Abstract
The operation of polymer electrolyte membrane (PEM)-based fuel cells involves numerous physicochemical processes and components actively governing its function and, among them, gas transport phenomena and gas diffusion layer (GDL) are noteworthy, and the present paper provides a comprehensive assessment on gas diffusion mechanism, geometry of GDL components and related modelling studies involved in GDL fabrication. The impact of GDL on diffusion of reactants, water management and the transport of ions has also been systematically dealt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
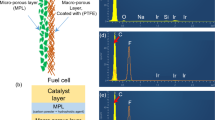
It is believed that there will be a time, in the future, when global energy demands will be met by sources other than fossil fuels, and fuel cells are expected to play a remarkable role in this because of their high fuel conversion efficiency and environmental compatibility [1]. Among the various fuel cells, PEM fuel cells are considered as one of the promising solutions and will attract numerous niche markets due to its superior characteristics and power density compared to conventional generators or advanced batteries. In a PEM fuel cell, hydrogen gas diffuses through the gas diffusion medium or gas diffusion layer (GDL) and reaches the anode catalyst site where it is electrochemically oxidized to protons and electrons. The membrane transports the protons to the cathode, but the electrons are forced to travel in an external circuit (since the membrane is electrically insulating) to generate electric power. At the cathode, oxygen/air gets electrochemically reduced and combines with protons, thereby producing water and heat as the by-products. The typical structure of a PEM fuel cell, with flow field, membrane-electrode-assembly (MEA), catalyst layer (CL), gas diffusion layer (GDL) and electrochemical reactions is illustrated in Fig. 1.
The vital components of a PEM fuel cell are bipolar plates, membrane and electrodes, catalyst and a GDL. The role of bipolar/flow field plates are to distribute the reactant gas over the surface of the electrodes through flow channels. They also collect the current and form the supporting structure of the fuel cell [2]. The function of the membrane is to conduct proton and impede electron. DuPont’s Nafion membranes are widely used due to their high proton conductivity and excellent chemical stability [3], and the dimension of state-of-the-art membranes are about 50 μm [4]. Integration of the gas diffusion layer with the microporous layer and catalyst layer is termed as gas diffusion electrode (GDE). The membrane, the catalyst (platinum supported on carbon particle) and the two GDEs are assembled into a sandwich structure to form a MEA [5]. GDL is an integral part of a MEA, and its principal functions are to efficiently transport the reactants and the products to and from the reaction sites as well as to conduct heat and current [6]. GDLs are typically porous composites and have a thickness in the range of 100 to 300 μm [7]. The GDL comprises of carbon for electrical conductivity and PTFE for hydrophobicity [8]. Figure 2 represents the cross section of a gas diffusion layer that provides a physical microporous support for the catalyst layer while allowing gas and water to transport to and from the catalyst layer.
Although, GDL significantly influences the performance of a PEM fuel cell in all the three polarization regions, the mass transport characteristics predominantly affect the performance of a gas diffusion layer (GDL) in the proton exchange membrane (PEM) fuel cell [9] as well its durability. An ideal GDL should offer properties such as superior gas diffusion with optimum bending stiffness, porosity, surface contact angle, air permeability, water vapour diffusion, hydrophobicity, hydrophilicity, corrosion resistance, crack-free surface morphology, high mechanical integrity and enhanced oxidative stability along with durability at various operating conditions including freezing [10–14]. Any weak spot in the GDL circuits will adversely impact the gas diffusion processes which in turn affect the performance. Paganin et al. [15] perceived that the diffusion layer has a small effect on the cell performance; however, later it has been revealed that altering the diffusion layer composition can even lead to substantial improvements in the PEMFC performance [16]. Understanding the design and functional characteristics of GDL may provide a significant contribution in the gas diffusion process and components so as to optimize the quality of MEAs and, consecutively, this kind of comprehensive review can lead to the commercialization of PEM fuel cell.
Gas diffusion and structural characteristics of GDL
Gas diffusive transport is a physical process involved in fuel cell stack; Fick’s law [17] predicts how diffusion causes the concentration to change with time, and the equation is governed as follows:
Where
- C i :
-
is the concentration.
- D eq i :
-
is the diffusion coefficient.
- Z :
-
is the position [length].
Fick’s law is insufficient in approximating the mass diffusion process due to its very fine pore sizes and as a result the effective diffusion coefficient is modified by Bruggeman correction. This alteration is employed in species transport of oxidants and fuels in the porous media of the PEM fuel cells. However, the texture of porous media is very complex and the relative influence of ordinary diffusion or Knudsen diffusion on species transport is governed by the pore geometry [18]. As presented by Nam and Kaviany [19], the effective diffusion coefficient in the porous media in PEM fuel cells is better depicted by using percolation theory, given as;
Where ε p is the percolation critical value and has been reported to be 0.11 and 0.13 by Pharoah et al. [20] and Liu and Wang [21], respectively. The results produced by the anisotropic diffusion coefficient reveal that the gas flow is much higher for in-plane direction than through-plane. Mass transfer can take place by Knudsen or Fickian diffusion if the pores are sufficiently small [22] as well as the diffusion characteristics of the macroporous layer can be examined by Fick’s laws, while the microporous layer generally exhibits Knudsen diffusion. The Knudsen number, K n used to characterize the regime of diffusion is defined as
Where λ g is the mean molecular free path and d p is the pore diameter.
An estimate of K n under practical cell operating conditions, shows that this usually occurs for the gases in a PEM fuel cell when the permeability is in the range of 10−16 to 10−17 m2 and for λ g /d p <<1 the Knudsen diffusion effect can be neglected [23].
The effective diffusion coefficient is related to the bulk diffusion coefficient through the MacMullin number and is governed by the equation
The MacMullin number is a parameter determined only by the morphology of the GDL and can be expressed as a generalized relationship with tortuosity (τ) and porosity (\( \varepsilon \)):
Where n and m are constants that depend on the geometrical model of the porous media; under some conditions of an operating fuel cell, pores in the GDL can be filled with liquid water, which effectively decreases the porosity for the gas stream. To account for this effect, an effective porosity is generally used [24] and described by:
Porosity quantifies the reduction in a cross-sectional area available for gaseous transport, while tortuosity characterizes the convoluted nature of the porous pathways followed by diffusing species. The tortuosity of each sample was estimated using the Bruggeman equation [25]:
The theoretical determination of tortuosity is model-dependent and extremely cumbersome for all but the simplest geometries. Studies by Springer et al. [26] reveal that the effective tortuous path length for gas diffusion in the cathode backing is about 2.6 times the thickness. The dependence of the permeability of a porous material on its porosity is often described by the Carman–Kozeny equation [27]:
Tamayol and Bahrami predicted that the in-plane permeability of GDLs is directly proportional to its porosity and the fibres diameter squared [28]. The geometry is also correlated to the water and thermal management and was good in a system with high permeability in at least one direction (in-plane or through-plane), while water and thermal management were poor in a system with low permeability in both directions [29]. Observations by Fishman et al. [30] revealed that the GDL has been shown to be an anisotropic and heterogeneous material with transport properties that vary significantly between the in-plane and through-plane directions. Ironically, Gostick et al. [27] claimed that most GDL materials were found to display higher in-plane than through-plane permeability. Measurement of relative permeability of GDL has received little attention; however, some early attempts to measure air relative permeability were reported by Koido et al. [31]. Properties of the GDL such as permeability, porosity, tortuosity and the hydrophobic treatment can affect the degree of flooding, thus changing the total fuel cell performance [8]. The absolute gas permeability of PEM fuel cells using numerous gas diffusion layer (GDL) materials was measured in three perpendicular directions to investigate the anisotropic properties, and it was observed that most materials were found to display higher in-plane permeability than through-plane. In their study, Hussaini et al. [32] measured the absolute permeability and air–water relative permeability functions for typical fuel cell GDL materials such as Toray carbon paper and E-Tek carbon cloth. Conclusions drawn from this study are that carbon paper materials and absolute permeability in the in-plane directions are found to be higher than their through-plane values by about 18 %, whereas for carbon cloth, through-plane permeability is found to be higher by about 75 %. PEM fuel cell performance may be strongly influenced by in-plane permeability of the GDL [33], and liquid water saturation is found to be a linear function of a capillary number. At a given capillary number, carbon papers show similar saturation in both directions, whereas carbon cloth shows higher saturation in the through-plane than in the in-plane. In general, GDL has a simpler structural configuration than the membrane and catalyst layer. A scanning electron microscope has been used to observe the morphology of the gas diffusion medium, namely carbon cloth and carbon paper as showed in Fig. 3.
Physical characterization of diffusion mediums (Table 1) are characterized by Benziger et al. [34], and their measurements provide details about the pore sizes of different gas diffusion media. Rofaiel et al. [35] presented a novel method for measuring heterogeneous through-plane PTFE distributions within the bulk of the GDL using energy dispersive X-ray spectrometry (EDS) imaging.
Electrochemical characteristics of GDL
Electrochemical impedance spectroscopy (EIS) is, perhaps, the most reliable tool for in situ fuel cell characterization [36]. Diffusion can also create impedance called Warburg impedance, which depends on the frequency of the potential perturbation at high frequencies; the Warburg impedance is small since diffusing reactants do not have to move very far and at low frequencies, and the reactants have to diffuse farther, increasing the impedance. The equation for the “infinite” Warburg impedance is:
σ is the Warburg coefficient defined as:
In which,
- ω :
-
radial frequency
- D O :
-
diffusion coefficient of the oxidant
- D R :
-
diffusion coefficient of the reductant
- A :
-
surface area of the electrode
- n :
-
number of electrons involved
AC impedance spectroscopy was performed by Springer et al. [26]; the impedance spectra of gas diffusion cathodes are measured under various conditions, and it is inferred that air cathode contains two features: a higher frequency loop or arc determined by interfacial charge–transfer resistance and a lower frequency loop determined by gas–phase transport limitations in the backing.
Carbon paper vs. carbon cloth
A GDL typically consists of a gas diffusion medium (GDM) and a microporous layer (MPL), and carbon cloth or non-woven carbon paper is widely used as a GDM due to its high gas permeability, electronic and heat conductivity. The suitable candidates for diffusion of reactant gases in PEM fuel cells is the carbon fibre-based products such as non-woven carbon papers and woven carbon cloths due to their high porosity (>70 %) and electrical conductivity. They are commercially available for many industrial applications and are now being extended to PEM fuel cell applications. The typical properties of these two materials are specified in Table 2.
Gallo Stampino et al. [38] studied the electrical performance of PEM fuel cells with gas diffusion layers made of carbon paper and carbon cloth and demonstrated that carbon paper substrate has superior performance in a vast range of current densities starting from open-circuit voltage to 0.8 A/cm2. Sasikumar et al. [39] investigated the performance of gas diffusion electrodes fabricated using carbon paper and carbon cloth and observed better performance when carbon paper was used as the backing material. Their studies showed that the limitation of mass transport was a concern with carbon cloth under non-pressurized operating conditions, especially at higher current densities, due to the higher thickness and density. Yuan-Kai Liao et al. [40] compared the performance of conventional carbon fibre cloth and PAN-based cloth that uses phenolic resin to improve the characteristics of the gas diffusion layer (GDL). Wang et al. studied the structure–performance relationship of carbon cloth and paper as GDM [37] and revealed that under dry conditions, the carbon paper is found to be better due to its higher tortuous pore structure, which retains product water in the MEA and enhances the membrane conductivity by reducing ohmic loss. However, they have observed that carbon cloth gives a better performance under humidified conditions. The experiment conducted by Williams et al. [41] was also in line with that of Wang et al. stating that the performance of the carbon cloth is superior to that of carbon paper at elevated humidity operations. Park and Popov [42] have analysed the influence of a GDL based on carbon paper/carbon cloth through various electrochemical techniques like mercury porosimetry, surface morphology analysis, polarization techniques, AC impedance spectroscopy, contact angle and water permeation measurement. They have observed that the MEA fabricated using carbon paper exhibited better performance when compared to carbon cloth, because of high water flow resistance owing to less permeable macro porous substrate, and more hydrophobic and compact microporous layer. The ac-impedance technique reveals that a microporous layer which has high volume of micropores and more hydrophobic property allows oxygen to diffuse freely towards the catalyst layer due to the effective removal of water from the catalyst layer to the gas flow channels.
Sahu et al. successfully synthesized GDL incorporating a mesoporous carbon with a high specific surface area and pore size [43]. Xie Zhi-yong et al. [44] compared the performance of PEM fuel cells with pyrocarbon and conventional carbon paper composites as GDM. The carbon paper was fabricated using a conventional precursor and coating it with pyrocarbon by pyrolyzing propylene via a chemical vapour deposition (CVD) method. For comparison, conventional carbon paper composites were prepared using PAN-based carbon felt as the precursor followed by impregnation with resin, moulding and thermal treatment. SEM characterization signposted that pyrocarbon was uniformly and tightly bonded on the surface of the fibre; in contrast, cracks were observed in the matrix and debonding of fibres was reported to occur due to carbonization shrinkage in the conventional carbon paper. Measurements showed that the former had much better conductivity and gas permeability than the latter. Additionally, current density–voltage performance revealed that the pyrocarbon coating can also improve the properties of carbon papers used as electrode materials. The carbon fibres of both the cloth and paper are slightly hydrophobic; work must be done to push the water into the hydrophobic pores. The larger the pore, the less work is required to overcome the unfavourable surface energy. Coating the carbon fibres with Teflon makes the pores highly hydrophobic and requires a higher pressure to push the water into the pores. The pressure, P that must be applied to force water into the pores of radius r pore is given by the Young and Laplace equation:
Where γ water is the surface tension of water, and θ is the contact angle of water with the surface of the pore [45]. Chunyu Du et al. [46] proposed a new method of fabricating a hierarchy carbon paper with CNTs uniformly grown on carbon fibres and observed it to be good for the self-humidifying PEM fuel cells. They claimed that carbon paper facilitated the self-humidifying characteristics and can be attributed to its higher hydrophobic nature.
Novel gas diffusion medium
So far, limited exploration with other gas diffusion medium apart from carbon cloth and carbon paper is available in the open literature. Few attempts have been made using metallic thin film as GDM by Fushinobu et al. [47], with micromachined titanium film as GDM due to its high endurance property. A similar sort of experiment was also performed by Hottinen et al. [48] using titanium sinter material and their investigation illustrated the applicability of titanium sinter as a GDM in free-breathing PEM fuel cells. Micromachined silicon has also been used as GDM for microPEM fuel cell applications tested with hydrogen/air [49]. The technique of incorporating sintered stainless steel fibre felt was implemented by Yi et al. [50] and they inferred that the compressive modulus and ductility of GDL were improved. In addition, they claimed that the characteristics of treated stainless steel fibre felt were comparable to carbon paper. Ironically, Glora et al. [51] and Long et al. [http://www.acs.omnibooksonline.com/data/papers/2004_I048.pdf] tried using aerogels to replace conventional GDMs and observed several advantages with aerogels over traditional carbon supports for fuel–cell catalysis including large surface areas (typically >500 m2/g), high-fractional mesoporous pore volumes for gas transport, synthetic control over structural properties and availability in monolithic forms [http://www.acs.omnibooksonline.com/data/papers/2004_I048.pdf]. Glora et al. [51] employed a resorcinol-formaldehyde aerogel with the thickness of less than 500 μm and the highest achieved electronic conductivity was about 28 S/cm in an 80 % porous GDL structure. Wang et al.’s experiment [52] was also inline with that of Glora et al. [51], and the carbon aerogel is prepared from a resorcinol-formaldehyde mix by a pyrolysis technique in an inert gas atmosphere, and their properties are in Table 3.
Variation in GDL porosity could result in non-uniform mass transportation and, as a consequence, even a small change in the porosity may severely lower the overall cell current density. In order to circumvent this problem, Roshandel et al. [53] mathematically calculated the porosity variation in the GDL by considering the applied pressure and the amount of water generated in the cell, and they concluded that a decrease in the average porosity causes the reduction in oxygen consumption, resulting in decreased electrical current density. Zhang et al. [54] developed a novel porous gas diffusion medium with improved thermal and electrical conductivity and controllable porosity using MEMS technology. The gas diffusion medium is fabricated with 12.5-μm thick copper foil and by applying a microporous layer (MPL) on it and enhancing the in-plane transport. This novel-designed material exhibited multi-functionality such as high thermal and electrical conductivity and controllable permeability.
Treatments in GDM
Treatment methods followed for GDM plays a crucial role in determining the performance of the PEMFCs, and water flooding takes place through the following ways [55]:
-
(i)
Water vapour from the humidified reactant feeds
-
(ii)
Product water from cathode side
-
(iii)
The electro-osmotic drag through the electrolyte membrane.
Carbon substrates used as diffusion media are not generally hydrophobic when received from the supplier, so they are teflonized by pretreating with hydrophobic material usually polytetrafluoroethylene (PTFE) in order to increase the hydrophobicity. Staiti et al. was a pioneer to reveal the relationship between the water transport and the amount of hydrophobic agent in the gas diffusion electrodes [56]. Teflonization is one of the most commonly used methods, by which the gas diffusion media is immersed into an aqueous PTFE suspension where the excess suspension is allowed to drip off, and the remaining solvent is removed by oven drying. Finally, the PTFE is heated above 350 °C to sinter and to bind the PTFE particles onto the GDL surface. The homogeneous PTFE distribution throughout the thickness of the gas diffusion media is very sensitive to the drying process. Rapid drying in a convective oven tends to result in PTFE concentrated in the exposed surfaces of the diffusion media. On the other hand, slow diffusive drying (e.g. air drying) results in the even distribution throughout the bulk [57]. Bevers et al.’s [58] exploratory analysis has led to an imperative conclusion that PTFE content and sinter temperature both correlate negatively with conductivity and positively with hydrophobicity. Park et al. [59] studied the effects of PTFE content in the carbon paper under various operating conditions in a H2/Air PEMFC system and investigated to explore which driving forces (capillary, shear or evaporation) are dominant in controlling water transport. Their results are summarized in Table 4 at various porosity, which shows that lesser thickness and larger pore diameter in GDM are the factors which support good reactant gas permeation and water management. A similar study by Prasanna et al. [60] was also in line and concluded that the gas permeability and pore diameter of the GDM are the vital factors to be controlled for achieving acceptable performance. In addition, they demonstrated that if hydrophobicity is less, then the reactant gas permeability is affected by poor water removal. However, there would be a severe gas diffusion loss if hydrophobicity is high. The technique of employing fluorinated ethylene propylene (FEP) for introducing hydrophobicity in GDM was examined by Lim and Wang [61], and they reported that lower polymer content (<10 %) is sufficient to facilitate liquid water removal but at the same time leaves the GDM surfaces relatively accessible for the reactants and product moving in and out. On the contrary, excess of FEP impregnation results in significant blockage of surface pores of carbon paper, highly restricted surface area for the reactant transport and product removal.
Lin and Nguyen [62] also investigated the effect of thickness and hydrophobic polymer content of GDM on flooding and observed that adding PTFE to the GDM could enhance reactant gas and water transport when a cell operates under flooding conditions. On the contrary, excess PTFE can reduce the hydrophilic pathway and make it more difficult for water to diffuse out of the catalyst layer and within the GDM, which results in electrode flooding. Generally, GDM with lower thickness is beneficial due to low gas diffusion loss than materials with higher thickness, whereas very thin GDM is susceptible to mass transfer limitation, contact resistance and losses in mechanical properties. A study by Park et al. [63] indicated that the optimized PTFE content of GDM resulted in an effective water management and improved oxygen gas diffusion kinetics in the membrane–electrode assembly. Pai et al. [64] observed that the usage of carbon tetra fluoride plasma will effectively improve the hydrophobic property of GDM and the plasma treatment can modify the surface morphologies of the wet-proofed GDL to enhance the fuel cell performance without the usage of expensive electro catalytic elements. The method of coating the GDL with an entirely new chemical, namely fluoro alkyl silane (FAS) having strong hydrophobic ligands as well as siloxane bonds was introduced by Yoon et al. [65] and they inferred that FAS-treated carbon paper has an extremely thin and uniform coating surface compared to PTFE, showing that they are firmly formed on carbon fibres without any flaking-off.
Significance of microporous layer (MPL) and its fabrication
A microporous layer (MPL) is a critical component sandwiched between the GDL and the CL and usually comprises of carbon black Teflon as a hydrophobic binder and pore-forming agent. The binders such as Teflon or PTFE serve two functions, namely (i) binding the high-surface-area carbon particles into a cohesive layer and (ii) imparting hydrophobicity to the layer in order to facilitate the removal of water [66]. The MPL also reduces the ohmic resistance between the catalyst layer and the GDM, providing non-permeable support during catalyst deposition and manages liquid water flow during fuel cell operation [15, 67–69]. The micropores in the layer provide sufficient surface pores and hydrophobicity to avoid flooding and also reduce the liquid saturation in the catalyst layer. In addition, the MPL on the anode side can serve as a diffusion barrier, preventing membrane dehydration under low humidity conditions [70]. Another positive impact of adding a MPL is the prevention of diffusion media fibre intrusion into the CL. Due to its critical role in improving water management, prior research efforts [63, 71–73] have been focused on investigating the effect of physical properties of the MPL (i.e. carbon black, hydrophobic agent, thickness, etc.) on fuel cell performance. In addition, it is also intended to prevent the catalyst ink from leaking into the GDM, thereby increasing the catalyst utilization. PEM fuel cell performance can be improved by altering the properties of MPL and this can be achieved by modifying the amounts, nature and characteristics of the above components. Several authors examined diverse properties of the microporous layer for near-saturated (∼100 % R.H.) operation in various aspects. PTFE has been the most commonly used hydrophobic agent in the diffusion layer and as its content increases, the porosity decreases, resulting in higher oxygen transport resistance. However, as the PTFE content gets too low, there is inadequate water removal capability and the optimum PTFE content for near-saturated operation was found between 15 and 20 wt.% [41]. If the thickness of the microporous layer is too low, the cell total resistance increases due to an insufficient carbon/PTFE layer to establish good electronic contact between the rough macroporous substrate and the catalyst layer. Thus, it is evident that the thickness of the microporous layer is a critical parameter for fuel cell performance [41]. Different kinds of carbon powder, besides the widely used Vulcan carbon black powder in the microporous layer, have been studied. Acetylene black was found to be superior just because of its characteristics involving surface area, pore volume and pore-size distribution [61]. The microporous carbon layer should have optimum hydrophobicity to remove the product water effectively from the active layer [74]. Pasaogullari and Wang [70] inferred that placing a MPL between GDL and membrane enhances liquid water removal and reduces the liquid saturation in the catalyst layer. However, analysis of MPL effect is limited to liquid water transport in the cathode gas diffusion medium. Wang et al. [75] performed an extensive characterization on the effect of various carbon powders such as acetylene black, black pearls 2000 and composite carbon black in the microporous layer (MPL) and inferred a superior fuel cell performance with peak power density of 0.91 W/cm2 with 10 wt.% Black Pearls 2000 in composite carbon black. Giorgi et al. [76] studied the performance operated with both oxygen and air as oxidants with the insertion of MPL in GDL fabrication and observed that GDL porosity decreases considerably with increasing PTFE content. In a similar study, Passalacqua et.al [77] established a better performance when a hydrophobic MPL was used with a hydrogen/air system. Investigation on several carbons, namely Vulcan XC-72, Shawinigan acetylene black (SAB), Mogul L and Asbury 850 graphite with different specific surface areas were used for the diffusion layers. SAB which has a high pore volume and a small average pore size exhibited better performance; this may be attributed to reduced mass transport problems, probably connected to improved water transport.
The remarkable effect of MPL on water management of fuel cells was also explained by Chen et al. [78]. It reveals a more uniform water profile throughout the fuel cell operation, when MPL was used than when it was absent. The effects were particularly imperative for a non-humidified fuel. Karan et al. [79] investigated the effect of a MPL at the cathode on the net water transport in a PEM fuel cell and established that the MPL on the cathode neither enhances back-diffusion nor water removal from the cathode catalyst layer to the gas diffusion medium; their experimental results were in contrary to the frequently asserted hypothesis that the MPL enhances back-diffusion of water from the cathode to the anode [80]. Studies on the effect of varying carbon loadings in MPL fabrication were carried out to achieve higher performance in PEM fuel cell operation, and there are numerous publications discussing various types of carbon for MPLs [49]. Table 5 shows the properties of various carbons used to fabricate MPL.
Passalacqua et al. [85] investigated the effect of using carbon blacks and graphite as candidates for diffusion layer fabrication in PEMFC electrode and a power density of about 360 mWcm−2 in hydrogen-/air-operated at 70 °C using SAB as carbon which has a high pore volume and a small average pore size. An improvement in the performance of PEM fuel cell was observed by Jordan [86] and Antolini et al. [87] using MEA with electrode diffusion-layers made from acetylene black as a substitute of Vulcan carbon. Literatures of Wang et al. [75] reveals that a novel MPL prepared with composite carbon black consisting of acetylene black and Black Pearls 2000 (10 wt.%) form an effective bi-functional pore structure and gave a maximum power density of 910 mWcm−2 in hydrogen/air operation. An article by Park et al. [88] adopted carbon nanotube (CNT) and carbon nanofibre (CNF) to facilitate thin microlayers and concluded that a composition of 25 % CNF and 75 % Vulcan XC-72 in the microporous layer gives higher performance by enhancing the electronic conductivity and gas permeability. Kannan et al. [89] have also studied the usage of single-walled CNT as an effective MPL for fabricating GDL. The GDL fabricated by the use of pure black carbon (jointly developed by a superior graphite company and Columbian Chemicals Company) exhibits superior performance in hydrogen/air system without any back pressure [64]. The PEM fuel cell performance has been compared using non-processed Ketjenblack EC-600JD and Vulcan XC-72 as the MPL material [90, 91], and better performances were observed with high-surface-area Ketjen black. Apart from the effect of various carbons in MPL preparation on PEM fuel cell performance, researchers have also focused on influence of carbon loading in MPL, and the results accomplished by various researchers in this area have been summarized in Table 6.
Han et al. [92] discussed the influence of PTFE content in the carbon-filled diffusion layer at optimal carbon loadings, and their result reveals that increasing the carbon loading can reduce the internal resistance of the cell and, hence, improve the fuel cell performance by decreasing the contact resistance between the interfaces of GDL/CL. In addition, it can also reduce the mass transport limitation due to the improvement of water management. However, they also suggest that excessive loading in such carbon-filled GDL would decrease the porosity and increase the concentration over-potential, in particular, in the H2/air-fed fuel cell. The results acquired by various researchers by varying PTFE loading in MPL are summarized in Table 7. The literature [67, 92, 93, 96] shows that the pre-eminent performances are obtained with the lowest PTFE loading, but it is not possible to totally eliminate it. Yan et al. [94] used fluorinated ethylene propylene (FEP) as a hydrophobic agent in GDL preparation and achieved the best performance with 20 % FEP content in MPL.
From diverse literatures [72, 74, 97–100], we infer that the use of MPL’s typically results in a better fuel cell performance, increases electrical conductivity and improves water management and in turn the power density. MPL improves performance by reducing mass transport limitations, especially with the air feed and also by reducing ohmic losses especially with oxygen feed [76]. Latorrata et al. [101] coated the microporous layers (MPLs) prepared with and without carboxymethylcellulose (CMC), a unique technique, and compared their electrical performances with a single fuel cell. They revealed that at high current density (CD), the CMC-based GDLs suffer water management, and such behaviour was attributed to a hydrophilic character of the GDL due to residual amounts of CMC in MPL coating [101].
Impact of pore former in MPL fabrication
Porosity of the GDL is a critical parameter for determining the mass transport process which in turn impacts PEM fuel cell performance especially at high current density regions for which a pore former such as ammonium bicarbonate, lithium carbonate and sucrose is included during the fabrication process of the diffusion layer. Chebbi et al. [102] studied the effects of pore-size distribution on reactant transport by introducing lithium carbonate as a pore former and suggested that pore distribution should be bi-modal, facilitating the water discharge through large pores and gas diffusion through small pores. However, complete removal of Li-ions from the electrode needs to be ensured and to circumvent these issues. Selvarani et al. [103] introduced sucrose as a pore former during the GDL fabrication process and reported that the un-leached pore former can be easily burned as a carbon during the gas diffusion electrode-backing process. They found the optimum content of pore former in the GDL to be 50 w/o for effective gas transport and product removal. Kong et al. controlled the pore-size distribution by adding isopropyl alcohol and lithium carbonate as a pore former, together with carbon powder and PTFE [98] and followed by the heat treatment led to a dual functionality, namely higher porosity and bimodal water transport. Tang et al. [104] examined the effect of porosity graded microporous layer prepared by using ammonium chloride as pore former. Their result disclosed that, MPL with graded porosity is beneficial for the electrode process of fuel cell reaction by facilitating the liquid water transportation through large pores, increasing the capillary force of graded microporous layer and gas diffusion via small porous in graded microporous layers. Kitahara et al. [105] made a physical analysis compared to the adding of chemical pore former by varying the mean pore diameter of MPL from 1 to 10 mm and observed that the through-plane permeability increases with MPL mean pore size. The performance was investigated at low and high cathode humidity with anode humidification to be 100 % in both cases. At low humidity, the performance was the best where the GDL had the smallest MPL pore size and lowest through-plane permeability because the MPL prevented MEA dehydration. At high humidity, the pre-eminent performance was found using GDLs with a mean MPL pore diameter of 3 mm and had an intermediate through-plane permeability value as it was also reported in [106]. Such kind of GDLs facilitates the transport of reagents from a gas channel to a catalyst surface as well as water transport from an electrode to the gas phase.
Assessment on compression and thermal conductivity of GDL
GDL compression and thermal conductivity are also significant factors that should be precisely controlled and, as a result, are briefly dealt with. The effects of changing the bolt torque on the performance of a PEM fuel cells gas diffusion layer have been investigated at fixed stoichiometric flow rates for the reactant by Lee et al. [107], and they observed that the thickness of the GDL may also be affected by the amount of torque exerted on the bolts. In addition, the change in GDL thickness during compression can be converted to porosity and the relationship between measured permeability and porosity can be compared. Jiabin Ge et al. [107] performed a similar correlation study on the GDL compression and fuel cell performance by evaluating two different GDL materials. Their experimental results implied that the fuel cell performance decreases with the increase in compression and, in addition, a unique fuel cell test fixture was designed; it was concluded that the effect of GDL compression is significant for both carbon cloth and paper and significantly in the high current density region. GDL is subjected to compressive stress at high temperatures along with polymer electrolyte membrane in the fabrication process and in assembling the fuel cell stacks. Lee et al. [107] asserted from experimental results that an optimal bolt torque was acquired for a soft commercial diffusion layer because of porosity and electrical contact resistance changes. In addition, both the bolt torque and the gas diffusion layer type are significant factors for the PEM fuel cell performance. A similar experiment by Senthi Velan et al. describes that compressive stress decreases the GDL thickness, electrical conductivity, permeability and affects the pores [108]. Lee and Merida studied [109] GDL compressive strain under steady-state and freezing conditions. GDL strain was measured to occur under steady-state aging conditions. An increase in in-plane and through-plane air permeability (18 and 80 %, respectively) was attributed to material loss during permeability measurements and the ex situ tests showed that convective airflow can cause material loss, resulting in increased permeability and further convection. Effective thermal conductivity is also a vital transport parameter that plays an important role in fuel cell performance analysis [110]. A recent study shows that the thermal conductivity increases gradually with the water content [111]. In another study, it has been shown experimentally as well as numerically that a temperature gradient across diffusion media induces additional water transport due to phase change [112–114]. Yablecki et al. examined the anisotropic thermal conductivity of the GDL using the two- and three-dimensional two-phase conjugate fluid–solid thermal lattice Boltzmann model and concluded that the anisotropic structure of the GDL causes anisotropic thermal conductivity, with a higher value for the in-plane thermal conductivity than the through-plane thermal conductivity [112]. Khandelwal and Mench [115] measured the through-plane thermal conductivity of GDLs by examining two dissimilar commercial GDLs with a variety of thicknesses and porosities. They studied the effect of temperature and polytetrafluoroethylene (PTFE) content on the effective thermal conductivity and obtained values in close agreement with the manufacturer data. Ironically, Sadeghi et al. [116] studied on the parameter’s that determine the effective thermal conductivity as well as the thermal contact resistance associated with the interface between the GDL and adjacent layers by building a test bed and observed that effective thermal conductivity increases with the compressive load and decreases with an increase with operating temperature, however, independent to ambient air pressure.
Durability of GDL
Durability is one of the most significant issues impeding successful commercialization of PEM fuel cell systems, and studies on GDL degradation and dry operation might provide more insight into fuel cell performance as there are only a limited number of studies currently in open literature. Specifically, the GDL plays an important role concerning the durability of the MEAs, which is a critical concern for the end users [117] as well as an abnormally high current density which significantly accelerates the deterioration of the gas diffusion medium. Also, corrosion on the GDL will increase resistance and decrease electrical conductivity. Wu et al. compared the physical characteristics of the GDLs before and after corrosion tests and validated that GDLs are susceptible to electrochemical oxidation [118]. Chen et al. [119] performed an effective ex situ method for characterizing electrochemical durability of a gas diffusion layer (GDL). Wood et al. [120] discussed the physical properties required to understand GDL durability and long-term performance for next-generation GDL components. Yi et al. [121] have studied a numerical model for predicting gas diffusion layer failure in proton exchange membrane fuel cells. Correlations between performance loss and deviations in GDL properties might contribute to enhanced understanding on durability.
Results extrapolated from various modelling studies
Dawn M. Bernardi and Mark W. Verbrugge [122] were the pioneers in performing the one-dimensional model on the gas diffusion medium. They modelled both membrane and gas diffusion electrodes so that it follows extensions to account for heat transfer and pressure gradients. In their subsequent research, they claimed that the anode diffusion layer needs to be included in the cell model to properly account for water transport. It is referred to as BV, and the value of the gas diffusion electrode thickness is 0.26 mm [123].
One of the initially used PEFC models capable of predicting both membrane resistance and water balance is described by Springer et al. [124]. Preliminary research papers on GDL modelling used the Bruggeman expression, which determines an effective diffusive coefficient in porous medium by multiplying the binary gas coefficient by ε 1.5 [124], and these approximations were initiated by De La Rue and Tobias [125] to hold for conduction through a heterogeneous mixture of random non-conducting spheres. Forthcoming, modelling works have revealed that mass transport in the gas diffusion porous medium constitutes a significant performance loss in the fuel cell, especially when liquid water is present [23, 68, 124].
Chiang and Chu [126] investigated the effects of transport phenomena and performance of PEM fuel cells by using a three-dimensional model and found that a thin GDL generates more current at low cell voltage due to the merits of better reactant gas transport and liquid water delivery.
Um et al. [127] developed a computational fuel cell dynamics model, and their results show that forced convection of gases through GDL helps to improve performance at high current densities. Gurau et al. [128] presented a computational fluid dynamic model to capture multiphase phenomena at the cathode gas diffusion layer–channel interface, and their analyses provides insight for designing diffusion media with controlled structural properties at the interface with the channel, such as pore-size distribution or pattern of orifices punctured during the fabrication process.
Yi et al. [121] studied a numerical model for predicting gas diffusion layer failure in proton exchange membrane fuel cells basic mechanism and concluded that assembly pressure on the bipolar plate should be below 2.0 MPa to ensure the integrity of GDL. Zhou et al. [129] developed a numerical model and observed that large GDL compression deformation and porosity variation reduced the transport ability of the reactant gas and liquid water in the GDL. Sadeghi et al. [116] also proposed a model to compute the thermal resistances, which was in line with experimental data over a wide range of compressive loads from 0.2 to 1.5 MPa. Table 8 provides the transport equations for the gas and liquid phase in GDL. Wang et al. [130] presented a novel model that encompasses both single- and two-phase regimes and concluded that transport of both liquid and vapour water is controlled by capillary action and molecular diffusion, respectively, due to negligible small air velocity within the porous GDL.
The potential distribution in the GDL is described by [135]:
The boundary conditions are
Nitta et al. [136] developed a two-dimensional model to study the effects of inhomogeneous compression of GDLs on local transport phenomena within a PEM fuel cell and predicted a uniform temperature profile along the active area, with a variation of ca. 1 °C. The compressed GDL thickness under the rib affects the current density distribution and the temperature profile. Su et al. [137] investigated numerically and developed a three-dimensional model to analyse the impact of transport properties of the GDL, specifically porosity and permeability. Three different configurations of transport properties were tested, i.e. uniform uncompressed GDL properties, uniform compressed GDL properties and non-homogeneous GDL properties and found that the non-homogeneous structure shows noticeable differences in predicted cell performance. Dotelli et al. [138] compared two gas diffusion layers of PEM fuel cells based on the same carbon cloth substrate, coated with microporous layers of different hydrophobicities assembled in. Each configuration of polarization curves were recorded; in order to evaluate the role of different GDLs, AC impedance spectroscopy of the running cell was also performed and inferred that higher compression ratio worsened the cell performances at a higher temperatures and the presence of the microporous layer onto the carbon cloth resulted in extremely beneficial operations especially at high current density. Moreover, it sensibly reduces the high frequency resistance. Pasaogullari et al. developed an analytical model and inferred that capillary transport is the dominant transport process to remove water from flooded GDLs. In addition, flooding diminishes the cell performance as a result of decreased oxygen transport and surface coverage of active catalyst by liquid water [139]. Modelling of GDL by lattice Boltzmann simulation technique for understanding the behaviour of two-phase flow of complex fluid in porous medium was studied by Koido et al. [31] and Tabe et al. [140], and they asserted that capillary–pressure saturation relationship and wettability of the channel are the two phenomenons that can severely influence the GDL performance. Investigation on the anisotropic permeability of a carbon cloth GDL [141] based on the integration of X-ray micro-tomography and lattice Boltzmann (LB) simulation was performed by Rama et al., and their results demonstrated that the simulated through-plane permeability is about four times higher than the in-plane permeability. The simulated results are also applied to generate a parametric coefficient for the Kozeny–Carman (KC) method of determining permeability. Niu et al. [4] examined a model to simulate water–gas transportations in the GDL based on the diffuse interface theory and employed two distributions so that multiphase flows with large density ratios and various viscosities can be controlled. To numerically realize the boundary conditions for the complicated structure like GDL, besides the standard bounce back condition used for the nonslip condition, an approximated average scheme based on the extrapolation method is derived to mimic wetting boundaries.
Summary and recommendation
Dynamic behaviour is a key property of PEM fuel cells to be used for automotive application [142], and the structural design of GDL significantly influences the dynamic response [143]. From the various studies in open literature [42–57], it is observed that though carbon cloth and carbon paper have their own pros and cons, carbon paper has been recommended by most of the authors especially for low humidity operation and carbon cloth for high humidity operation. The worst performance of the carbon cloth is likely to be attributed to contact resistances; however, at high current density, carbon papers have some issues pertaining to water management. The modified carbon fibre cloth can eliminate assembly difficulties with a relatively low resin content and exhibits good through-plane resistance, resulting in good cell performance [40]. Carbon papers are brittle and quite compressible and, as results, are good for designs where a tighter tolerance is permitted in the compression and where the thin GDL is a critical factor. However, in general characteristics such as fraction of hydrophobic pore-size distribution, gas permeability, surface morphology and electronic resistivity predominantly defines the functionality of the GDL, despite the fact that some limitations may occur for measurement of each quantities. Table 9 provides a difference in characteristics of carbon cloth and paper perceived from various literatures.
The cathode electrochemical reactions produce a large amount of liquid water at low-operating voltages, and if the liquid water is not appropriately purged it may accumulate in the pores of the diffusion layers and restrict the oxygen transport to the gas diffusion and the catalyst layer, thereby reducing the reaction rate. Diffusion media characterization and development still rely heavily on in situ testing because well-established correlations between in situ performance results and ex situ characterization data are not yet available. The carbon cloth is the most flexible and is generally robust, but results in higher ohmic loss due to its thickness and, in contrast, novel materials like pyrocarbons are yet to achieve significant performance levels. Modified carbon paper can be a clear winner among the gas diffusion layer if precise water management strategies and gas flow designs are formulated. Increased carbon loadings in MPL can reduce contact resistance between the interfaces of GDL/catalyst layer and hence improve the fuel cell performance. However, excessive carbon loading in GDL tends to decrease the porosity and increase the concentration over potential, in particular, for hydrogen-/air-fed PEM fuel cell, which is due to an increased diffusion path in carbon-filled GDL. Hence, care should be taken for carbon loading in GDL fabrication for attaining optimal performance. An optimized carbon loading had a significant positive impact on the PEMFC performance. Acetylene black was chosen for most MPLs because of the favourable combination of water management and corrosion resistance [144]. Assessment of cathode and anode GDL has been performed by several authors, and it has been concluded that the cathode GDL determines the maximum possible current and power density [26, 145, 146]. Transport equations pertinent to GDL [13, 128, 131–134] clarified the impact of operational parameters on the thermal properties of GDLs and provided new insights on the importance of a key interfacial phenomenon. Flooding can drastically decline the fuel cell performance by hindering the gas diffusion and blocking the electro-catalyst sites [139]. In specific, cathode flooding can result in a catastrophic decrease of performance by reducing the oxygen transport to the reaction sites and decreasing the effective catalyst area which has been observed over a wide range of operating conditions. Cathode flooding can be detected experimentally and significantly depends on GDL properties (e.g. porosity and hydrophobicity) [147–150]. In a hydrophilic GDL, there would be no restriction to liquid flow and it would begin with any applied pressure and, for example, thick diffusion layer attributes to a long reactant transport passage and the flooding problem, whereas thin layers are susceptible to mass transfer and contact resistance losses. Another significant factor studied from the review is that the contact resistance could be higher between the bipolar plates and the GDLs due to the flow channels compared to that between GDLs and catalysts layers. Materials with the most highly aligned fibres showed the highest anisotropy and the permeability. There is a lack of fundamental experiments on water transport in GDLs, and the most challenging issue in the GDL fabrication is to develop a compatible GDL for both hydrophilic and hydrophobic operations. GDL components of PEMFCs degrade in different manners, and the mechanisms involved in the degradation are not completely implicit. This might be because there are different techniques employed in preparing those functional components and operating conditions are not well declared. The various mechanisms are related, so one degradation mechanism may trigger or exacerbate another. Future designs could integrate the GDL, current collector and flow field to be manufactured seamlessly by automated MEMS processes [54], and this would be a key to address numerous challenges including the hydrophilic and hydrophobic conditions. The aforementioned details evidently validate the role of GDL in the design and performance of the PEM fuel cells, especially pertaining to the characteristics, fabrication techniques and related components. Incorporation of carbon nanotube as gas diffusion electrode for the next-generation PEM fuel cells due to its exceptionally high transport rates are shown to be as a result of the inherent smoothness of the nanotubes [151] and will be one of the cornerstones of the cutting edge research and may guide PEM fuel cells to a successful level of commercialization.
Conclusions
Despite its critical function, the role of GDL in PEM fuel cell performance is not well asserted and this has been an impetus for the authors to review the GDL characteristics, geometry, fabrication techniques and related components. The best components for each functionality cannot make the best GDL; a trade-off between the properties will evolve the ideal GDL. The review of the authors may pave the way for the future research to be concentrated on a holistic approach in the evolution of GDL.
References
Pethaiah SS, Kalaignan GP, Ulaganathan M, Arunkumar J (2011) Preparation of durable nanocatalyzed MEA for PEM fuel cell applications. Ionics 17(4):361–366
Chiu L-Y, Diong B, Gemmen RS (2004) An improved small-signal model of the dynamic behavior of PEM fuel cells. IEEE Trans Ind Appl 40(4):970–977
Tang H, Peikang S, Jiang SP, Wang F, Pan M (2007) A degradation study of Nafion proton exchange membrane of PEM fuel cells. J Power Sources 170(1):85–92. doi:10.1016/j.jpowsour.2007.03.061
Niu X-D, Munekata T, Hyodo S-A, Suga K (2007) An investigation of water-gas transport processes in the gas-diffusion-layer of a PEM fuel cell by a multiphase multiple-relaxation-time lattice Boltzmann model. J Power Sources 172(2):542–552
Berning T, Lu DM, Djilali N (2002) Three-dimensional computational analysis of transport phenomena in a PEM fuel cell. J Power Sources 106(1–2):284–294. doi:10.1016/S0378-7753(01)01057-6
Neergat M, Shukla A (2002) Effect of diffusion-layer morphology on the performance of solid-polymer-electrolyte direct methanol fuel cells. J Power Sources 104(2):289–294
Secanell M, Songprakorp R, Djilali N, Suleman A (2010) Optimization of a proton exchange membrane fuel cell membrane electrode assembly. Struct Multidiscip Optim 40(1–6):563–583
Shimpalee S, Beuscher U, Van Zee JW (2007) Analysis of GDL flooding effects on PEMFC performance. Electrochim Acta 52(24):6748–6754. doi:10.1016/j.electacta.2007.04.115
Cho J, Oh H, Park J, Min K, Lee E, Jyoung J-Y (2014) Effect of the micro porous layer design on the dynamic performance of a proton exchange membrane fuel cell. Int J Hydrog Energy 39(1):459–468
Wilson MS, Valerio JA, Gottesfeld S (1995) Low platinum loading electrodes for polymer electrolyte fuel cells fabricated using thermoplastic ionomers. Electrochim Acta 40(3):355–363
Spiegel C (2011) PEM fuel cell modeling and simulation using MATLAB. Academic
Jordan L, Shukla A, Behrsing T, Avery N, Muddle B, Forsyth M (2000) Effect of diffusion-layer morphology on the performance of polymer electrolyte fuel cells operating at atmospheric pressure. J Appl Electrochem 30(6):641–646
Gurau V, Bluemle MJ, De Castro ES, Tsou Y-M, Zawodzinski TA Jr, Mann JA Jr (2007) Characterization of transport properties in gas diffusion layers for proton exchange membrane fuel cells: 2. Absolute permeability. J Power Sources 165(2):793–802. doi:10.1016/j.jpowsour.2006.12.068
Wishart J, Dong Z, Secanell M (2006) Optimization of a PEM fuel cell system based on empirical data and a generalized electrochemical semi-empirical model. J Power Sources 161(2):1041–1055
Paganin V, Ticianelli E, Gonzalez E (1996) Development and electrochemical studies of gas diffusion electrodes for polymer electrolyte fuel cells. J Appl Electrochem 26(3):297–304
Tan Z, Jia L, Zhang Z (2011) A study on the transport process in gas diffusion layer of proton exchange membrane fuel cells. J Therm Sci 20(5):449–453
Rohling J, Shen J, Wang C, Zhou J, Gu C (2007) Determination of binary diffusion coefficients of gases using photothermal deflection technique. Appl Phys B 87(2):355–362
Johnson MF, Stewart WE (1965) Pore structure and gaseous diffusion in solid catalysts. J Catal 4(2):248–252
Nam JH, Kaviany M (2003) Effective diffusivity and water-saturation distribution in single-and two-layer PEMFC diffusion medium. Int J Heat Mass Transf 46(24):4595–4611
Pharoah J, Karan K, Sun W (2006) On effective transport coefficients in PEM fuel cell electrodes: anisotropy of the porous transport layers. J Power Sources 161(1):214–224
Liu F, Wang C-Y (2006) Optimization of cathode catalyst layer for direct methanol fuel cells: part II: computational modeling and design. Electrochim Acta 52(3):1409–1416
Kast W, Hohenthanner C-R (2000) Mass transfer within the gas-phase of porous media. Int J Heat Mass Transf 43(5):807–823
Djilali N, Lu D (2002) Influence of heat transfer on gas and water transport in fuel cells. Int J Therm Sci 41(1):29–40
Martínez-Rodríguez MJ, Cui T, Shimpalee S, Seraphin S, Duong B, Van Zee JW (2012) Effect of microporous layer on MacMullin number of carbon paper gas diffusion layer. J Power Sources 207:91–100. doi:10.1016/j.jpowsour.2012.01.132
Van Brakel J, Heertjes P (1974) Analysis of diffusion in macroporous media in terms of a porosity, a tortuosity and a constrictivity factor. Int J Heat Mass Transf 17(9):1093–1103
Springer T, Zawodzinski T, Wilson M, Gottesfeld S (1996) Characterization of polymer electrolyte fuel cells using AC impedance spectroscopy. J Electrochem Soc 143(2):587–599
Gostick JT, Fowler MW, Pritzker MD, Ioannidis MA, Behra LM (2006) In-plane and through-plane gas permeability of carbon fiber electrode backing layers. J Power Sources 162(1):228–238. doi:10.1016/j.jpowsour.2006.06.096
Tamayol A, Bahrami M (2011) In-plane gas permeability of proton exchange membrane fuel cell gas diffusion layers. J Power Sources 196(7):3559–3564
Ahmed DH, Sung HJ, Bae J (2008) Effect of GDL permeability on water and thermal management in PEMFCs—I. Isotropic and anisotropic permeability. Int J Hydrog Energy 33(14):3767–3785
Fishman Z, Hinebaugh J, Bazylak A (2010) Microscale tomography investigations of heterogeneous porosity distributions of PEMFC GDLs. J Electrochem Soc 157(11):B1643–B1650
Koido T, Furusawa T, Moriyama K, Takato K (2006) Two-phase transport properties and transport simulation of the gas diffusion layer of a PEFC. ECS Trans 3(1):425–434
Hussaini I, Wang C (2010) Measurement of relative permeability of fuel cell diffusion media. J Power Sources 195(12):3830–3840
Feser J, Prasad A, Advani S (2006) Experimental characterization of in-plane permeability of gas diffusion layers. J Power Sources 162(2):1226–1231
Benziger J, Nehlsen J, Blackwell D, Brennan T, Itescu J (2005) Water flow in the gas diffusion layer of PEM fuel cells. J Membr Sci 261(1):98–106
Rofaiel A, Ellis J, Challa P, Bazylak A (2012) Heterogeneous through-plane distributions of polytetrafluoroethylene in polymer electrolyte membrane fuel cell gas diffusion layers. J Power Sources 201:219–225
Hirschorn B, Tribollet B, Orazem ME (2008) On selection of the perturbation amplitude required to avoid nonlinear effects in impedance measurements. Isr J Chem 48(3–4):133–142
Wang Y, Wang C-Y, Chen K (2007) Elucidating differences between carbon paper and carbon cloth in polymer electrolyte fuel cells. Electrochim Acta 52(12):3965–3975
Stampino PG, Omati L, Dotelli G (2011) Electrical performance of PEM fuel cells with different gas diffusion layers. J Fuel Cell Sci Technol 8(4):041005
Sasikumar G, Ryu H (2003) Comparison of electrode backing materials for polymer electrolyte membrane fuel cells. J Korean Electrochem Soc 6(No.3):183–186
Liao Y-K, Ko T-H, Liu C-H (2008) Performance of a polymer electrolyte membrane fuel cell with fabricated carbon fiber cloth electrode. Energy Fuel 22(5):3351–3354
Williams MV, Begg E, Bonville L, Kunz HR, Fenton JM (2004) Characterization of gas diffusion layers for PEMFC. J Electrochem Soc 151(8):A1173–A1180
Park S, Popov BN (2011) Effect of a GDL based on carbon paper or carbon cloth on PEM fuel cell performance. Fuel 90(1):436–440
Sahu A, Nishanth K, Selvarani G, Sridhar P, Pitchumani S, Shukla A (2009) Polymer electrolyte fuel cells employing electrodes with gas-diffusion layers of mesoporous carbon derived from a sol–gel route. Carbon 47(1):102–108
Xie Z-Y, Jin G-Y, Zhang M, Su Z-A, Zhang M-Y, Chen J-X, Huang Q-Z (2010) Improved properties of carbon fiber paper as electrode for fuel cell by coating pyrocarbon via CVD method. Trans Nonferrous Metals Soc China 20(8):1412–1417
Wang C-Y (2004) Fundamental models for fuel cell engineering. Chem Rev 104(10):4727–4766
Du C, Wang B, Cheng X (2009) Hierarchy carbon paper for the gas diffusion layer of proton exchange membrane fuel cells. J Power Sources 187(2):505–508. doi:10.1016/j.jpowsour.2008.11.046
Fushinobu K, Takahashi D, Okazaki K (2006) Micromachined metallic thin films for the gas diffusion layer of PEFCs. J Power Sources 158(2):1240–1245
Hottinen T, Mikkola M, Mennola T, Lund P (2003) Titanium sinter as gas diffusion backing in PEMFC. J Power Sources 118(1):183–188
Modroukas D, Modi V, Fréchette LG (2005) Micromachined silicon structures for free-convection PEM fuel cells. J Micromech Microeng 15(9):S193
Yi P, Peng L, Lai X, Li M, Ni J (2012) Investigation of sintered stainless steel fiber felt as gas diffusion layer in proton exchange membrane fuel cells. Int J Hydrog Energy 37(15):11334–11344. doi:10.1016/j.ijhydene.2012.04.161
Glora M, Wiener M, Petričević R, Pröbstle H, Fricke J (2001) Integration of carbon aerogels in PEM fuel cells. J Non-Cryst Solids 285(1):283–287
Wang J, Glora M, Petricevic R, Saliger R, Proebstle H, Fricke J (2001) Carbon cloth reinforced carbon aerogel films derived from resorcinol formaldehyde. J Porous Mater 8(2):159–165
Roshandel R, Farhanieh B, Saievar-Iranizad E (2005) The effects of porosity distribution variation on PEM fuel cell performance. Renew Energy 30(10):1557–1572
Zhang F-Y, Advani SG, Prasad AK (2008) Performance of a metallic gas diffusion layer for PEM fuel cells. J Power Sources 176(1):293–298
Ren X, Gottesfeld S (2001) Electro-osmotic drag of water in poly (perfluorosulfonic acid) membranes. J Electrochem Soc 148(1):A87–A93
Staiti P, Poltarzewski Z, Alderucci V, Maggio G, Giordano N, Fasulo A (1992) Influence of electrodic properties on water management in a solid polymer electrolyte fuel cell. J Appl Electrochem 22(7):663–667
Quick C, Ritzinger D, Lehnert W, Hartnig C (2009) Characterization of water transport in gas diffusion media. J Power Sources 190(1):110–120
Bevers D, Rogers R, Von Bradke M (1996) Examination of the influence of PTFE coating on the properties of carbon paper in polymer electrolyte fuel cells. J Power Sources 63(2):193–201
Park G-G, Sohn Y-J, Yang T-H, Yoon Y-G, Lee W-Y, Kim C-S (2004) Effect of PTFE contents in the gas diffusion media on the performance of PEMFC. J Power Sources 131(1):182–187
Prasanna M, Ha H, Cho E, Hong S-A, Oh I-H (2004) Influence of cathode gas diffusion media on the performance of the PEMFCs. J Power Sources 131(1):147–154
Lim C, Wang C (2004) Effects of hydrophobic polymer content in GDL on power performance of a PEM fuel cell. Electrochim Acta 49(24):4149–4156
Lin G, Van Nguyen T (2005) Effect of thickness and hydrophobic polymer content of the gas diffusion layer on electrode flooding level in a PEMFC. J Electrochem Soc 152(10):A1942–A1948
Park S, Lee J-W, Popov BN (2008) Effect of PTFE content in microporous layer on water management in PEM fuel cells. J Power Sources 177(2):457–463
Pai Y, Ke J, Huang H, Lee C, Zen J, Shieu F (2006) CF4 plasma treatment for preparing gas diffusion layers in membrane electrode assemblies. J Power Sources 161(1):275–281
Yoon GH, Park SB, Kim EH, Kim S, Park Y-I (2007) Effect of fluoroalkylsilane coating on the properties of gas diffusion layer in PEMFCs. In: Meeting Abstracts. vol 20. The Electrochemical Society, pp 898–898
Thoben B, Siebke A (2004) Influence of different gas diffusion layers on the water management of the PEFC cathode. J New Mater Electrochem Syst 7(1):13–20
Lee H-K, Park J-H, Kim D-Y, Lee T-H (2004) A study on the characteristics of the diffusion layer thickness and porosity of the PEMFC. J Power Sources 131(1):200–206
Mathias M, Roth J, Fleming J, Lehnert W (2003) Diffusion media materials and characterisation. Handbook of fuel cells
Rajalakshmi N, Velayutham G, Ramya K, Subramaniyam C, Dhathathreyan K Characterisation and optimisation of low cost activated carbon fabric as a substrate layer for PEMFC electrodes. In: ASME 2005 3rd International Conference on Fuel Cell Science, Engineering and Technology, 2005. American Society of Mechanical Engineers, pp 169–173
Pasaogullari U, Wang C-Y (2004) Two-phase transport and the role of micro-porous layer in polymer electrolyte fuel cells. Electrochim Acta 49(25):4359–4369
Nakajima H, Konomi T, Kitahara T (2007) Direct water balance analysis on a polymer electrolyte fuel cell (PEFC): effects of hydrophobic treatment and micro-porous layer addition to the gas diffusion layer of a PEFC on its performance during a simulated start-up operation. J Power Sources 171(2):457–463. doi:10.1016/j.jpowsour.2007.06.004
Weber AZ, Newman J (2005) Effects of microporous layers in polymer electrolyte fuel cells. J Electrochem Soc 152(4):A677–A688
Ihonen J, Mikkola M, Lindbergh G (2004) Flooding of gas diffusion backing in PEFCs physical and electrochemical characterization. J Electrochem Soc 151(8):A1152–A1161
Velayutham G, Kaushik J, Rajalakshmi N, Dhathathreyan K (2007) Effect of PTFE content in gas diffusion media and microlayer on the performance of PEMFC tested under ambient pressure. Fuel Cells 7(4):314–318
Wang X, Zhang H, Zhang J, Xu H, Tian Z, Chen J, Zhong H, Liang Y, Yi B (2006) Micro-porous layer with composite carbon black for PEM fuel cells. Electrochim Acta 51(23):4909–4915
Giorgi L, Antolini E, Pozio A, Passalacqua E (1998) Influence of the PTFE content in the diffusion layer of low-Pt loading electrodes for polymer electrolyte fuel cells. Electrochim Acta 43(24):3675–3680
Passalacqua E, Lufrano F, Squadrito G, Patti A, Giorgi L (1998) Influence of the structure in low-Pt loading electrodes for polymer electrolyte fuel cells. Electrochim Acta 43(24):3665–3673
Chen J, Matsuura T, Hori M (2004) Novel gas diffusion layer with water management function for PEMFC. J Power Sources 131(1):155–161
Karan K, Atiyeh H, Phoenix A, Halliop E, Pharoah J, Peppley B (2007) An experimental investigation of water transport in PEMFCs the role of microporous layers. Electrochem Solid-State Lett 10(2):B34–B38
Lin G, Van Nguyen T (2006) A two-dimensional two-phase model of a PEM fuel cell. J Electrochem Soc 153(2):A372–A382
Ong AL, Bottino A, Capannelli G, Comite A (2008) Effect of preparative parameters on the characteristic of poly (vinylidene fluoride)-based microporous layer for proton exchange membrane fuel cells. J Power Sources 183(1):62–68
Chen J, Xu H, Zhang H, Yi B (2008) Facilitating mass transport in gas diffusion layer of PEMFC by fabricating micro-porous layer with dry layer preparation. J Power Sources 182(2):531–539
Hung T, Huang J, Chuang H, Bai S, Lai Y, Chen-Yang Y (2008) Highly efficient single-layer gas diffusion layers for the proton exchange membrane fuel cell. J Power Sources 184(1):165–171
Andersen SM, Borghei M, Lund P, Elina Y-R, Pasanen A, Kauppinen E, Ruiz V, Kauranen P, Skou EM (2013) Durability of carbon nanofiber (CNF) & carbon nanotube (CNT) as catalyst support for proton exchange membrane fuel cells. Solid State Ionics 231:94–101
Passalacqua E, Squadrito G, Lufrano F, Patti A, Giorgi L (2001) Effects of the diffusion layer characteristics on the performance of polymer electrolyte fuel cell electrodes. J Appl Electrochem 31(4):449–454
Jordan L, Shukla A, Behrsing T, Avery N, Muddle B, Forsyth M (2000) Diffusion layer parameters influencing optimal fuel cell performance. J Power Sources 86(1):250–254
Antolini E, Passos R, Ticianelli EA (2002) Effects of the carbon powder characteristics in the cathode gas diffusion layer on the performance of polymer electrolyte fuel cells. J Power Sources 109(2):477–482
Park G-G, Sohn Y-J, Yim S-D, Yang T-H, Yoon Y-G, Lee W-Y, Eguchi K, Kim C-S (2006) Adoption of nano-materials for the micro-layer in gas diffusion layers of PEMFCs. J Power Sources 163(1):113–118
Kannan AM, Menghal A, Barsukov IV (2006) Gas diffusion layer using a new type of graphitized nano-carbon PUREBLACK® for proton exchange membrane fuel cells. Electrochem Commun 8(5):887–891
Song Y, Wei Y, Xu H, Williams M, Liu Y, Bonville LJ, Russell Kunz H, Fenton JM (2005) Improvement in high temperature proton exchange membrane fuel cells cathode performance with ammonium carbonate. J Power Sources 141(2):250–257
Park S, Lee J-W, Popov BN (2006) Effect of carbon loading in microporous layer on PEM fuel cell performance. J Power Sources 163(1):357–363
Han M, Chan S, Jiang S (2006) Development of carbon-filled gas diffusion layer for polymer electrolyte fuel cells. J Power Sources 159(2):1005–1014
Qi Z, Kaufman A (2002) Improvement of water management by a microporous sublayer for PEM fuel cells. J Power Sources 109(1):38–46
Yan W-M, Hsueh C-Y, Soong C-Y, Chen F, Cheng C-H, Mei S-C (2007) Effects of fabrication processes and material parameters of GDL on cell performance of PEM fuel cell. Int J Hydrog Energy 32(17):4452–4458
Song J, Cha S, Lee W (2001) Optimal composition of polymer electrolyte fuel cell electrodes determined by the AC impedance method. J Power Sources 94(1):78–84
Wang X, Zhang H, Zhang J, Xu H, Zhu X, Chen J, Yi B (2006) A bi-functional micro-porous layer with composite carbon black for PEM fuel cells. J Power Sources 162(1):474–479
Lufrano F, Passalacqua E, Squadrito G, Patti A, Giorgi L (1999) Improvement in the diffusion characteristics of low Pt-loaded electrodes for PEFCs. J Appl Electrochem 29(4):445–448
Kong CS, Kim D-Y, Lee H-K, Shul Y-G, Lee T-H (2002) Influence of pore-size distribution of diffusion layer on mass-transport problems of proton exchange membrane fuel cells. J Power Sources 108(1):185–191
Cindrella L, Kannan A, Lin J, Saminathan K, Ho Y, Lin C, Wertz J (2009) Gas diffusion layer for proton exchange membrane fuel cells—a review. J Power Sources 194(1):146–160
Janssen GJM, Overvelde MLJ (2001) Water transport in the proton-exchange-membrane fuel cell: measurements of the effective drag coefficient. J Power Sources 101(1):117–125. doi:10.1016/S0378-7753(01)00708-X
Latorrata S, Stampino PG, Amici E, Pelosato R, Cristiani C, Dotelli G (2012) Effect of rheology controller agent addition to micro-porous layers on PEMFC performances. Solid State Ionics 216:73–77. doi:10.1016/j.ssi.2012.03.030
Chebbi R, Wan Daud WR, Mohamad AB, Kadhum AAH (2011) Review of parameters affecting performance of (Pt/C) electrode for proton exchange membrane fuel cells (PEMFCS). Adv Mater Res 233:43–49
Selvarani G, Sahu A, Sridhar P, Pitchumani S, Shukla A (2008) Effect of diffusion-layer porosity on the performance of polymer electrolyte fuel cells. J Appl Electrochem 38(3):357–362
Tang H, Wang S, Pan M, Yuan R (2007) Porosity-graded micro-porous layers for polymer electrolyte membrane fuel cells. J Power Sources 166(1):41–46
Kitahara T, Konomi T, Nakajima H (2010) Microporous layer coated gas diffusion layers for enhanced performance of polymer electrolyte fuel cells. J Power Sources 195(8):2202–2211
Tseng C-J, Lo S-K (2010) Effects of microstructure characteristics of gas diffusion layer and microporous layer on the performance of PEMFC. Energy Convers Manag 51(4):677–684
W-k L, Ho C-H, Van Zee J, Murthy M (1999) The effects of compression and gas diffusion layers on the performance of a PEM fuel cell. J Power Sources 84(1):45–51. doi:10.1016/S0378-7753(99)00298-0
Senthil Velan V, Velayutham G, Rajalakshmi N, Dhathathreyan K (2014) Influence of compressive stress on the pore structure of carbon cloth based gas diffusion layer investigated by capillary flow porometry. Int J Hydrog Energy 39(4):1752–1759
Lee C, Mérida W (2007) Gas diffusion layer durability under steady-state and freezing conditions. J Power Sources 164(1):141–153
Radhakrishnan A, Lu Z, Kandlikar SG (2010) Effective thermal conductivity of gas diffusion layers used in PEMFC: measured with guarded-hot-plate method and predicted by a fractal model. ECS Trans 33(1):1163–1176
Xu G, LaManna JM, Clement JT, Mench MM (2014) Direct measurement of through-plane thermal conductivity of partially saturated fuel cell diffusion media. J Power Sources 256:212–219. doi:10.1016/j.jpowsour.2014.01.015
Yablecki J, Nabovati A, Bazylak A (2012) Modeling the effective thermal conductivity of an anisotropic gas diffusion layer in a polymer electrolyte membrane fuel cell. J Electrochem Soc 159(6):B647–B653
Weber AZ, Hickner MA (2008) Modeling and high-resolution-imaging studies of water-content profiles in a polymer-electrolyte-fuel-cell membrane-electrode assembly. Electrochim Acta 53(26):7668–7674. doi:10.1016/j.electacta.2008.05.018
Wang Y, Wang C-Y (2006) A nonisothermal, two-phase model for polymer electrolyte fuel cells. J Electrochem Soc 153(6):A1193–A1200
Khandelwal M, Mench MM (2006) Direct measurement of through-plane thermal conductivity and contact resistance in fuel cell materials. J Power Sources 161(2):1106–1115. doi:10.1016/j.jpowsour.2006.06.092
Sadeghi E, Djilali N, Bahrami M (2011) Effective thermal conductivity and thermal contact resistance of gas diffusion layers in proton exchange membrane fuel cells. Part 1: effect of compressive load. J Power Sources 196(1):246–254
Escribano S, Blachot J-F, Ethève J, Morin A, Mosdale R (2006) Characterization of PEMFCs gas diffusion layers properties. J Power Sources 156(1):8–13
Wu J, Yuan XZ, Martin JJ, Wang H, Zhang J, Shen J, Wu S, Merida W (2008) A review of PEM fuel cell durability: degradation mechanisms and mitigation strategies. J Power Sources 184(1):104–119. doi:10.1016/j.jpowsour.2008.06.006
Chen G, Zhang H, Ma H, Zhong H (2009) Electrochemical durability of gas diffusion layer under simulated proton exchange membrane fuel cell conditions. Int J Hydrog Energy 34(19):8185–8192
Wood DL III, Borup RL (2009) Durability aspects of gas-diffusion and microporous layers. In: Polymer electrolyte fuel cell durability. Springer, New York, pp 159–195
Yi P, Ni J, Peng L, Lai X (2011) A numerical model for predicting gas diffusion layer failure in proton exchange membrane fuel cells. J Fuel Cell Sci Technol 8(1):011011
Bernardi DM, Verbrugge MW (1991) Mathematical model of a gas diffusion electrode bonded to a polymer electrolyte. AICHE J 37(8):1151–1163
Bernardi DM, Verbrugge MW (1992) A mathematical model of the solid‐polymer‐electrolyte fuel cell. J Electrochem Soc 139(9):2477–2491
Springer T, Wilson M, Gottesfeld S (1993) Modeling and experimental diagnostics in polymer electrolyte fuel cells. J Electrochem Soc 140(12):3513–3526
Tobias CW (1959) Effect of gas evolution on current distribution and ohmic resistance in electrolyzers. J Electrochem Soc 106(9):833–838
Chiang M-S, Chu H-S (2006) Numerical investigation of transport component design effect on a proton exchange membrane fuel cell. J Power Sources 160(1):340–352
Um S, Wang C (2004) Three-dimensional analysis of transport and electrochemical reactions in polymer electrolyte fuel cells. J Power Sources 125(1):40–51
Gurau V, Mann JA (2010) Effect of interfacial phenomena at the gas diffusion layer-channel interface on the water evolution in a PEMFC. J Electrochem Soc 157(4):B512–B521
Zhou P, Wu C (2007) Numerical study on the compression effect of gas diffusion layer on PEMFC performance. J Power Sources 170(1):93–100
Wang Z, Wang C, Chen K (2001) Two-phase flow and transport in the air cathode of proton exchange membrane fuel cells. J Power Sources 94(1):40–50
Gurau V, Zawodzinski TA, Mann JA (2008) Two-phase transport in PEM fuel cell cathodes. J Fuel Cell Sci Technol 5(2):021009
Hartnig C, Manke I, Kuhn R, Kardjilov N, Banhart J, Lehnert W (2008) Cross-sectional insight in the water evolution and transport in polymer electrolyte fuel cells. Appl Phys Lett 92(13):134106
Pasaogullari U, Wang C-Y (2005) Two-phase modeling and flooding prediction of polymer electrolyte fuel cells. J Electrochem Soc 152(2):A380–A390
Meng H, Wang C-Y (2005) Model of two-phase flow and flooding dynamics in polymer electrolyte fuel cells. J Electrochem Soc 152(9):A1733–A1741
Grigoriev S, Kalinnikov A, Fateev V, Wragg A (2006) Numerical optimization of bipolar plates and gas diffusion layers for PEM fuel cells. J Appl Electrochem 36(9):991–996
Nitta I, Karvonen S, Himanen O, Mikkola M (2008) Modelling the effect of inhomogeneous compression of GDL on local transport phenomena in a PEM fuel cell. Fuel Cells 8(6):410–421
Su Z, Liu C, Chang H, Li C, Huang K, Sui P (2008) A numerical investigation of the effects of compression force on PEM fuel cell performance. J Power Sources 183(1):182–192
Dotelli G, Omati L, Gallo Stampino P, Grassini P, Brivio D (2011) Investigation of gas diffusion layer compression by electrochemical impedance spectroscopy on running polymer electrolyte membrane fuel cells. J Power Sources 196(21):8955–8966. doi:10.1016/j.jpowsour.2011.01.078
Pasaogullari U, Wang C (2004) Liquid water transport in gas diffusion layer of polymer electrolyte fuel cells. J Electrochem Soc 151(3):A399–A406
Tabe Y, Lee Y, Chikahisa T, Kozakai M (2009) Numerical simulation of liquid water and gas flow in a channel and a simplified gas diffusion layer model of polymer electrolyte membrane fuel cells using the lattice Boltzmann method. J Power Sources 193(1):24–31. doi:10.1016/j.jpowsour.2009.01.068
Rama P, Liu Y, Chen R, Ostadi H, Jiang K, Zhang X, Gao Y, Grassini P, Brivio D (2011) Determination of the anisotropic permeability of a carbon cloth gas diffusion layer through X‐ray computer micro‐tomography and single‐phase lattice Boltzmann simulation. Int J Numer Methods Fluids 67(4):518–530
Kim Y, Kang S (2010) Time delay control for fuel cells with bidirectional DC/DC converter and battery. Int J Hydrog Energy 35(16):8792–8803
Cho J, Park J, Oh H, Min K, Lee E, Jyoung J-Y (2013) Analysis of the transient response and durability characteristics of a proton exchange membrane fuel cell with different micro-porous layer penetration thicknesses. Appl Energy 111:300–309. doi:10.1016/j.apenergy.2013.05.022
Mändle M, Wilde P (2001) SGL Carbon Group. SGL TECHNOLOGIES GmbH, Private communication
Bultel Y, Wiezell K, Jaouen F, Ozil P, Lindbergh G (2005) Investigation of mass transport in gas diffusion layer at the air cathode of a PEMFC. Electrochim Acta 51(3):474–488. doi:10.1016/j.electacta.2005.05.007
Baker DR, Wieser C, Neyerlin KC, Murphy MW (2006) The use of limiting current to determine transport resistance in PEM fuel cells. ECS Trans 3(1):989–999
Berning T, Djilali N (2003) A 3D, multiphase, multicomponent model of the cathode and anode of a PEM fuel cell. J Electrochem Soc 150(12):A1589–A1598
Gloaguen F, Durand R (1997) Simulations of PEFC cathodes: an effectiveness factor approach. J Appl Electrochem 27(9):1029–1035
Yu HM, Ziegler C, Oszcipok M, Zobel M, Hebling C (2006) Hydrophilicity and hydrophobicity study of catalyst layers in proton exchange membrane fuel cells. Electrochim Acta 51(7):1199–1207. doi:10.1016/j.electacta.2005.06.036
Li H, Tang Y, Wang Z, Shi Z, Wu S, Song D, Zhang J, Fatih K, Zhang J, Wang H, Liu Z, Abouatallah R, Mazza A (2008) A review of water flooding issues in the proton exchange membrane fuel cell. J Power Sources 178(1):103–117. doi:10.1016/j.jpowsour.2007.12.068
Skoulidas AI, Ackerman DM, Johnson JK, Sholl DS (2002) Rapid transport of gases in carbon nanotubes. Phys Rev Lett 89(18):185901
Acknowledgments
This work was supported by the IBTec, Auckland University of Technology (AUT) grant by the Doctoral Fellowship and the authors would like to acknowledge their support. The authors are also grateful to Ms. Jo Stone for an effective proofreading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayakumar, A., Sethu, S.P., Ramos, M. et al. A technical review on gas diffusion, mechanism and medium of PEM fuel cell. Ionics 21, 1–18 (2015). https://doi.org/10.1007/s11581-014-1322-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1322-x