a Land Battery Tester (Land CT 2001A, Wuhan, China). The cyclic voltammetry (CV) was carried on a Chi604e electrochemical workstation at a scan rate of 0.1 mV s−1 in the voltage range of 0.01–3 V.
Results and discussion
The crystal structure of the MoO2@MoS2 was characterized by XRD measurement. The pattern of MoO2 with three major diffractions peaking at 26.2°, 36.8°, 53.7° shown in Fig. 1a could be assigned to the (-111), (-211), (-312) facet of typical monoclinic MoO2 (JCPDS card 32-0671). It should be mentioned that the three obvious broaden peaks indicate the MoO2 would have very small subgrain. Fig. 1b presents the XRD pattern of as-obtained MoO2@MoS2. It can be seen that the main three peaks were remained indicating the presence of MoO2.
Besides, the three other pronounced peaks located at 14.4°, 32.3°, and 59.5° can be predominantly indexed to the (002), (100), and (110) planes of hexagonal 2H-MoS2 (37-1492). The representative (002) peak of MoS2 can also be used to calculate the interlamellar distance of the two-dimensional structure. According to the Bragg equation, the interlayer spacing is 6.2 Å. No diffraction peaks of other phase or impurities have been found proving the high-purity of the MoO2@MoS2.
FESEM was used to observe the morphology of the materials and the results were shown in Fig. 2.
As shown in Fig. 2a, MoO2 has homogeneous spheres with uniform size [37]. Magnified image of MoO2 was shown in Fig. 2b. It can be clearly observed that the surface of the spheres is relatively smooth and the size of as-formed spheres was about 500 nm. Figure 2c–d show the morphology of MoO2@MoS2; uniform spheres can also be found in Fig. 2c of low-magnified FESEM image. In contrast to MoO2, it has a larger sphere size than MoO2. Magnified FESEM image revealed that the size of MoO2@MoS2 is approximate 700–800 nm. Unlike the relatively smooth surface of MoO2, MoO2@MoS2 has a rough surface. More specifically, the surface consists of randomly assembled ultrathin nanosheets. In our work, thiourea as the sulfur source will release sulfuretted hydrogen in the process of reaction and will react with MoO2. The resultant is on the basis of MoO2 spheres with MoS2 formed and in-situ grown on MoO2. Thus, the surface of as-formed MoO2@MoS2 consists of MoS2 nanosheets and forms the hierarchical structure. Meanwhile, it can be clearly seen, there are relatively large interspaces between the MoS2 subunits supplying extra spaces for the volume expansion in the cycling process [26]. Compared to the relatively smooth surface of as-obtained MoO2, such secondary structure can fully utilize the two-dimension structure of MoS2, to increase the contact areas with the electrolyte. It can boost the electrochemical reaction rates and shorten the distance, the diffusion of the ions, and electrons thus improve the electrochemical performance of the materials [38,39,40,41]. To determine the composition of MoO2@MoS2, EDX was carried out and the result was shown in Fig. S1. The molar ration of MoO2 and MoS2 is 0.31:0.69. The specific surface area was calculated by using BET method. N2 adsorption/desorption was performed as shown in Fig. S2. The measured specific area is about 28.64 m2 g−1. Besides, it indicates a type IV curve with H3 type hysteresis. The behavior is related to the secondary capillary condensation [42, 43].
To further reveal the microstructure and morphology of the materials, TEM was applied to observe MoO2 and MoO2@MoS2 and the results were shown in Fig. 3.
From the TEM image of MoO2 in Fig. 3a, it can be observed that MoO2 sphere is a solid structure in nature. Besides, its surface is not fully smooth and there are some small particles around the surface. In the high-resolution TEM shown in the inset of Fig. 3a, it revealed that MoO2 is made up of small particles with the size of 20–30 nm. Such secondary structures are corresponding to the broader peaks in the XRD pattern of MoO2. TEM image of MoO2@MoS2 was shown in Fig. 3b demonstrating the hollow sphere structure of as-formed MoO2@MoS2. Magnified images of MoO2@MoS2 illustrate the shell of the spheres which are composed of MoS2 nanosheets in agreement with the SEM images, and the thickness of the shell is around 180–200 nm. High-resolution TEM image shown in Fig. 3d provides further insights into the MoS2 nanosheets of the shell. The thickness of the nanosheets is about 4–10 nm consisting of dozens of lattice planes. The lattice spacing with a distance of around 0.62 nm is consistent with the (002) facets distance value calculated from the XRD. To better understand the composition of the MoO2@MoS2, the scanning transmission electron microscopy and the element mapping images of MoO2@MoS2 are provided as shown in Fig. 3e–h. The presence of Mo, O, and S element demonstrates their uniform distribution. The intense of O is weaker because MoO2 was in the inner shell and was coated by MoS2. Such hollow structure could increase the contact area between electrode and electrolyte, in favor of maintaining its structure by the mean time [44, 45]. On the one side, MoO2 in the shell can utilize the high conductivity of MoO2 to overcome the poor conductivity of MoS2, and the synergistic effect could also promote the reactivity of MoO2@MoS2 [29, 46]. On the other side, MoS2 nanosheets with dozens of layers randomly assembled hollow sphere can improve kinetic rates of the materials and shorten the distance of ions and electrons during charging/discharging. Besides, hierarchical structure with relatively large space between nanosheets would provide extra space during charge/discharge and promote cycling stability.
To investigate the charge/discharge process of as-obtained materials, CV and GCD tests at a current density of 100 mA g−1 were performed as shown in Fig. 4a–b. In the first cathodic sweep, the very pronounced peak at 0.4 V can be attributed to the SEI formation and the conversion reaction of LixMoS2 to Mo and Li2S [47, 48]. It disappeared in the subsequent sweep, which indicates that it is an irreversible reaction. Except for the first discharge curve, the other curves of later charge/discharge overlap well. Strong oxidation peak at 2.25 V and reduction peak at 1.85 V are derived from the reversible reaction of Li2S and S\( {}_8^{2-} \) [49, 50]. The other peaks at 1.74, 1.5, and 1.47 V are corresponding to a four-step monoclinic-orthorhombic phase transformation of LixMoO2 and MoO2 during the charge/discharge process [51, 52]. In the first discharge process shown in Fig. 4b, a potential plateau around 0.5 V corresponding to the conversion reaction in agreement with the first CV cathodic scan which indicates LixMoS2 converts to Mo and Li2S. Similar to the CV curve, other curves which overlap well indicate high reversibility except for the first discharge curve. Besides, the first discharge, specific capacity is 1122 mA h g−1 with the coulombic efficiency of 75% which mainly due to the irreversible reaction and formation of SEI [53, 54].
GCD under a current density of 200 mA g−1 was applied to investigate the electrochemical performances of materials as shown in Fig. 4c. The first discharge capacity of MoO2@MoS2 is 953 mA h g−1, and then, the capacity fades to 902 mA h g−1 that lost about 5.35% which mostly originated from SEI formation and irreversible reaction. The coulomnbic efficiency in the first cycle was 85% and then maintain at around 100% showing excellent charge/discharge performances. Along with the cycling test, the capacity will slightly increase which was ascribed to the activation process of MoO2 during the charge/discharge. It is mainly because only a certain amount of MoO2 undergoes the conversion reaction due to the poor kinetics of LixMoO2 at the beginning. But the reaction leads to partially crystallinity degradation of the MoO2 or convert it into amorphous structures. Thus, it boosts the ion diffusion kinetics and causes more MoO2 to undergo the reaction [55, 56]. After 50 cycles, the capacity of 917 mA h g−1 still can be remained with high capacity retention of 96.2% and only 3.8% decay on the basis of the first discharge capacity. In addition, rate performance is exhibited in Fig. 4d at the current density of 0.1, 0.2, 0.3, 0.5, 1.0 A g−1. The first discharge capacity is 1150, 950, 890, 830, and 700 mA h g−1. After current density back to 0.1 A g−1, its capacity could gradually restore to about 1000 mA h g−1 demonstrating good capacity restore performances.
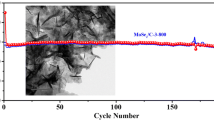
In order to compare the electrochemical performance of MoO2 and MoO2@MoS2, the charge/discharge cycling test was evaluated at a current density of 500 mA g−1 in the voltage range of 0.01–3 V and the result is shown in Fig. 5.
The specific discharge capacity of MoO2@MoS2 in the first cycle was 929.7 mA h g−1. A fade occurs in the second and the third cycle mainly because of the irreversible reaction. From the third cycle onwards, the capacity starts to increase due to the activation process of MoO2. After 100 cycles, its capacity still remains as 820.7 mA h g−1, showing excellent cycle performance. In addition, SEM was carried out to investigate the MoO2@MoS2 electrode after 40 cycles as shown in Fig. S3. It can be observed that the sphere morphology could be maintained after charge/discharge process. As a contrast, the first discharge capacity of MoO2 was 622.3 mA h g−1. After that, MoO2 underwent an activating stage, and the capacity began to rise up to the 21 cycles; the capacity reaches its peak of 752.7 mA h g−1. After 100 cycles, the capacity of MoO2 faded to 557.3 mA h g−1. In contrast to MoO2, MoO2@MoS2 delivers higher capacity accompanying with less fade and displayed better cycling stability and energy storage performance.
Conclusions
In summary, we successfully synthesized hierarchical MoO2@MoS2 hollow-nanostructure consisting of ultrathin MoS2 nanosheets and a hollow MoO2 nanospheres. This strategy only involves a facile method via thiourea reaction with uniform solid MoO2 nanospheres, which leads ultrathin MoS2 nanosheets in-situ grown on hollow MoO2 nanospheres. The obtained hierarchical MoO2@MoS2 hollow-nanostructure integrated the advantages of the high conductivity of MoO2 and the two-dimensional structure of MoS2 to improve the electrochemical reaction activity. When evaluated as anode materials of lithium batteries, it delivers the specific capacity of 1150 mA h g−1 in the first cycle at the current density of 100 mA g−1 and still remains the specific capacity of 820.7 mA h g−1 after charge/discharge for 100 cycles at a current density of 500 mA g−1. Compared to MoO2, it exhibits better energy storage performances owing to the advantages of unique secondary hierarchical hollow structures.
References
Zhao C, Kong J, Yao X, Tang X, Dong Y, Phua SL, Lu X (2014) Thin MoS2 nanoflakes encapsulated in carbon nanofibers as high-performance anodes for lithium-ion batteries. ACS Appl Mater Interfaces 6:6392–6398
Li HZ, Yang LY, Liu J, Li ST, Fang LB, Lu YK, Yang HR, Liu SL, Lei M (2016) Improved electrochemical performance of yolk-shell structured SnO2@void@C porous nanowires as anode for lithium and sodium batteries. J Power Sources 324:780–787
Pan X, Li S, Wang Z, Yang L-Y, Zhu K, Ren L, Lei M, Liu J (2017) Core–shell MoO2/C nanospheres embedded in bubble sheet-like carbon film as lithium ion battery anodes. Mater Lett 199:139–142
Lu Y, Wu J, Liu J, Lei M, Tang S, Lu P, Yang L, Yang H, Yang Q (2015) Facile synthesis of Na0.33V2O5 nanosheet-graphene hybrids as ultrahigh performance cathode materials for lithium ion batteries. ACS Appl Mater Interfaces 7:17433–17440
Zhou J, Qin J, Zhang X, Shi C, Liu E, Li J, Zhao N, He C (2015) 2D space-confined synthesis of few-layer MoS2 anchored on carbon nanosheet for lithium-ion battery anode. ACS Nano 9:3837–3848
Tan C, Zhang H (2015) Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem Soc Rev 44:2713–2731
Zhong W, Tu W, Feng S, Xu A (2019) Photocatalytic H2 evolution on CdS nanoparticles by loading FeSe nanorods as co-catalyst under visible light irradiation. J Alloy Compounds 772:669–674
Huang X, Zeng Z, Zhang H (2013) Metal dichalcogenide nanosheets: preparation, properties and applications. Chem Soc Rev 42:1934–1946
Huang X, Cai X, Xu D, Chen W, Wang S, Zhou W, Meng Y, Fang Y, Yu X (2018) Hierarchical Fe2- O3@CNF fabric decorated with MoS2 nanosheets as a robust anode for flexible lithium-ion batteries exhibiting ultrahigh areal capacity. J Mater Chem A 6:16890–16899
Wang Z, Chen JS, Zhu T, Madhavi S, Lou XW (2010) One-pot synthesis of uniform carbon-coated MoO2 nanospheres for high-rate reversible lithium storage. Chem Commun 46:6906–6908
Petnikota S, Teo KW, Chen L, Sim A, Marka SK, Reddy MV, Srikanth VV, Adams S, Chowdari BV (2016) Exfoliated graphene oxide/MoO2 composites as anode materials in lithium-ion batteries: an insight into intercalation of Li and conversion mechanism of MoO2. ACS Appl Mater Interfaces 8:10884–10896
Zhang P, Zou L, Hu H, Wang M, Fang J, Lai Y, Li J (2017) 3D hierarchical carbon microflowers decorated with MoO2 nanoparticles for lithium ion batteries. Electrochim Acta 250:219–227
Wang S, Zhang Z, Yang Y, Tang Z (2017) Efficient lithium-ion storage by hierarchical core-shell TiO2 nanowires decorated with MoO2 quantum dots encapsulated in carbon nanosheets. ACS Appl Mater Interfaces 9:23741–23747
Yu XY, Yu L, Lou XW (2016) Metal sulfide hollow nanostructures for electrochemical energy storage. Adv Energy Mater 6:1501333
Nguyen QH, Hur J (2019) MoS2–TiC–C nanocomposites as new anode materials for high-performance lithium-ion batteries. J Nanosci Nanotechnol 19:996–1000
Li X, Zai J, Xiang S, Liu Y, He X, Xu Z, Wang K, Ma Z, Qian X (2016) Regeneration of metal sulfides in the delithiation process: the key to cyclic stability. Adv Energy Mater 6:1601056
Jiang H, Ren D, Wang H, Hu Y, Guo S, Yuan H, Hu P, Zhang L, Li C (2015) 2D monolayer MoS2-carbon interoverlapped superstructure: engineering ideal atomic interface for lithium ion storage. Adv Mater 27:3687–3695
Wang PP, Sun H, Ji Y, Li W, Wang X (2014) Three-dimensional assembly of single-layered MoS2. Adv Mater 26:964–969
Wang M, Li G, Xu H, Qian Y, Yang J (2013) Enhanced lithium storage performances of hierarchical hollow MoS2 nanoparticles assembled from nanosheets. ACS Appl Mater Interfaces 5:1003–1008
Wang Y, Yu L, Lou XW (2016) Synthesis of highly uniform molybdenum–glycerate spheres and their conversion into hierarchical MoS2 hollow nanospheres for lithium-ion batteries. Angew Chem Int Ed 55:7423–7426
Zhang L, Wu HB, Yan Y, Wang X, Lou XW (2014) Hierarchical MoS2 microboxes constructed by nanosheets with enhanced electrochemical properties for lithium storage and water splitting. Energy Environ Sci 7:3302–3306
Yan Y, Ge X, Liu Z, Wang JY, Lee JM, Wang X (2013) Facile synthesis of low crystalline MoS2 nanosheet-coated CNTs for enhanced hydrogen evolution reaction. Nanoscale 5:7768–7771
Zhu C, Mu X, van Aken PA, Yu Y, Maier J (2014) Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage. Angew Chem Int Ed 53:2152–2156
Chen YM, Yu XY, Li Z, Paik U, Lou XW (2016) Hierarchical MoS2 tubular structures internally wired by carbon nanotubes as a highly stable anode material for lithium-ion batteries. Sci Adv 2:e1600021
Deng Z, Hu Y, Ren D, Lin S, Jiang H, Li C (2015) Reciprocal hybridization of MoO2 nanoparticles and few-layer MoS2 for stable lithium-ion batteries. Chem Commun 51:13838–13841
Xu Z, Wang T, Kong L, Yao K, Fu H, Li K, Cao L, Huang J, Zhang Q (2017) MoO2@MoS2 Nanoarchitectures for high-loading advanced lithium-ion battery anodes. Part Part Syst Charact 34:1600223
Xiao D, Zhang J, Li X, Zhao D, Huang H, Huang J, Cao D, Li Z, Niu C (2016) Nano-carved MoS2-MoO2 hybrids fabricated using in situ grown MoS2 as nano-masks. ACS Nano 10:9509–9515
Chen B, Liu E, Cao T, He F, Shi C, He C, Ma L, Li Q, Li J, Zhao N (2017) Controllable graphene incorporation and defect engineering in MoS2-TiO2 based composites: towards high-performance lithium-ion batteries anode materials. Nano Energy 33:247–256
Zhang L, Lou XW (2014) Hierarchical MoS2 shells supported on carbon spheres for highly reversible lithium storage. Chem Eur J 20:5219–5223
Zhang G, Xia BY, Xiao C, Yu L, Wang X, Xie Y, Lou XW (2013) General formation of complex tubular nanostructures of metal oxides for the oxygen reduction reaction and lithium-ion batteries. Angew Chem Int Ed 125:8916–8916
Yu L, Zhang L, Wu HB, Lou XW (2014) Formation of NixCo3-xS4 hollow nanoprisms with enhanced pseudocapacitive properties. Angew Chem Int Ed 53:3711–3714
Wang S, Guan BY, Yu L, Lou XW (2017) Rational design of three-layered TiO2@ carbon@ MoS2 hierarchical nanotubes for enhanced lithium storage. Adv Mater 29:1702724
Lu J, Xia G, Gong S, Wang C, Jiang P, Lin Z, Wang D, Yang Y, Chen Q (2018) Metallic 1T phase MoS2 nanosheets decorated hollow cobalt sulfide polyhedrons for high-performance lithium storage. J Mater Chem A 6:12613–12622
Li P, Jeong JY, Jin B, Zhang K, Park JH (2018) Vertically oriented MoS2 with spatially controlled geometry on nitrogenous graphene sheets for high-performance sodium-ion batteries. Adv Energy Mater 8:1703300
Zhang Y, Wang C, Hou H, Zou G, Ji X (2017) Nitrogen doped/carbon tuning yolk-like TiO2 and its remarkable impact on sodium storage performances. Adv Energy Mater 7:1600173
Weng W, Lin J, Du Y, Ge X, Zhou X, Bao J (2018) Template-free synthesis of metal oxide hollow micro-/nanospheres via Ostwald ripening for lithium-ion batteries. J Mater Chem A 6:10168–10175
Min J, Wang K, Liu J, Yao Y, Wang W, Yang L, Zhang R, Lei M (2017) Facile synthesis of uniform MoO2/Mo2CTx heteromicrospheres as high-performance anode materials for lithium-ion batteries. J Power Sources 363:392–403
Wang Y, Ma Z, Chen Y, Zou M, Yousaf M, Yang Y, Yang L, Cao A, Han RPS (2016) Controlled synthesis of core-shell carbon@MoS2 nanotube sponges as high-performance battery electrodes. Adv Mater 28:10175–10181
Teng Y, Zhao H, Zhang Z, Li Z, Xia Q, Zhang Y, Zhao L, Du X, Du Z, Lv P, Świerczek K (2016) MoS2 nanosheets vertically grown on graphene sheets for lithium-ion battery anodes. ACS Nano 10:8526–8535
Chang K, Chen W (2011) L-cysteine-assisted synthesis of layered MoS2 graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 5:4720–4728
Jing Y, Ortiz-Quiles EO, Cabrera CR, Chen Z, Zhou Z (2014) Layer-by-layer hybrids of MoS2 and reduced graphene oxide for lithium ion batteries. Electrochim Acta 147:392–400
Sing KS, Williams RT (2004) Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt Sci Technol 22:773–782
Xie T, Min J, Liu J, Chen J, Fu D, Zhang R, Zhu K, Lei M (2018) Synthesis of mesoporous Co3O4 nanosheet-assembled hollow spheres towards efficient electrocatalytic oxygen evolution. J Alloys Compounds 754:72–77
Chang K, Geng D, Li X, Yang J, Tang Y, Cai M, Li R, Sun X (2013) Ultrathin MoS2/nitrogen-doped graphene nanosheets with highly reversible lithium storage. Adv Energy Mater 3:839–844
Zhong W, Shen S, Feng S, Lin Z, Wang Z, Fang B (2018) Facile fabrication of alveolate Cu2−xSe microsheets as a new visible-light photocatalyst for discoloration of Rhodamine B. CrystEngComm 20:7851–7856
Chen B, Liu E, He F, Shi C, He C, Li J, Zhao N (2016) 2D sandwich-like carbon-coated ultrathin TiO2-@defect-rich MoS2 hybrid nanosheets: synergistic-effect-promoted electrochemical performance for lithium ion batteries. Nano Energy 26:541–549
Jeong JM, Lee KG, Chang SJ, Kim JW, Han YK, Lee SJ, Choi BG (2015) Ultrathin sandwich-like MoS2@N-doped carbon nanosheets for anodes of lithium ion batteries. Nanoscale 7:324–329
Hu L, Ren Y, Yang H, Xu Q (2014) Fabrication of 3D hierarchical MoS2/polyaniline and MoS2/C architectures for lithium-ion battery applications. ACS Appl Mater Interfaces 6:14644–14652
Kong D, He H, Song Q, Wang B, Lv W, Yang Q-H, Zhi L (2014) Rational design of MoS2@graphene nanocables: towards high performance electrode materials for lithium ion batteries. Energy Environ Sci 7:3320–3325
Fang X, Hua C, Guo X, Hu Y, Wang Z, Gao X, Wu F, Wang J, Chen L (2012) Lithium storage in commercial MoS2 in different potential ranges. Electrochim Acta 81:155–160
Liu J, Tang S, Lu Y, Cai G, Liang S, Wang W, Chen X (2013) Synthesis of Mo2N nanolayer coated MoO2 hollow nanostructures as high-performance anode materials for lithium-ion batteries. Energy Environ Sci 6:2691–2697
Yang Q, Liang Q, Liu J, Liang S, Tang S, Lu P, Lu Y (2014) Ultrafine MoO2 nanoparticles grown on graphene sheets as anode materials for lithium-ion batteries. Mater Lett 127:32–35
Ma L, Huang G, Chen W, Wang Z, Ye J, Li H, Chen D, Lee JY (2014) Cationic surfactant-assisted hydrothermal synthesis of few-layer molybdenum disulfide/graphene composites: microstructure and electrochemical lithium storage. J Power Sources 264:262–271
Choi SH, Kang YC (2015) Synergetic effect of yolk-shell structure and uniform mixing of SnS-MoS2 nanocrystals for improved Na-ion storage capabilities. ACS Appl Mater Interfaces 7:24694–24702
Guo B, Fang X, Li B, Shi Y, Ouyang C, Hu YS, Wang Z, Stucky GD, Chen L (2012) Synthesis and lithium storage mechanism of ultrafine MoO2 nanorods. Chem Mater 24:457–463
Reddy MV, Rao GVS, Chowdari BVR (2013) Metal oxides and oxysalts as anode materials for Li ion batteries. Chem Rev 113:5364–5457
Funding
This work is supported by the National Natural Science Foundation of China (Grant Nos. 51772331, 51472271), the National Basic Research Program of China (973 Program) grant No. 2013CB932901, and the Project of Innovation-driven Plan in Central South University (2016CX002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 280 kb)
Rights and permissions
About this article
Cite this article
Xie, J., Zhu, K., Min, J. et al. In-situ grown ultrathin MoS2 nanosheets on MoO2 hollow nanospheres to synthesize hierarchical nanostructures and its application in lithium-ion batteries. Ionics 25, 1487–1494 (2019). https://doi.org/10.1007/s11581-019-02863-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-02863-3