Abstract
A series of cross-linkable sulfonated poly(arylene ether nitrile)s (SPEN) membranes with different ratios of sulfonated poly(vinyl alcohol) (SPVA) have been prepared through thermal heating. This experiment had two advantages: expectant low methanol permeability and swelling ratio obtained after being cross-linked. Besides, the cross-linked membranes also exhibited excellent thermal stability than that of pure SPEN. Low swelling ratio and water uptake membranes had been obtained after being cross-linked. The methanol permeability showed a minimum value of 8.08 × 10−8 cm2 s−1 of SPEN-SPVA-40%, and the membrane also showed higher selectivity than Nafion 117. The result suggested that the SPEN-SPVA membranes are potential candidates as PEM in DMFCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, direct methanol fuel cells (DMFCs) have attracted more and more attention due to their advantages of clean, renewable, and high-energy conversion efficiency. As a core component in the system, the proton exchange membrane (PEM) provides proton exchange channels and acts as a barrier to the fuel crossover between the electrodes [1–6]. Up to now, perfluorosulfonic acid polymer membranes such as Nafion have been widely applied for PEM due to their high proton conductivity and excellent chemical stability [7–10]. However, the proton conductivity of Nafion117 decreases significantly at high temperature (>100 °C) [11], and high cost and high methanol permeability hinder their extensive applications in fuel cells [12–18]. Therefore, great efforts have been devoted to seek alternative membrane materials which show excellent proton conductivity and low methanol permeability simultaneously.
In recent years, the kinds of sulfonated polymers such as sulfonated poly(arylene ether nitrile) (SPEN) [19–22] and sulfonated poly(ether ether ketone) are being widely studied, owning to high proton conductivity, excellent chemical stability, and mechanical strength [23–25]. However, proton conductivity has a great dependence on the proportion of sulfonic acid groups. When they have high sulfonic acid content, the polymers exhibit high conductivity while unfavorable excessive swelling and dramatic degradation in the mechanical strength are present, causing the weak adhesion of the catalyst layer and membrane [26, 27]. Therefore, the membranes possessed high proton conductivity, low swelling, and low methanol permeability are urgent to develop.
Cross-linking is an effective means to lower the methanol permeability of proton exchange membranes and attract more and more attention [28]. However, the low proton conductivity always accompanies the formation process of cross-linking membrane and limits its further development. Poly(vinyl alcohol) (PVA) has been regarded as an attractive material for preparing cross-linked proton exchange membranes and is used as a cross-linking agent because of its outstanding film-forming capacity [29]. To compensate the loss of proton conductivity in the cross-linking, sulfonated poly(vinyl alcohol) (SPVA) was synthetized by using the post-sulfonated method. Besides, the membranes used macromolecule cross-linker that exhibits better toughness than that of micromolecule cross-linker membranes [30]. Therefore, in order to simultaneously obtain the high conductivity, low swelling, and methanol permeability performances, we prepared the cross-linked membrane of SPEN by using SPVA as a cross-linking agent. The membranes with different cross-linking degrees were obtained by controlling the ratio of SPVA. The structure of SPVA was characterized by 1H NMR and FTIR. Besides, the performances of cross-linked membranes of SPEN-SPVA were studied in detail, such as thermal stability, proton conductivity, methanol permeability, etc.
Experimental
Materials
The hydroquinonesulfonic acid potassium salt (SHQ), 2,6-difluorobenzonitrile (DFBN), and PVA (Mw ∼ 125, 000, 87.5% hydrolyzed) used in this study were purchased from Aldrich. Sulfuric acid (H2SO4) was purchased from Chengdu Kelong Chemicals, China. Phenolphthalin (PPL) had been synthesized by phenolphthalein (PP), and Zn (PP and Zn were purchased from Chengdu Haihong Chemicals) and NaOH were purchased from Tianjin Bodi Chemicals. N-Methyl-2-pyrrolidinone (NMP) (99%purity), N,N′-dimethylacetamide (DMAc, AR), toluene, and potassium carbonate K2CO3 (AR grade) were purchased from Tianjin Bodi Chemicals.
Synthesis
Synthesis of sulfonated poly(vinyl alcohol)
SPVA was synthesized via a sulfonation reaction. First, 3 g PVA was added in a 250-mL three-necked flask and dissolved in 30 mL deionized water with a mechanical stirrer for about 4 h at a certain temperature. Then, excessive concentrated sulfuric acid was added to three-necked flask drop wise at 0 °C with a mechanical stirrer for about 1 h. After that, the mixture temperature was risen to 40 °C for 3 h. Finally, the solution had been poured into ethanol, and the precipitate was collected by filtration and washed with ethanol until pH = 6–7. The product was dried in in a vacuum oven at 40 °C for 48 h. The DS of SPVA was 2.38% [11]. The synthesis of SPVA is shown in Scheme 1.
Synthesis of SPEN
The sulfonated poly(arylene ether nitrile) copolymers were synthesized by nucleophilic aromatic substitution reactions. First, DFBN (20.85 g, 0.15 mol), SHQ (20.52 g, 0.09 mol), and PPL (19.2 g, 0.06 mol) were added to a 250-mL three-necked flask equipped with a mechanical stirrer. Next, the NMP (70 ml) and K2CO3 (37.5 g, 0.27 mol) were added and toluene (30 ml) as the dehydrating agent. The reaction mixture was refluxed for 2 h at 140 °C to distill the dehydrating agent. After the toluene was distilled out completely, the oil bath temperature was raised slowly to 190 °C until the viscosity did not change obviously, and the resulting viscous solution was slowly poured onto the ethanol. The copolymer powders were washed with ethanol and deionized water for several times. Finally, the copolymer powders were dried in vacuum oven at 80 °C for 24 h.
Cross-linked membrane preparation
A solution casting and evaporation method had been used to prepare cross-linked membrane. The SPEN copolymer and SPVA were dissolved in DMAc, which was stirred until hyaline solution was formed at a certain temperature. Then, the resulting homogeneous solution was poured onto a glass plate. The membranes were allowed to dry in vacuum oven using a temperature program at 80, 100, 120, 140, and 160 °C (each for 2 h) respectively. After that, the membranes with different SPVA content had been prepared and marked as SPEN − SPVA − X, where X represents weight percentages of SPVA/SPEN (X = 0, 5, 10, 20, 30, and 40 wt%). The completely dried membranes were peeled off from the glass plate. Finally, the membranes were obtained after being immersed in 1 M H2SO4 for 24 h. The cross-linked procedure can be seen in Scheme 2.
The characterization of the cross-linked SPEN-SPVA membranes
The FTIR spectra were recorded using Shimadzu (Kyoto, Japan) in KBr pellets between 4000 and 400 cm−1 in air. 1H NMR spectra for SPVA were recorded using a 400-MHz Bruker Avance III spectrometer at 298 K, and the deuterated dimethyl sulfoxide (DMSO-d6) was used as solvent. Thermogravimetric analysis (TGA) of the membranes was conducted on TA Instruments TGA-Q50 module. Under N2 atmosphere, the samples were heated to 600 °C at a heating rate of 20 °C min−1. The mechanical properties were measured using SANS (Shenzhen, China) with the operating rate of 5 mm/min. The membrane specimens (10 × 1 mm2) had been immersed in deionized water for about 24 h. For each test, the value was reported by using an average value of three samples. The oxidative stability of the membranes had been measured by Fenton reagent (3%H2O2 solution containing 2 ppm FeSO4). Before immersed in Fenton reagent, the samples were dry in vacuum oven at 100 °C for 24 h and weighed. The samples had been immersed in Fenton reagent about 1 h at 80 °C, dried, and weighed.
Both the water uptake value and swelling ratio value were calculated between dry film and wet film. Prior to immersion in deionized water, the membranes were dry in vacuum oven at 100 °C for 24 h, weighted, and measured lengths as W dry and L dry. Then, the membranes were immersed in deionized water at 20, 50, and 80 °C respectively for 24 h to ensure the film fully absorbs the water. Subsequently, the water was quickly wiped out with filter paper and weighted and measured lengths as W wet and L wet.
The water uptake was calculated using the following formula:
The swelling ratio was calculated using the following formula:
Proton conductivity of the polymer membranes was measured by the AC impedance method which, using an electrochemical impedance spectroscopy technique over the frequency range of 0.1 Hz to 1 M Hz, the proton conductivity (σ) was determined using the formula:
Where L is the distance between the two electrode, A is the cross-sectional area of sample, R is the surface resistance, and σ is the proton conductivity.
The ion exchange capacity (IEC) of the cross-linked membranes had been tested by calculating the concentration of H+. The samples were dried in vacuum oven at 100 °C for 24 h and weighted, prior to immersion in 2 M NaCl (about 50 ml) for 48 h to making sure H+ ions in the membrane were replaced by Na+ ions. Afterwards, the liquor was titrated by 0.01 M NaOH solution and used phenolphthalein as indicator. The IEC computation formula is as follows:
Where V NaOH and C NaOH represent the volume and concentration of NaOH, respectively. Wdry represents the weight of drying membrane.
The methanol permeability was measured using a glass diffusion cell comprising two compartments of capacity 16 ml each, separated by a vertical membrane with an effective area of 0.9π cm2. Before testing, the membranes were immersed in deionized water for 24 h. Initially, one compartment (cell A) was loaded with 10 M methanol solution (16 ml) and the other (cell B) was loaded with ultrapure water (16 ml). After standing 2 h, the concentration of the permeating methanol was analyzed using SHIMADZU GC-8A chromatograph. Methanol permeability was estimated by following formula:
Where DK represents methanol permeability coefficient, C A , C B represent methanol concentration of cell A and cell B, respectively, A represents effective area on mold (cm2), L represents the thickness of membrane (cm), and VB represents the volume of ultrapure water. The difference value between t and t 0 was the time of permeation.
Results and discussion
1H NMR
As shown in Fig. 1, the 1H NMR spectrum of the SPVA copolymers obtained to verify the −SO3 had been connected up successfully. The protons of CH2 in the SPVA had shown at δ 1.35 ppm. The peaks of δ 4.339 ppm were attribute to the CH protons. The peaks at 3.802 ppm can be attributed to the OH protons in SO3H. The peaks at 3.351 ppm were attributed to the H2O [11].
The 1H NMR spectrum of the SPEN copolymers is shown in Fig. 2. The peaks at δ 3.34 ppm were an overlapping peak which can be assigned to the tertiary base protons and H2O. The peaks at δ 6.713 ppm was attributed to the H at 4 and 5 which was influenced by −CN and −O−. The peaks at δ 6.566 can be assigned to H at 1 which suffers lower attraction by −CN in contrast to the H at 4 and 5. The peaks at δ 7.288 and δ 7.531 ppm can be assigned to the contiguous proton to the carboxylic acid and sulfonic groups, respectively.
FTIR
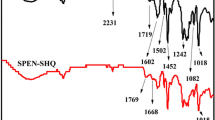
The FTIR spectra of PVA and SPVA was obtained to verify whether the –SO3 had been connected up. The FTIR of PVA and SPVA is shown in Fig. 3. Around 3273 cm−1, a characteristic strong and broad absorption band that was exhibited belonged to O–H stretching vibration. The asymmetric stretching CH2 can be showed at 2921 cm−1. The absorption band at 1324 cm−1 was attributed to the C–O stretching vibration. There are two obvious differences at 1197 and 1031 cm−1. For the SPVA, the absorption band at 1197 and 1031 cm−1 can be assigned to the asymmetrical and symmetrical stretching vibration of S═O in SO3H groups. The result suggested the SPVA presence of sulfonic acid groups [11].
Thermal properties
The thermal stability of a PEM is a key property to guarantee a stable fuel cell operation. TGA curve is shown in Figs. 4 and 5. Prior to the test, all the membranes had been dried at 150 °C for 5 min to remove residual solvent. As shown in Fig. 4, the first weight loss appeared about 250–320 °C. It was attributed to the thermal decomposition of the sulfonic acid groups. It is obvious there was a second weight loss at 350–400 °C. This was attributed to the cracking of cross-linking network and polymer main chain. In spite of the count of SPVA in each membrane which was different, the TGA curves had no obvious change. It was obvious that the curve of SPEN-SPVA-0% lost weight faster than other membranes. The phenomenon had been explained that the cross-linked membrane had better stability. Anyhow, all membranes exhibited good thermal stability that the initial decomposition temperature was higher than 250 °C. It was revealed that the SPEN-SPVA membranes could satisfy the need of PEMs in DMFCs.
Ion exchange capacity
The IEC provides an indication of the count of sulfonic acid group in the membrane. The specific date is shown in Table 1. As shown in Fig. 6, the IEC values of all membranes showed decreasing trend with the amount of SPVA increase. It could be attributed to the H+ that is hard to be replaced by Na+ with the increase of network density which was influenced by the amount of SPVA and the dilutions of H+ by introducing SPVA [30]. Besides, the IEC value of SPVA was 0.655 mmol g−1 [31].
Mechanical properties
Excellent mechanical property was a crucial factor for PEMs. Figure 7 showed the tensile strengths and Young’s modulus and the detailed values of mechanical property were explained in Table 2. It was found that all the membranes showed exhibited outstanding mechanical properties. Competed with cross-linked SPVA membranes which had a tensile strength of 10.14–27.44 MPa and Young’s modulus of 0.52–0.71 GPa as studied by Chi-Yung Tseng et al. [33], whether the SPVA addition or not, the tensile strength was greater than 43 MPa and the Young’s modulus was over 1450 MPa. Further, the membranes, when the loading of SPVA was 10 wt%, 20%, and 30%, exhibited better tensile strength and Young’s modulus than other membranes. It might be caused by cross-linked network and water uptake. The membranes of SPEN-SPVA-X (X = 10, 20, and 30%) had low water uptake and high density of cross-linked network. In spite of the sample of SPEN-SPVA-40% that had high density of cross-linked network, the mechanical property had been limited by high water uptake. Anyhow, the membranes of SPEN-SPVA fit the requirement for PEM using as DMFCs.
Oxidative stability
The oxidative stability was a key index for PEM which could influence the lifetime of DMFC. The oxidative stability of SPEN-SPVA membranes is recorded in Table 2. As shown in Table 2, after being immersed in Fenton’s reagent for about 1 h at 80 °C, all SPEN-SPVA membranes had exhibited excellent oxidative stability (>80%). With increasing loading of SPVA, the oxidative stability had decreased. This phenomenon had been attributed by more SPVA, which increases the instability of PVA aliphatic chain. Therefore, the sample which had more SPVA showed lower resistance-to-oxidation.
Water uptake and swelling ratio
Water uptake and swelling ratio significantly has a profound impact on the properties of PEMs. For proton conductivity and methanol permeability, they were improved with high water uptake. As shown in Figs. 8 and 9, in both water uptake and swelling ratio, all membranes explained a decreasing trend with increasing loading of SPVA at room temperature [33]. In general, competed with low temperature, higher water uptake had been obtained at 50 and 80 °C for the same samples. The samples which contain SPVA exhibited lower water uptake than pure SPEN. It was obvious that the water uptake and swelling ratio had a minimum value when the count of SPVA at 20% was at 50 and 80 °C. The water uptake had been affected by two factors: the content of SPVA and cross-linking density. The SPVA had strong hydrophilia at high temperature (>50 °C). The SPVA cross-linked membranes possessed more stable dimensional stability that reduces the water mobility. Further, the water uptake was first decreased at high temperature, due to the factor of cross-linking which had more influence on water uptake than SPVA. However, with the count of SPVA over 20%, the water uptake increased. This phenomenon had been attributed by a large number of SPVA which had strong hydrophilia at high temperature (>50 °C).
Proton conductivity
The proton conductivity was a key property which used to estimate the excellent performance for the membrane. Prior to the test, the samples had been immersed in deionized water for 24 h at 20, 50, and 80 °C respectively. The result is shown in Figs. 10 and 11. It was obvious that the proton conductivity had been improved with the increase of temperature. The hydrophilia of sulfonic acid group had been improved at high temperature, which increases proton conductivity. It is worth noting that with increasing count of SPVA, the proton conductivities decreased obviously. The proton had two channels which was provided by water and sulfonic acid group in the membrane. The first channel is hopping mechanism, wherein the proton is transferred from one sulfonic acid group to the other. The second channel was provided by water, forming a complex like H3O+ or CH3OH2 + [34, 35]. Despite the count of hydrophilia of sulfonic acid group had increased, the cross-linked membranes had poor water uptake. Besides, the turning space had been limited by cross-linked network, and the channel provided by sulfonic acid group had been affected when passing the proton [11]. The detail values is shown in Table 1. Compared with PVA-g-GO/SPVA membranes which is a compound by Myeongyeol Yoo, etc. [31] (0.0097∼0.0206S cm−1 at 25 °C), the SPEN-SPVA membranes exhibited more excellent property.
Methanol permeability
Methanol permeability coefficient is the major parameter used to estimate the property of membranes. The results are shown in Fig. 12. It was obvious that with the increased count of SPVA, the methanol permeability had decreased. This phenomenon was attributed by cross-linking. The cross-link degree had been improved with the count of SPVA. More methanols had been obstructed with the high-density cross-linked network which easily forms complex methanol barrier. When the content of SPVA was 40 wt%, the methanol permeability shows a minimum value of 8.08 × 10−8 cm2 s−1. These samples had lower values than Nafion 117 which exhibited a value of 1.41 × 10−6 cm2 s−1. Competed with pure SPEN, the samples of SPEN-SPVA with loading of SPVA exhibited lower methanol permeability. The detailed date is shown in Table 1. This result suggested that the SPEN-SPVA cross-linked membranes were potential proton exchange membranes for DMFC applications.
Selectivity
The selectivity of membrane had been defined with the ratio of proton conductivity and methanol permeability. This value was a very important index for DMFCs. High selectivity symbolized excellent property. As shown in Fig. 13, competed with pure SPEN, the selectivity of cross-linked membranes had certain increase. For cross-linked membranes, when the count of SPVA at 20%, there was a minimum value of about 1.22 × 105 S·s·cm−3, which was 2.7 times higher than Nafion117. The sample of SPEN-SPVA-40% exhibited best selectivity of about 1.51 × 105 S·s·cm−3. This result suggested that the SPVA membrane could be a suitable alternate as PEM in DMFCs.
Conclusions
In summary, here we demonstrate the preparation and characterization of cross-linked SPEN. In order to prepare low water uptake membrane with methanol permeability resistance, we used a direct cross-linking method. An interpenetrating network structure had been obtained in membrane. The result had found that the cross-linked membranes showed excellent performance. This experiment had two advantages: expectant low methanol permeability and swelling ratio had been obtained after cross-linked. As is well known, the proton conductivity had reduced after being cross-linked, which was the drawback of cross-linked membrane. In this study, cross-linked membrane had been prepared with using SPVA as a cross-linker which had an advantage on decreasing the degree of reduce. The SPEN-SPVA-40% showed a significant decrease in swelling ratio and methanol permeability. Competed with Nafion 117 1.41 × 10−6 cm2 s−1, the SPEN-SPVA-40% exhibits an excellent value of 8.08 × 10−8 cm2 s−1. In general, the experimental results suggest that the SPVA membrane is potentially useful for DMFCs.
References
Yan XH, Wu R, Xu JB, Luo Z, Zhao TS (2016) A monolayer graphene—Nafion sandwich membrane for direct methanol fuel cells. J Power Sources 311:188–194
Li, Q., Wang, T., Havas, D., Zhang, H., Xu, P., Han, J., … & Wu, G. (2016). High-performance direct methanol fuel cells with precious-metal-free cathode. Adv Sci
Wang Y, Cheng Q, Yuan T, Zhou Y, Zhang H, Zou Z et al (2016) Controllable fabrication of ordered Pt nanorod array as catalytic electrode for passive direct methanol fuel cells. Chin J Catal 37(7):1089–1095
Zheng J, Bi W, Dong X, Zhu J, Mao H, Li S, Zhang S (2016) High performance tetra-sulfonated poly (p-phenylene-co-aryl ether ketone) membranes with microblock moieties for passive direct methanol fuel cells. J Membr Sci 517:47–56
Liu, G., Zhou, H., Ding, X., Li, X., Zou, D., Li, X., … & Lee, J. K. (2016a) Effect of fabrication and operating parameters on electrochemical property of anode and cathode for direct methanol fuel cells. Energy Convers Manag 122:366–371
Cheng H, Xu J, Ma L, Xu L, Liu B, Wang Z, Zhang H (2014) Preparation and characterization of sulfonated poly (arylene ether ketone) copolymers with pendant sulfoalkyl groups as proton exchange membranes. J Power Sources 260:307–316
Wang C, Li N, Shin DW et al (2011) Fluorene-based poly (arylene ether sulfone) s containing clustered flexible pendant sulfonic acids as proton exchange membranes[J]. Macromolecules 44(18):7296–7306
Wang C, Shen B, Zhou Y, Xu C, Chen W, Zhao X, Li J (2015a) Sulfonated aromatic polyamides containing nitrile groups as proton exchange fuel cell membranes. Int J Hydrog Energy 40(19):6422–6429
Wang C, Shen B, Dong H, Chen W, Xu C, Li J, Ren Q (2015b) Sulfonated poly (aryl sulfide sulfone) s containing trisulfonated triphenylphosphine oxide moieties for proton exchange membrane. Electrochim Acta 177:145–150
Shen B, Wang CY, Xu C, Chen WT, Li J, Ren Q (2016) Synthesis and characterization of a side-chain type sulfonated poly (arylene ether sulfone) s for proton exchange membranes. Acta Polym Sin 10:1409–1417
Xu J, Ni H, Wang S, Wang Z, Zhang H (2015) Direct polymerization of a novel sulfonated poly (arylene ether ketone sulfone)/sulfonated poly (vinylalcohol) crosslinked membrane for direct methanol fuel cell applications. J Membr Sci 492:505–517
Ong, B. C., Kamarudin, S. K., Masdar, M. S., & Hasran, U. A. (2016) Applications of graphene nano-sheets as anode diffusion layers in passive direct methanol fuel cells (DMFC). Int J Hydrog Energy
Kim, S. M., Kang, Y. S., Ahn, C., Jang, S., Kim, M., Sung, Y. E., … & Choi, M. (2016) Prism-patterned Nafion membrane for enhanced water transport in polymer electrolyte membrane fuel cell. J Power Source 317:19–24
Antonucci PL, Arico AS, Cretı P, Ramunni E, Antonucci V (1999) Investigation of a direct methanol fuel cell based on a composite Nafion®-silica electrolyte for high temperature operation. Solid State Ionics 125(1):431–437
Lee, S. J., Muthuchamy, N., Gopalan, A. I., & Lee, K. P. (2016) New nafion/conducting polymer composite for membrane application
Wasmus S, Küver A (1999) Methanol oxidation and direct methanol fuel cells: a selective review. J Electroanal Chem 461(1):14–31
Li L, Zhang J, Wang Y (2003) Sulfonated poly (ether ether ketone) membranes for direct methanol fuel cell. J Membr Sci 226(1):159–167
Dimitrova P, Friedrich KA, Stimming U, Vogt B (2002) Modified Nafion®-based membranes for use in direct methanol fuel cells. Solid State Ionics 150(1):115–122
Chen L., Pu Z., Long Y., Tang H., and Liu X (2014) Synthesis and properties of sulfonated poly (arylene ether nitrile) copolymers containing carboxyl groups for proton-exchange membrane materials. J Appl Polym Sci 131(9)
Feng MN, Pu ZJ, Zheng PL, Jia K, Liu XB (2015a) Sulfonated carbon nanotubes synergistically enhanced the proton conductivity of sulfonated polyarylene ether nitriles. RSC Adv 5(43):34372–34376
Feng M, Meng F, Pu Z, Jia K, Liu X (2015b) Introducing magnetic-responsive CNT/Fe3O4 composites to enhance the mechanical properties of sulfonated poly (arylene ether nitrile) proton-exchange membranes. J Polym Res 22(3):1–9
Feng M, Jin F, Huang X, Jia K, Liu X (2015c) In situ fabrication of MWCNTs reinforce dielectric performances of polyarylene ether nitrile nanocomposite. J Mater Sci Mater Electron 26(1):1–10
Reyna-Valencia A, Kaliaguine S, Bousmina M (2005) Tensile mechanical properties of sulfonaed poly (ether ether ketone) (SPEEK) and BPO4/SPEEK membranes. J Appl Polym Sci 98(6):2380–2393
Dang HS, Kim D (2013) Cross-linked poly (arylene ether ketone) membranes sulfonated on both backbone and pendant position for high proton conduction and low water uptake. J Power Sources 222:103–111
Tas S, Zoetebier B, Hempenius MA, Vancso GJ, Nijmeijer K (2016) Monovalent cation selective crown ether containing poly (arylene ether ketone)/SPEEK blend membranes. RSC Adv 6(60):55635–55642
Kreuer KD (2001) On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J Membr Sci 185(1):29–39
Wang F, Hickner M, Kim YS, Zawodzinski TA, McGrath JE (2002) Direct polymerization of sulfonated poly (arylene ether sulfone) random (statistical) copolymers: candidates for new proton exchange membranes. J Membr Sci 197(1):231–242
Lutkenhaus JL, Hammond PT (2007) Electrochemically enabled polyelectrolyte multilayer devices: from fuel cells to sensors. Soft Matter 3(7):804–816
Xiong Y, Fang J, Zeng QH, Liu QL (2008) Preparation and characterization of cross-linked quaternized poly (vinyl alcohol) membranes for anion exchange membrane fuel cells. J Membr Sci 311(1):319–325
Liu, J., Zheng, P., Feng, M., & Liu, X. (2016b) Preparation and properties of crosslinked hybrid proton exchange membrane based on sulfonated poly (arylene ether nitrile) with improved selectivity for fuel cell application. Ionics 1–9
Yoo M, Kim M, Hwang Y, Kim J (2014) Fabrication of highly selective PVA-g-GO/SPVA membranes via cross-linking method for direct methanol fuel cells. Ionics 20(6):875–886
Mandal AK, Bera D, Banerjee S (2016) Sulfonated polyimides containing triphenylphosphine oxide for proton exchange membranes. Mater Chem Phys 181:265–276
Tseng CY, Ye YS, Kao KY, Joseph J, Shen WC, Rick J, Hwang BJ (2011) Interpenetrating network-forming sulfonated poly (vinyl alcohol) proton exchange membranes for direct methanol fuel cell applications. Int J Hydrog Energy 36(18):11936–11945
Pivovar BS, Wang Y, Cussler EL (1999) Pervaporation membranes in direct methanol fuel cells. J Membr Sci 154(2):155–162
Kreuer KD (1996) Proton conductivity: materials and applications. Chem Mater 8(3):610–641
Acknowledgements
The authors gratefully thank the financial support from National Natural Science Foundation of China (Project Nos. 51373028 and 51403029), “863” National Major Program of High Technology (2012AA03A212), South Wisdom Valley Innovative Research Team Program, and Ningbo Major Science and Technology Research Plan (2013B06011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Feng, M. & Liu, X. Cross-linked sulfonated poly(arylene ether nitrile)s membranes based on macromolecule cross-linker for direct methanol fuel cell application. Ionics 23, 2133–2142 (2017). https://doi.org/10.1007/s11581-017-2054-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2054-5