Abstract
Electro-reduction of carbon dioxide (CO2) to carbon monoxide (CO) has been extensively studied on metal and alloy electrodes for many decades. However, owing to their disadvantages of low current density and high over-potential, the practical application of these electrodes has been limited. Hence, it is highly desirable to explore new and high efficient electrode for CO2 reduction to CO. Ag2S has been widely studied as electrode material in electrochemistry due to its unique properties, such as high conductivity, chemical stability, and easy to be prepared. In this work, we have fabricated an Ag2S electrode via electro-oxidation of Ag in aqueous solution. X-ray diffraction (XRD) and scanning electron microscope (SEM) confirm that Ag2S has been modified on Ag foil, which made the electrode surface roughness. And then, we have evaluated the performance of Ag2S electrode as the cathode for CO2 reduction in propylene carbonate/tetrabutylammonium perchlorate. The cathodic current density reaches to 9.85 mA/cm2, with the faradic efficiency for CO formation remaining stable at 92% during 4 h long-term electrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrochemical reduction of CO2 to CO is the first step in the synthesis of more complex carbonaceous products [1,2,3,4]. In the past decades, numerous works have been done to explore an efficient electrode to convert CO2 into CO with high efficiency. Most of the works focused on metal and alloy electrodes. However, owing to their disadvantages of low current density and high over-potential, the practical application has been limited. Hence, it is highly desirable to develop new electrode to improve the efficiency of CO2 reduction [5,6,7,8,9,10].
Silver sulfide (Ag2S) has been widely used as electrode materials in electrochemistry, because it possesses many unique properties such as high conductivity, low price, and anti-poisonous characteristics [11,12,13,14,15]. In recent years, it was found that Ag2S has high catalytic effects towards hydrogen evolution [16, 17]. But little attention has been given to investigate electro-reduction CO2 on Ag2S. In present work, we intend to study the electrochemical property of Ag2S towards CO2 reduction.
CO2 is a non-polar molecule and is highly soluble in organic electrolyte. Therefore, it would be beneficial to conduct the electrochemical reduction of CO2 in an organic electrolyte. Selection of suitable supporting electrolyte and organic solvent is essential to CO2 reduction. Because CO2 is a non-polar molecule and has high solubility in organic solution, it would be beneficial to conduct CO2 reduction in organic media. Among the commonly used organic solvents (AN, DMF, DMSO, and PC), acetonitrile (AN) is not suitable for practical application, because it is toxic and easily volatilize at ambient condition, it would result in hazardous effect to human health and environment. N,N-Dimethylformamide (DMF) is not suitable for practical application also, because it is poisonous and readily hydrolysis. Towards dimethyl sulfoxide (DMSO), because its melting point is 18 °C, CO2 reduction cannot be conducted at ambient condition below 18 °C. Propylene carbonate (PC) [18] is normally used as CO2 absorber in industry, and also, it is a widely used medium in organic electrochemical, which has many advantages, such as non-toxicity, wide electrochemical window, low volatility, high CO2 solubility, high boiling point, and high dielectric constant [19]. Thereby, we select PC as the organic solvent. Imidazolium ionic liquids (ILs) has been extensively studied as a novel support electrolyte for CO2 reduction [20,21,22], owing to that they have some unique properties, such as negligible vapor pressure, wide electrochemical window, low volatility, and adjustable physical and chemical properties. Nevertheless, due to their disadvantages of high cost and readily decomposition at very negative potential (electro-reduction of CO2 requires large negative potential) [23], it is not ideal for industry application. Tetrabutylammonium perchlorate (TBAP) [24, 25] is a commonly used supporting electrolyte in the organic electrochemistry, which possesses many outstanding advantages, such as low cost, easily to be prepared, and wide electrochemical windows. Hence, in this work, we select TBAP as the supporting electrolyte.
Herein, for the first time, we have reported CO2 reduction on Ag2S in PC/TBAP using two-compartment cell. Since electro-reduction of CO2 to CO naturally generates H2O, the presence of water in PC/TBAP is inevitable, and water plays in crucial role during CO2 reduction process. Thereby, we have also discussed water effects on CO2 reduction on Ag2S.
Experimental section
Materials
Propylene carbonate (PC, analytical grade) was purchased from Meryer Chemicals and distilled before use. Tetrabutylammonium perchlorate (TBAP, analytical grade) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Ethanol absolute (AR, analytical grade), sulfuric acid (H2SO4, 98.08%), and sodium sulfide (Na2S, 99.99%) were used as received from Aladdin Ltd. (Shanghai, China) without further purification. Ion-exchange membrane (Nafion 117) was purchased from DuPont Company. CO2 and Ar (99.99%, purity) were purchased from Messer Company (Hainan, China). All the water in the experiment is double distilled.
Preparation of Ag2S electrode
The Ag electrode was polished with 0.05 μm alumina slurry and then sonicated in double-distilled water for 3 min, subsequently pretreated by degreasing in acetone, immersed in 10% nitric acid 3 min, and rinsed with a copious amount of doubly distill water. Ag2S was prepared by anodic oxidation method in 0.1 M Na2S solution. During this process, Ag+ resolved in Na2S solution via anodization of Ag electrode at 0.9 V (NHE). It reacts with S2− and formed a film of Ag2S, which was deposited on Ag electrode. The electrode reaction is as follows:
Electrochemical experiments
A two-compartment electrolysis cell [26] has been designed for CO2 reduction in propylene carbonate (PC)/tetrabutylammonium perchlorate (TBAP) electrolyte. The electrolysis cell is separated into two compartments by an ion-exchange membrane (Fig. 1). In this cell, CO2-saturated organic electrolyte was used as the catholyte, and 0.1 M H2SO4 aqueous solution was used as the anolyte. The required proton and electrons for CO2 reduction through from the anolyte, the reaction can be carried out more easily. Ag2S modified Ag electrode (99.99%, 2 mm × 2 mm) was used as the cathode. A graphite rod (99.99%, 5 mm in diameter and 15 cm in length) was used as the anode [27]. An I3−/I− electrode was used as the reference electrode [28]. This reference electrode was constructed by immersing a Pt wire in a 0.1-M PC/TBAP solution containing 0.05 M I2 and 0.1 M Bu4NI. The I3−/I− reference electrode was end with a porous Teflon cylinder and isolated from the cathode compartment by a salt bridge. The salt bridge contained the same solution as the catholyte. The salt bridge was separated from the catholyte by an ultra-fine glass firt. The tip of the salt bridge was placed 3 mm away from the cathode. The graphite rod was polished with 1200 grid sandpaper, and then, the Ag2S electrode and graphite rod are cleaned using ultrasonication in distilled water and rinsed thoroughly with double-distilled water. During the electrolysis process, the generated protons from H2O oxidized transfer through the proton-exchange membrane and diffuse to the cathode. On the cathode, CO2 is reduced to CO with the participation of proton. The half reactions of anodic and cathodic are as follows in Fig. 1.
Measurements
All the electrochemical experiments are performed on CHI660D electrochemical working station at room temperature. PT-101 ultrasonic cleaner was purchased (Germany Branson Company, China). During the electrolysis process, the cathodic gas products are collected in a gas collector and analyzed by a gas chromatograph (Agilent-7890B, Westeast Analytical Instruments, China). The temperatures of the injector, the oven, and the detector are maintained at 130 °C, 120 °C, and 110 °C. X-ray diffraction (XRD) analysis of the samples was performed on the X-ray diffractometer (Model D/MAX2500, Rigaka Denki Co., Ltd., Japan) with Cu-Kα radiation (λ = 1.54184 Å) at a voltage/current of 40 kV/40 mA, and the scan speed was 2°min−1 and a step size of 0.02°. Variable temperature XRD data were collected using a Shimadzu XRD-7000 with Cu-Kα radiation (λ = 1.54184 Å). The morphologies of Ag2S electrode was characterized by a HITACHI S-4800 scanning electron microscope (SEM) (Hitachi, S-4800, Tokyo, Japan) with a magnification of 100 k and emission voltage of 10 kV.
Results and discussion
XRD analysis
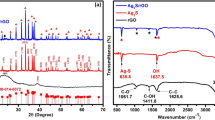
Figure 2a shows the pictures of Ag electrode which were taken before and after the electro-oxidation of Ag electrode in Na2S solution. Before the electro-oxidation, Ag electrode displayed mirror-like surface. After the electro-oxidation, a film of black substance was formed and deposited on the surface of Ag electrode. To determine the ingredient of the black adsorbates, X-ray diffraction (XRD) was performed (Fig. 2b). Comparing with the pure Ag, several characteristic monoclinic planes of Ag2S have been observed, which located at 28.1°, 34.3°, and 36.4°, corresponding to 200, − 121, and 022. These results demonstrated that Ag2S was formed and deposited on Ag foil. The formation process of Ag2S should be explained as follows: when the Ag electrode was electro-oxidation in Na2S aqueous solution, Ag+ was generated and entered into the aqueous solution, which further reacted with S2−, resulting in the formation of Ag2S. Because the solubility of the Ag2S is very low, it was crystalled on Ag foil and formed a film. Thus, Ag2S-modified Ag electrode was obtained.
Linear sweep voltammetry
Figure 3 shows linear sweep voltammetry (LSV) measured on Ag2S. In Ar-saturated PC/TBAP, the negative limiting potential was detected as − 2.35 V (Fig. 3a). After purged with CO2, the onset potential shifted positively to − 1.70 V (vs. Fc/Fc+), and the current density increased obviously (Fig. 3b), indicating that CO2 electro-reduction took place on Ag2S-modified electrode. Because electro-reduction of CO2 to CO naturally produces H2O, the accumulation of H2O in PC/TBAP is inevitable [29]. When the content of H2O exceeds saturation level, it would separate from PC/TBAP due to the hydrophobic nature of PC/TBAP. The saturation content of H2O in PC/TBAP is 6.8 wt% (measured via Karl Fisher titration) [29]. We have also conducted LSV measurements in the PC/TBAP + 6.8 wt% H2O. The results are shown in Fig. 3c. As can be seen, the current density detected on Ag2S increased sharply up to 14.18 mA/cm2, and the onset potential shifted positively to − 1.50 V(vs. Fc/Fc+). These results verified that H2O has catalytic effects on CO2 reduction.
Kinetic analysis
Tafel curves have been measured on Ag2S in PC/TBAP. Since CO2 is a thermodynamically stable molecule, electrochemical reduction CO2 needs high over-potential. Hence, the equilibrium potential of CO2 reduction was − 1.72 V (Fig. 4a). After adding 6.8 wt% of H2O in 0.1 M PC/TBAP, the onset potential shifted positively to − 1.50 V (Fig. 4b). These results further demonstrated that H2O has catalytic effects on CO2 reduction.
Tafel curves for CO2 reduction to CO on Ag2S in 0.1 M a PC/TBAP; b PC/TBAP/ + 6.8 wt% H2O (condition same as Fig. 3)
The dynamic parameters of CO2 reduction were calculated according to Eqs. (3)–(5) [30]. The results are listed in Table 1.
Where η is the activation over-potential, a and b are Tafel constant, α is the charge transfer coefficient, n is the number of electron transfer, i0 is the exchange current density, F is Faraday’s constant, R is universal gas constant, T is the absolute temperature, and Rct is the polarization resistance.
As can be seen, the exchange current density i0 measured on Ag2S in PC/TBAP is 0.6144 × 10−4 A·cm−2. The polarization resistance Rct is 209.2812 Ω·cm−2 (Table. 1). These results are consistent with LSV (Fig. 3). After adding 6.8 wt% of H2O in 0.1 M PC/TBAP, i0 increased to 0.9784 × 10−4 A·cm−2, and Rct decreased to 98.2960 Ω·cm−2. The increase of i0 and decrease of Rct are caused by the catalytic effects of H2O.
Long-term stability
Long-term potentiostatic electrolysis was carried out on Ag2S in PC/TBAP. To avoid the decomposition of the electrolyte, the electrode potential was controlled at − 2.3 V (vs. Fc/Fc+). The results are shown in Fig. 5. As can be seen, the cathodic current density kept stable at 6.63 mA/cm2 (Fig. 5a). After adding 6.8 wt% of H2O in 0.1 M PC/TBAP, the current density increased significantly. It reached to 9.85 mA/cm2 and remained stable at this level until the completion of the electrolysis experiment (Fig. 5b).
The faradaic efficiency (η) of CO formation had been calculated according to Eq. (6) [31, 32].
Where n is the amount of substance of CO produced, F is the faradaic constant (96,485 C/mol), and Q is the total charge passed. The results are shown in Fig. 4b. In the initial period, the faradaic efficiency of CO formation is low (both in PC/TBAP and PC/TBAP/ + 6.8 wt% H2O). This is because a part of CO dissolved in the catholyte [33], which could not be collected for analysis. After 40 min of electrolysis, the faradaic efficiency of CO formation increased significantly and kept stable at high level until the end of the electrolysis. In CO2-saturated PC/TBAP, the faradaic efficiency of CO formation is high (85%). Owing to the presence of residual water, hydrogen was generated on the cathode, the faradaic efficiency for H2 formation arrived to 15%. After long-term electrolysis, because CO2 electro-reduction into CO naturally produces H2O, the accumulation of H2O increased. The faradaic efficiency of H2 formation increased with time. When H2O content reached to 6.8 wt% (saturated concentration), the faradaic efficiency of CO and H2 arrived to 92% and 8%, respectively, which remained stable until the end of the electrolysis. Because the electrochemical reduction of CO2 to CO naturally produces H2O, H2 has been detected on the cathode from water electro-reduction.
SEM observation
To further understand the high performance of Ag2S electrode for CO2 reduction, scanning electron microscope (SEM) was employed to observe the morphology of the cathode. Because Ag electrode was polished with aluminum oxide before the tests, it showed mirror surface (Fig. 6a). After deposition of Ag2S on the Ag electrode by electro-oxidation, the surface of the Ag electrode turned into roughness (Fig. 6b). A layer of black adsorbates adhered to Ag electrode. During the electrolysis process, a part of loosened Ag2S split away from the cathode. A layer of strongly absorbed Ag2S was exposed (Fig. 6c). When the image was enlarged, uniformly distributed Ag2S nanoparticles could be observed (Fig. 6d). This nanostructure results in a comparatively lager surface area and catalytic effects towards CO2 reduction. As a consequence, the potentiostatic electrolysis current density increased significantly (Fig. 5), and the onset potential shift positively (Fig. 3).
Conclusions
In this work, in order to improve the current density and decreased the onset potential of CO2 reduction, we have fabricated Ag2S-modified Ag electrode using the electro-oxidation method. The catalytic performance of the Ag2S electrode was assessed in 0.1 M PC/TBAP/6.8 wt% H2O solution. It was found that Ag2S-modified Ag electrode exhibited high catalytic activity, selectivity towards CO2 reduction. The faradaic efficiency of CO and the current density reached to 92% and 9.85 mA/cm2, respectively. Ag2S exhibits a promising in CO2 reduction for practical application.
References
Shen J, Kolb MJ, Göttle AJ, Koper MT (2016) DFT study on the mechanism of the electrochemical reduction of CO2 catalyzed by cobalt porphyrins. J Phys Chem C 120:15714–15721
Liu M, Pang Y, Zhang B, De Luna P, Voznyy O, Xu J, Zheng X, Dinh CT, Fan F, Cao C, de Arquer FP, Safaei TS, Mepham A, Klinkova A, Kumacheva E, Filleter T, Sinton D, Kelley SO, Sargent EH (2016) Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537:382–386
Hu B, Guild C, Suib SL (2013) Corrigendum to “Thermal, electrochemical and photochemical conversion of CO2 to fuels and value-added products”. J CO2 Util 2:18–27
Ramakrishnan S, Chidsey CED (2017) Initiation of the electrochemical reduction of CO2 by a singly reduced ruthenium(II) bipyridine complex. Inorg Chem 56:8326–8333
Yang D-w, Li Q-y, Shen F-x, Wang Q, Li L, Song N, Dai Y-n, Shi J (2016) Electrochemical impedance studies of CO2 reduction in ionic liquid/organic solvent electrolyte on Au electrode. Electrochim Acta 189:32–37
Sarfraz S, Garcia-Esparza AT, Jedidi A, Cavallo L, Takanabe K (2016) Cu–Sn bimetallic catalyst for selective aqueous electroreduction of CO2 to CO. ACS Catal 6:2842–2851
Cheng T, Xiao H, Goddard WA (2016) Reaction mechanisms for the electrochemical reduction of CO2 to CO and formate on the Cu(100) surface at 298K from quantum mechanics free energy calculations with explicit water. J Am Chem Soc 138:13802–13805
Wang F, Cao B, To W-P, Tse C-W, Li K, Chang X-Y, Zang C, Chan SL-F, Che C-M (2016) The effects of chelating N4 ligand coordination on Co(ii)-catalysed photochemical conversion of CO2 to CO: reaction mechanism and DFT calculations. Catal Sci Technol 6:7408–7420
Choi SY, Jeong SK, Kim HJ, Baek I-H, Park KT (2016) Electrochemical reduction of carbon dioxide to formate on Tin–Lead alloys. ACS Sustain Chem Eng 4:1311–1318
H. Y, Electrochemical CO2 reduction on metal electrodes. Springer New York (2008) 89–189
Catriona O’Sullivan, Robert D. Gunning, Ambarish Sanyal, Christopher A. Barrett, Hugh Geaney, Fathima R. Laffir, Shafaat Ahmed, K. M. Ryan, [11] Spontaneous room temperature elongation of CdS and Ag2S nanorods via oriented attachment. J Am Chem Soc 131 (2009) 12250–12257
Fan W, Jewell S, She Y, Leung MK (2014) In situ deposition of Ag-Ag2S hybrid nanoparticles onto TiO2 nanotube arrays towards fabrication of photoelectrodes with high visible light photoelectrochemical properties. Phys Chem Chem Physics: PCCP 16:676–680
Bozanic DK, Djokovic V, Blanusa J, Nair PS, Georges MK, Radhakrishnan T (2007) Preparation and properties of nano-sized Ag and Ag2S particles in biopolymer matrix. Eur Phys J E 22:51–59
Li P, Li Z, Zhang L, Shi E, Shang Y, Cao A, Li H, Jia Y, Wei J, Wang K, Zhu H, Wu D (2012) Bubble-promoted assembly of hierarchical, porous Ag2S nanoparticle membranes. J Mater Chem 22:24721
Basu M, Nazir R, Mahala C, Fageria P, Chaudhary S, Gangopadhyay S, Pande S (2017) Ag2S/Ag Heterostructure: a promising electrocatalyst for the hydrogen evolution reaction. Langmuir 33:3178–3186
Adams N W H, K. J. R., Potentiometric determination of silver thiolate formation constants using a Ag2S electrode. Pdf>, Aquat Geochem 5 (1999) 1–11
Eckert W (1998) Electrochemical identification of the hydrogen sulfide system using a pH2S (glass/Ag°, Ag2S) electrode. J Electrochem Soc 1:77–79
Izutsu K, Kolthoff IM, Fujinaga T, Hattori M, Chantooni MK (1977) Acid-base equilibria of some acids in propylene carbonate.Pdf>. Anal Chem 49:503–508
Murrieta-Guevara F, Trejo A (1984) Solubility of carbon dioxide, hydrogen sulfide and methane in pure and mixed solvents. J Chem Eng Data 29:456–460
Rosen BA, Salehi-Khojin A, Thorson MR, Zhu W, Whipple DT, Kenis PJ, RI M (2011) Ionic liquid–mediated selective conversion of CO2 to CO at low overpotentials. Science (New York, N.Y.) 334:643–644
Galiński M, Lewandowski A, Stępniak I (2006) Ionic liquids as electrolytes. Electrochim Acta 51:5567–5580
Wang Y, Hatakeyama M, Ogata K, Wakabayashi M, Jin F, Nakamura S (2015) Activation of CO2 by ionic liquid EMIM-BF4 in the electrochemical system: a theoretical study. Phys Chem Chem Phys: PCCP 17:23521–23531
Neubauer SS, Schmid B, Reller C, Guldi DM, Schmid G (2017) Alkalinity initiated decomposition of mediating imidazolium ions in high current density CO2 electrolysis. Chem Electro Chem 4:160–167
Oh Y, Hu X (2013) Organic molecules as mediators and catalysts for photocatalytic and electrocatalytic CO2 reduction. Chem Soc Rev 42:2253–2261
House HO, Feng E, Peet NP (1971) A comparison of various tetraalkylammonium salts as supporting electrolytes in organic electrochemical reactions. J Org Chem 36:2371–2375
Shi J, Shi F, Song N, Liu J-X, Yang X-K, Jia Y-J, Xiao Z-W, Du P (2014) A novel electrolysis cell for CO2 reduction to CO in ionic liquid/organic solvent electrolyte. J Power Sources 259:50–53
Andrews E, Katla S, Kumar C, Patterson M, Sprunger P, Flake J (2015) Electrocatalytic reduction of CO2 at Au nanoparticle electrodes: effects of interfacial chemistry on reduction behavior. J Electrochem Soc 162:F1373–F13F8
Shi J, Li Q-Y, Shi F, Song N, Jia Y-J, Hu Y-Q, Shen F-x, Yang D-w, Dai Y-N (2016) Design of a two-compartment electrolysis cell for the reduction of CO2 to CO in tetrabutylammonium perchlorate/propylene carbonate for renewable electrical energy storage. J Electrochem Soc 163:G82–GG7
Shi J, Shen F-x, Shi F, Song N, Jia Y-J, Hu Y-Q, Li Q-Y, Liu J-x, Chen T-Y, Dai Y-N (2017) Electrochemical reduction of CO2 into CO in tetrabutylammonium perchlorate/propylene carbonate: water effects and mechanism. Electrochim Acta 240:114–121
Fang Y-H, Liu Z-P (2014) Tafel kinetics of electrocatalytic reactions: from experiment to first-principles. ACS Catal 4:4364–4376
Shaharun MS, Mukhtar H, Yusup S, Dutta BK (2008) Kinetics of hydroformylation of higher olefins using rhodiumphosphite catalyst in a thermomorphic solvent system. Aiche Meeting 1:1–9
Mosher BW, Czepiel PC, Shorter J, Allwine E, Harriss RC, Kolb C, Lamb B (1996) Mitigation of methane emissions at landfill sites in New England, USA. Energy Convers Manag 37:1093–1098
Shen F-X, Shi J, Chen T-Y, Shi F, Li Q-Y, Zhen J-Z, Li Y-F, Dai Y-N, Yang B, Qu T (2018) Electrochemical reduction of CO2 to CO over Zn in propylene carbonate/tetrabutylammonium perchlorate. J Power Sources 378:555–561
Funding
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC 51164020, 51062009), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the Analysis and Testing Foundation of Kunming University of Science and Technology (20152102004, 20060130), and Free Exploration Fund for Academician of Chinese Academy of Engineering in Yunnan (2017HA006).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shen, Fx., Shi, J., Shi, F. et al. Fabrication of Ag2S electrode for CO2 reduction in organic media. Ionics 25, 1921–1927 (2019). https://doi.org/10.1007/s11581-018-2728-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2728-7