Abstract
In this work, a novel holey graphene oxide bonded with sulfonic acid groups (S-HGO) was developed using a facile and eco-friendly strategy: erosion and oxidation of graphene oxide (GO) by H2O2 and sulfonation by 4-benzenediazoniumsulfonate. The freestanding paper fabricated by S-HGO shows a high proton conductivity of 4.97 × 10−2 S cm−1 at 60 °C and 100% relative humidity (RH), which is comparable to Nafion 115 (7.67 × 10−2 S cm−1) at the same condition. The holes on S-HGO provide shortcut channels for proton transfer across the GO nanosheet. The chemical-linked sulfonic acid groups afford additional hop sites for proton conduction and endow itself an enhanced thermostability. Under 100% RH and ambient pressure, the freestanding S-HGO deliver a peak power density of 103 mW cm−2 at a load current density of 273 mA cm−2, which is about three times more than that of GO at 35 °C. The results demonstrate that this S-HGO paper is a promising nanoscale inorganic material for proton exchange membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proton exchange membrane fuel cells (PEMFCs) have received attracted attention due to the outstanding conversion efficiency, prominent reliability, high power density, and free pollution [1]. The performance of PEMFCs is greatly dependent on proton exchange membranes (PEMs). PEMs are not only the barrier to impede fuel migration but also the solid electrolyte for proton transport. The ideal PEMs should have excellent proton conduction, high stability, good mechanical property, and low fuel permeability [2, 3].
As an oxidized derivative of graphene, graphene oxide (GO) has some similar characters with graphene, such as sheet-like structure, outstanding thermostability, and mechanical property. What is more, there are abundant hydrophilic oxygen-containing groups attached on GO, such as carbonyl, hydroxyl, and carboxyl groups, which endow itself a proton conductivity [4, 5]. Recently, several works have addressed the incorporation of GO into the sulfonated PEMs. Because of the strong interactions between GO and sulfonated polymers, the stability of PEMs is increased distinctly. At the same time, the fuel permeability is decreased due to the prevention of fuel permeation paths [6, 7]. Tseng et al. blended GO and sulfonated polyimide (SPI) to obtain SPI/GO composite membrane. The 0.5 wt% content of GO in this SPI/GO membrane reported to have higher proton conductivity than Nafion117 and showed lower methanol permeability and better mechanical property [8]. Bhat et al. prepared sulfonated poly (ether ether ketone) (SPEEK) nanocomposite membranes with aspartic acid functionalized GO, and the composite membranes show enhanced proton conductivity, mitigate methanol permeability, and improved PMFC performance [9].
In consideration of the influence of organic solvent that used to prepare PEMs on the environment, GO and its derivatives could become a carbon-based, non-polymeric alternative to organic PEMs for fuel cells. GO solution was used to prepare GO paper by Ravi Kumar et al. [10]. However, the fuel cell performance was poor because of its low proton conductivity. The research by Gao et al. demonstrated that the chemical modification of GO via ozone results in a higher percentage of oxidation, which leads to improved proton conductivity [11]. To enhance the proton conductivity and mechanical stability of GO, several works proposed the sulfonic acid functionalized GO (SGO) electrolyte PEMFCs [12, 13]. Because the two-dimensional GO nanosheets are perpendicular to the through-plane proton diffusion pathway, there is a limit for the proton diffusion across the SGO membranes. Jiang et al. fabricated holey GO (HGO) by ultrasonic irradiation of GO in concentrated HNO3 solution and then adsorption of sodium dodecylbenzenesulfonate (SDBS) onto HGO [14]. The holey structure provides additional short through-plane proton transport pathways, and the sulfonic acid groups in SDBC also provide high proton conductivity. However, there is a risk for the loss of SDBS because of the weak intermolecular interaction between SDBS and HGO.
In this paper, we investigated a facile and eco-friendly strategy to prepare sulfonic acid bonding holey graphene oxide (S-HGO) paper as a PEM. The S-HGO nanosheet was fabricated via two procedures: preparation of holey GO (HGO) by the erosion and oxidation of GO in the presence of H2O2 and sulfonation of HGO by 4-benzenediazoniumsulfonate. The morphology, structure, and composition of this S-HGO paper were studied, and the proton conductivity was measured and compared with Nafion membranes. The proton conductivity of S-HGO membrane increases almost one order of magnitude than GO, and nearly is close to Nafion 115 at 60 °C and saturation humidity. In addition, the S-HGO membrane exhibits excellent thermostability. When used in the H2/O2 fuel cell, the cell with the S-HGO membrane could exhibit an excellent maximum power density, which is 2.5 times larger than that of GO.
Experimental section
Materials
Chemicals
Graphite powder (carbon content ≥ 99%, average size 40 μm) was supplied by Guyu Graphite Co., Qingdao, China. Sodium nitrate (NaNO3), sodium nitrite (NaNO2), and potassium permanganate (KMnO4) were obtained from Yuanli Chemical Co., Tianjin, China. Concentrated sulfuric acid (H2SO4, 98 wt%), concentrated hydrochloric acid (HCl, 37 wt.%), and ethanol were supplied from Tianjin Kermel Chemical Reagent Company. Sulfanilic acid, hypophosphorous acid, and hydrogen peroxide (H2O2, 30 wt%) were supplied by Aladdin Chemical Co.
Preparation of GO and GO paper
Graphene oxide (GO) was synthesized from the graphite flakes according to the modified Hummers method [15]. Graphite (2.0 g) and sodium nitrate (1.0 g) were mixed with 46 mL of concentrated sulfuric acid in an ice bath under magnetic stirring. Potassium permanganate (6.0 g) was added into the solution slowly after 30 min; during the process, the reaction mixture should be kept lower than 20 °C. Then, the above mixture was moved to a 35 ± 3 °C water bath for 30 min, followed by adding water (120 mL) dropwise under stirring and increasing temperature to 95 °C for another 30 min. Successively, a 500 mL of water was added and then 30 mL of 30 wt% H2O2 was also added to the reaction system, which changed the color of the solution from brown to yellow. After cooled down to room temperature, the mixture was washed by centrifugation with hydrochloric acid (10 wt%) to remove metal ions and then repeated with water until the acid was removed. Finally the obtained solid was dispersed in water by ultrasonication for 2 h to make a GO aqueous dispersion, and then the aqueous dispersion was cast onto a glass mold and dried under vacuum at 70 °C for 12 h to obtain the solid GO. The GO paper is obtained by filtering the GO aqueous dispersion (2 mg mL−1) through a membrane filter (polyvinylidene fluoride membrane filter of 50 mm in diameter, 0.45-μm pore size), followed by air drying and peeling from the filter.
Preparation of HGO and HGO paper
Holey graphene oxide (HGO) was prepared by the oxidation of H2O2 [16]. Briefly, 5 mL 30 wt% H2O2 and 50 mL GO aqueous dispersion (2 mg/mL) were added to 100-mL flask and kept at 100 °C for 4 h under stirring. A homogeneous HGO aqueous dispersion was obtained by ultrasonication for 10 min. The GO and HGO papers were acquired by filtering GO and HGO, respectively, through a polyvinylidene fluoride membrane filter (0.45-μm pore size). After drying at 25 °C for 24 h, the papers were peeled from the filter.
Preparation of S-HGO and HGO paper
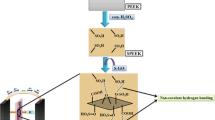
The holey graphene oxide bonded with sulfonic acid (S-HGO) was prepared as follows. Firstly, 4-benzenediazoniumsulfonate was prepared by diazotization of sulfanilic acid [17]. Specifically, 2.6 g of sulfanilic acid was added to 1 M 150 mL HCl aqueous solution under magnetic stirring in an ice bath. Then, 1 M 16.5 mL NaNO2 solution was mixed to the reaction mixture drop by drop; the solution became clear after the NaNO2 was added. Then, the above solution was stirred for 1 h; a white precipitate was obtained followed by filtering off and washing with deionized water. Then, 3 g 4-benzenediazoniumsulfonate, 60 mL ethanol, 10 mL deionized water, and 50 mL HGO aqueous dispersion (2 mg mL−1) were mixed in a beaker. Secondly, 60 mL 50 wt% H3PO2 aqueous solution was mixed with the solution under stirring at 5 °C for 30 min. Then, another 60 mL 50 wt% H3PO2 solution was added again and stirred for 1 h. The obtained dispersion was washed with deionized water by centrifugation, and then dispersed in water by ultrasonication. The S-HGO paper was obtained after filtering. For comparison, graphene oxide bonded with sulfonic acid (S-GO) and its paper were prepared by the same way. The schematic of diagrams to synthesize S-HGO is shown in Scheme 1.
Characterization
The morphology and microstructure of the paper were imaged via scanning electron microscopy (SEM, Hitachi S4800) and transmission electron microscopy (TEM, JEOL JEM 2100F). The TEM samples were prepared by immersing 0.001 g of samples in 100 mL of deionized water via ultrasonication for 30 min at room temperature; a droplet of the dispersion was cast onto a copper grid, then dried at room temperature. Energy dispersive X-ray spectroscopy (EDX) analysis was carried out via electron microscopy (SEM, Hitachi S4800). The samples were also analyzed via X-ray photoelectron spectroscope (XPS, Thermo Electron PHI-5000 II), Fourier transform infrared spectroscopy (FTIR, FTIR-650), and X-ray diffraction (XRD, D/Max-2500VPC, Rigaku). Raman spectra were recorded to detect the defects of GO (Renishaw 2000, 523 nm). Thermal stability was measured by a thermogravimetric analyzer (STA 409 PC, N2 atmosphere) with the heating rate of 10 °C min−1.
Water uptake and proton conductivity measurement
Water uptake of membrane was obtained by the calculation according to the following:
where wwet and wdry are corresponding to the weights of the membrane in wet and dry states, respectively. Wet samples were soaked in ultrapure water (HGO, S-GO and S-HGO papers) or in water vapor (GO paper) at a specified temperature for at least 24 h to reach swelling equilibrium.
The proton conductivity of the papers was tested using a membrane test system (MTS-740, Scribner) and an electrochemical workstation (Parstat 2273, Princeton). The impedance spectrum of membrane was obtained under an oscillating voltage of 10 mV with frequency sweeping from 2 MHz to 1 Hz. The proton conductivity σ was calculated using the following equation:
where L is the thickness of the membrane, S is the membrane area, and R is the ohmic resistance of the membrane obtained according to the impedance spectrum.
Ion exchange capacity
The ion exchange capacity (IEC) of the paper was measured with the classical titration technique [12]. The paper was firstly soaked into a certain volume of 0.1 mol L−1 HCl. After washed by a large distilled water to remove excess HCl, the paper was then soaked in 1 mol L−1 NaCl to release H+ into the solution. The released protons were titrated with a 0.01 mol L−1 NaOH using phenolphthalein as an indicator. The IEC (mmol g−1) of paper was obtained by the calculation according to the following:
where VNaOH and CNaOH are corresponding to the volume of NaOH consumed and the concentration of NaOH, respectively. W is the weight of the dry paper.
Membrane electrode assembly preparation and fuel cell testing
Three hundred milligrams of Pt/C (20 wt% Pt, Johnson Matthey) was added into 300 mg of water, then mixed with 300 mg Nafion solution (5 wt%, DuPont) and 1500 mg of isopropanol to form an ink. The gas diffusion layer electrodes were obtained by spread on 2 × 2 cm carbon paper (HCP120, Hesen) with the ink. The catalyst loadings of the anode and cathode are 7.2 mg and 14.4 mg, respectively. The 4 × 4 cm membrane was sandwiched between the anode and cathode and hot pressed at 120 °C and 9.0 MPa for 120 s to fabricate the membrane electrode assembly. Next, the membrane electrode assembly was assembled into a single cell, and the cell was tested at 30 °C and an ambient pressure with the 100% RH 100 mL min−1 of H2 and 200 mL min−1 of O2 in anode and cathode [18].
Results and discussion
The morphology and microstructure
From Fig. 1a–d, S-GO and S-HGO papers show a relatively rougher surface than that of GO and HGO papers. That is because the sulfonic acid groups make the layered structure disordered during the formation of paper [19]. The cross-section of all the samples shows similar layer-by-layer structure (the inset of Fig. 1). From Fig. 2, the obvious holes, with the diameter of 10 nm, are distributed on the surface of HGO and S-HGO nanosheets uniformly. These holes are fabricated by the oxidation and etching of H2O2. GO is easily reacted with oxygen from H2O2 at the defects, so blank sites could form and then these blank sites could be larger and the holes could be obtained as the reaction proceed [20]. The elemental mapping of S-HGO (Fig. 3) illustrates the sulfonic acid groups are distributed on the whole surface of GO homogeneously. Quantitative analysis is shown in the inset in Fig. 4. The presence of sulfur in S-GO and S-HGO indicated that sulfonic acid functionalized groups are reacted on the GO successfully. Besides the ratio of oxygen to carbon increases in HGO and S-HGO paper because of the enhancement of oxygen-containing functional groups from oxidization of GO by H2O2.
By analysis of binding energy (BE) in XPS C1s spectra (Fig. 4), we can confirm the new chemical bond C-S was generated during the sulfonation of GO and HGO [21]. There is no nitrogen in S-GO and S-HGO samples, suggesting that the element N in 4-benzenediazoniumsulfonate was released as N2 during this chemical crosslinking reaction. The FTIR spectra in Fig. 5 also confirm the successful sulfonation of GO and HGO. The peaks at 1740, 1628, 1400, and 1055 cm−1 represent the O–H stretching, C=O stretching, C–O deformation, and C–O stretching vibrations, respectively. These oxygen-containing functional groups in GO are existing in all the samples. While there is a slight absorption peak at 1346 cm−1 in S-GO and S-HGO, which is corresponding to the vibration of sulfonic acid functional groups [12].
The defects of GO could be increased after making holes and sulfonation, and they can be detected by Raman spectroscopy. The peak at about 1350 cm−1 represents the vibrations of disordered carbon at edge and defect sites of the graphitic structure named D-band peak, and the G-band peak near 1580 cm−1 reflects the in-plane bond stretching of sp2 carbon atoms. Generally, the intensity ratio of the D-band peak to G-band peak (ID/IG) was used to reflect the defects of GO, and the defect density is in direct proportion to the value of ID/IG [22]. As shown in Fig. 6a, these in-plane holes are from the defects of GO nanosheet, there is hardly more defects after making holes, while ID/IG reflects the defects on the sheet, so the value of ID/IG is almost unchanged after making holes on sheets. Compared with GO (or HGO), the value of ID/IG slightly is enhanced in S-GO (or S-HGO), indicating more disordered carbon on the surface of GO after sulfonation [23]. X-ray diffraction (XRD) was used to study the interlayer distance of these papers and to demonstrate the surface functionalization of GO and HGO. In Fig. 6b, XRD patterns of GO and HGO exhibit the sharp peak at 11.0° and 11.2° with an average inter-planar spacing of 0.805 nm and 0.790 nm. Nevertheless, the peaks of S-GO and S-HGO shift to 11.3°and 11.4° with an interlayer spacing of 0.785 nm and 0.776 nm respectively. It is worth to note that the interlayer spacing of the S-GO and S-HGO paper is respectively smaller than GO and HGO paper. That is because the attractive interaction between the adjacent nanosheets is increased by the sulfonic acid groups which could make the layers tighter and make the material more stable [24, 25] .
Thermal characteristics
The thermogravimetric analysis of GO, HGO, S-GO, and S-HGO as shown in Fig. 7 indicated all of them underwent three-stage weight losses. The first is below 150 °C where the adsorbed water was evaporated. The weight loss is more than the previous at second-stage from 150 to 230 °C due to the loss of oxygen-containing functionalities such as CO, CO2, or H2O. Finally, the weight loss is relatively moderate above 230 °C which could be attributed to the disruption the stacking of GO sheets and decomposing of framework of the GO. Compared with GO and HGO, S-GO and S-HGO exhibit more thermostability which was reflected in the less loss after 230 °C. The thermogravimetric result shows that S-HGO has the least weight loss after 230 °C, suggesting it exhibits more thermostability than others. That is because the sulfonation can increase the attractive interaction between the GO layers and decrease the disruption of GO structure [26].
Water uptake and proton conductivity
For PEMFC applications, one of the most significant properties is water uptake for sulfonic acid based PEMs. Figure 8 shows the water uptake of HGO, S-GO, and S-HGO in water and GO in vapor at different temperatures. Because the structure of GO paper was not as stable as others when immersed in water, the water uptake of GO was obtained in vapor. It worth to note that water uptake of S-HGO paper is higher than others. Compared with HGO, the sulfonic acid groups in S-HGO could adsorb more water. In the case of high humidity, more water uptake is benefit to form continue channels for proton transfer and increase the rate of conductivity.
The ion exchange capacity (IEC) and proton conductivity are presented in Figs. 9 and 10. The GO possesses a low proton conductivity because of the weakly acidic oxygen-containing functional groups, including hydroxyl, carboxyl, and epoxy groups [5]. The IEC and conductivity of S-GO paper is higher than those of GO paper, due to the sulfonic acid groups on the nanosheets which create more interconnected transfer channels and sits for proton transport [27]. HGO exhibits higher IEC than GO, because lots of oxygen-containing functional groups are generated around the holes during the preparation of holes. The conductivity of HGO paper is also higher than that of GO paper, due to the in-plane holes which provide shortcut channels for protons as shown in the Scheme 1. It could be seen that the conductivity of S-HGO paper is almost two orders of magnitude higher than GO paper. That means that combined effect of holes and sulfonic acid groups could give much influence on proton conductivity. On the one hand, sulfonic acid and oxygen-containing groups could adsorb more water, which is the vehicular for proton. On the other hand, proton could be transported through in-plane holes across the nanosheet and hop between sulfonic acid groups [28]. In particular, the S-HGO paper exhibited a higher IEC of 1.78 mmol g−1 than that of Nafion 115 (0.93 mmol g−1 [12]) and proton conductivity of 4.97 × 10−2 S cm−1 at 60 °C, which is comparable to Nafion 115 (7.67 × 10−2 S cm−1) and other GO membranes (Table S1).
Single-cell performance
A series hydrogen/oxygen fuel cell tests were operated to compare the electrochemical performance of GO, S-HGO, and Nafion 115. The results of polarization and power density for these three materials were shown in Fig. 11. The GO exhibited a maximum power density of 29 mW cm−2, while the S-HGO showed 103 mW cm−2 which was about 3.5 times as large as that of GO. Sulfonation and holes on GO could improve the electrochemical performance, but the performance was lower than Nafion 115 because S-HGO did not have enough sulfonic acid functional groups (see in Fig. 4d) compared with Nafion 115. Thus, it can be seen S-HGO has the potential as PEMs, but future work is needed to improve the degree of sulfonation.
Conclusion
The freestanding holey GO paper bonded with sulfonic acid was fabricated by a facile method: oxidation by H2O2 and sulfonation by 4-benzenediazoniumsulfonate. Holes and bonded sulfonic acid groups on GO can modify the property of GO remarkably. Without holes on GO, proton transport needs to travel a longer distance through the edge of GO nanosheet, while holes on the GO nanosheet could provide much proton transport shortcuts between the two adjacent layers to speed up proton transport across the GO nanosheet. The presence of sulfonic acid functionalized groups could create more proton transfer sites to improve the proton conductivity and enhance the attractive interaction between the neighboring layers to make the material more stable. This S-HGO paper is a promising PEM for PEMFC application.
References
Zhang W, Zheng H, Zhang C, Li B, Fang F, Wang Y (2017) Strengthen the performance of sulfonated poly (ether ether ketone) as proton exchange membranes with phosphonic acid functionalized carbon nanotubes. Ionics 23:2103–2112
Beydaghi H, Javanbakht M, Kowsari E (2016) Preparation and physicochemical performance study of proton exchange membranes based on phenyl sulfonated graphene oxide nanosheets decorated with iron titanate nanoparticles. Polymer 87:26–37
Pan H, Chen S, Jin M, Chang Z, Pu H (2018) Preparation and properties of sulfonated polybenzimidazole-polyimide block copolymers as electrolyte membranes. Ionics 24:1629–1638
Karim MR, Hatakeyama K, Matsui T, Takehira H, Taniguchi T, Koinuma M, Matsumoto Y, Akutagawa T, Nakamura T, Noro S, Yamada T, Kitagawa H, Hayami S (2013) Graphene oxide nanosheet with high proton conductivity. J Am Chem Soc 135:8097–8100
Hatakeyama K, Karim MR, Ogata C, Tateishi H, Funatsu A, Taniguchi T, Koinuma M, Hayami S, Matsumoto Y (2014) Proton conductivities of graphene oxide nanosheets: single, multilayer, and modified nanosheets. Angew Chem Int Ed 53:6997–7000
Qiu X, Dong T, Ueda M, Zhang X, Wang L (2017) Sulfonated reduced graphene oxide as a conductive layer in sulfonated poly (ether ether ketone) nanocomposite membranes. J Membrane Sci 524:663–672
Cheng T, Feng M, Huang Y, Liu X (2017) SGO/SPEN-based highly selective polymer electrolyte membranes for direct methanol fuel cells. Ionics 23:2143–2152
Tseng C, Ye Y, Cheng M, Kao K, Shen W, Rick J, Chen J, Hwang B (2011) Sulfonated polyimide proton exchange membranes with graphene oxide show improved proton conductivity, methanol crossover impedance, and mechanical properties. Adv Energy Mater 1:1220–1224
Rambabu G, Bhat SD (2018) Amino acid functionalized graphene oxide based nanocomposite membrane electrolytes for direct methanol fuel cells. J Membrane Sci 551:1–11
Kumar R, Mamlouk M, Scott K (2011) A graphite oxide paper polymer electrolyte for direct methanol fuel cells. Int J Electrochem 2011:1–7
Gao W, Wu G, Janicke MT, Cullen DA, Mukundan R, Baldwin JK, Brosha EL, Galande C, Ajayan PM, More KL, Dattelbaum AM, Zelenay P (2014) Ozonated graphene oxide film as a proton-exchange membrane. Angew Chem Int Ed 53:3588–3593
Ravikumar SK (2012) Freestanding sulfonated graphene oxide paper: a new polymer electrolyte for polymer electrolyte fuel cells. Chem Commun 48:5584–5586
Hou H, Hu X, Liu X, Hu W, Meng R, Li L (2015) Sulfonated graphene oxide with improved ionic performances. Ionics 21:1919–1923
Jiang Z, Shi Y, Jiang Z, Tian X, Luo L, Chen W (2014) High performance of a free-standing sulfonic acid functionalized holey graphene oxide paper as a proton conducting polymer electrolyte for air-breathing direct methanol fuel cells. J Mater Chem A 2:6494
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Xu Y, Lin Z, Zhong X, Huang X, Weiss NO, Huang Y, Duan X (2014) Holey graphene frameworks for highly efficient capacitive energy storage. Nat Commun 5:4554
Ji J, Zhang G, Chen H, Wang S, Zhang G, Zhang F, Fan X (2011) Sulfonated graphene as water-tolerant solid acid catalyst. Chem Sci 2:484–487
Bayer T, Bishop SR, Nishihara M, Sasaki K, Lyth SM (2014) Characterization of a graphene oxide membrane fuel cell. J Power Sources 272:239–247
Heo Y, Im H, Kim J (2013) The effect of sulfonated graphene oxide on sulfonated poly (ether ether ketone) membrane for direct methanol fuel cells. J Membrane Sci 425:11–22
Xu Y, Chen C, Zhao Z, Lin Z, Lee C, Xu X, Wang C, Huang Y, Shakir MI, Duan X (2015) Solution processable holey graphene oxide and its derived macrostructures for high-performance supercapacitors. Nano Lett 15:4605–4610
Nishimura O, Yabe K, Iwaki M (1989) X-ray photoelectron spectroscopy studies of high-dose nitrogen ion implanted-chromium: a possibility of a standard material for chemical state analysis. J Electron Spectrosc Relat Phemon 49:335–342
Vinothkannan M, Kannan R, Kim AR, Kumar GG, Nahm KS, Yoo DJ (2016) Facile enhancement in proton conductivity of sulfonated poly (ether ether ketone) using functionalized graphene oxide—synthesis, characterization, and application towards proton exchange membrane fuel cells. Colloid Polym Sci 294:1197–1207
Kumar R, Mamlouk M, Scott K (2014) Sulfonated polyether ether ketone - sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv 4:617–623
Beydaghi H, Javanbakht M, Bagheri A, Salarizadeh P, Ghafarian-Zahmatkesh H, Kashefi S, Kowsari E (2015) Novel nanocomposite membranes based on blended sulfonated poly (ether ether ketone)/poly (vinyl alcohol) containing sulfonated graphene oxide/Fe3O4 nanosheets for DMFC applications. RSC Adv 5:74054–74064
Zhao Y, Tang K, Ruan H, Xue L, Van der Bruggen B, Gao C, Shen J (2017) Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J Membrane Sci 536:167–175
Sharma PP, Kulshrestha V (2015) Synthesis of highly stable and high water retentive functionalized biopolymer-graphene oxide modified cation exchange membranes. RSC Adv 5:56498–56506
Neelakandan S, Jacob NK, Kanagaraj P, Sabarathinam RM, Muthumeenal A, Nagendran A (2016) Effect of sulfonated graphene oxide on the performance enhancement of acid-base composite membranes for direct methanol fuel cells. RSC Adv 6:51599–51608
Chien H, Tsai L, Huang C, Kang C, Lin J, Chang F (2013) Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int J Hydrogen Energ 38:13792–13801
Funding
This work was supported by the National Natural Science Foundation of China (11705126) and Science and Technology Program of Tianjin (16JCQNIC06000 and 16JCYBJC21100).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOC 71 kb)
Rights and permissions
About this article
Cite this article
Zhang, C., Zhang, W., Mu, Y. et al. Facile fabrication of holey graphene oxide paper bonded with sulfonic acid for highly efficient proton conduction. Ionics 25, 573–581 (2019). https://doi.org/10.1007/s11581-018-2789-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2789-7