Abstract
In this report, the dielectric properties of polyarylene ether nitrile (PEN)/multi-walled carbon nanotubes (MWCNTs) polymer composites were reinforced via in situ fabrication protocol for MWCNTs. The silanized MWCNTs were surface grafted with phenolphthalin (PPL), which is also one monomer involved in PEN synthesis. This is the premise for PPL can initiate cross-link behavior with the complex PEN and we called it as reactive MWCNTs. Therefore, the composite of PEN and reactive MWCNTs was readily fabricated by solution-casting method and the performance of this unique system was characterized by a range of different techniques. Fourier transform infrared spectroscopy confirmed that the MWCNTs have been bonded with PPL and silane functionalization agent. Based on the observation of scanning electron microscope, it was noted that the forming of reactive MWCNTs could improve the dispersion interfacial compatibility of PEN/MWCNTs nanocomposites. Consequently, the dielectric properties were improved, as lower dielectric loss and higher dielectric constant simultaneously obtained using reactive MWCNTs filler. Moreover, the results of thermal and mechanical performance tests provided additional evidences that the reactive MWCNTs synthesized via in situ fabrication can better reinforce PEN nanocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
While CNTs were initially reported by Bacon in the 1960s, their structures were not given enough attention until the discovery of multi-walled carbon nanotubes (MWCNTs) by Iijima in 1991. Since then MWCNTs have been used in a variety of engineering applications [1]. Due to the special structure, large aspect ratio, extraordinary mechanical, unique electrical conductivity and the potential applications, MWCNTs have attracted considerable attention and are deemed as remarkable materials [2–6]. Especially, the dielectric properties of MWCNTs can be readily tuned by using various surface modification, thus the MWCNTs have been employed as the effective building blocks for polymer nanocomposites used in electronic industry. With the information and technology reform, new electronics devices appear from one generation to another and the speed is faster than we expect. In the information-based society, the electronic material will develop gradually towards functionalization. MWCNTs that possessed above advantages more and more become the focal point of attention and blend with polymers to achieve synergistic effects of matrices and fillers. PEN, as a kind of high performance engineering thermoplastics, is finding increasing application in polymer composites due to their outstanding properties such as excellent thermal, thermo-oxidative stability and radiation resistance [7–10]. It is believed that the high performance dielectric material will be fabricated by using PEN as matrix and MWCNTs as fillers. However, bare MWCNTs have natural tendency of aggregation due to the strong intertubular van der Waals attractions, resulting to poor dispersion of polymer matrices and high dielectric loss [11–14]. Normally, dielectric materials possessed large dielectric constant and small loss are widely required in high energy storage capacitor and cable terminal materials. Therefore, the method of decreasing dielectric loss of MWCNTs is essential for widen their application in energy storage related areas. Under these circumstances, plenty of researchers have proposed a series of useful methods to improve the situation, such as chemical vapor deposition (CVD) [15] and ball milling, etc. [16]. In addition, concentrated acid treatment is a most common method to modify the surface of MWCNTs [17, 18], but the large dielectric loss also appears consequently. The considerable polar groups on the surface of MWCNTs are one of the reasons for the increased dielectric loss. Therefore, in this research, we utilized in situ fabrication protocol to modify MWCNTs to reduce the free vacancies in whole system, further optimize the dispersion and dielectric properties of PEN/MWCNTs nanocomposites. According to synthesize reactive MWCNTs, the dielectric properties of PEN nanocomposites were improved, as lower dielectric loss and higher dielectric constant were simultaneously obtained. Other properties such as thermal and mechanical properties were also investigated in detail.
2 Experimental
2.1 Materials
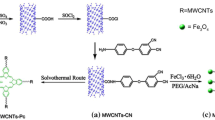
3-aminopropyltriethoxysilane (A1100 or APTES) was used as the silane functionalization agent. The MWCNTs (purity over 95 wt%, provided by Chengdu organic Chemicals Co., Ltd, Chinese Academy of Science), about 50 nm in outer diameter and 20 µm in length, were purified by sulfate acid and nitric acid (3:1) before use to remove the metal catalyst (Fe, etc.). Phenolphthalein (PP) and N-methylpyrrolidone (NMP, purity 99 %) were supplied by Tianjin Bodi Chemical Holding Co., Ltd, Tianjin, China. Sodium hydroxide (NaOH), zinc powder (Zn), anhydrous calcium chloride (CaCl2), pyridine (Py), triphenyl phosphate (TPP) and toluene were all purchased from Chengdu Kelong Chemical Co., Ltd, Sichuan, China. PEN (HQ/PPL) was synthesized in pilot production via polycondensation of 2,6-dichlorobenzonitrile (DCBN), with various phenolphthalin monomers using anhydrous K2CO3 as catalyst in NMP medium as the procedure described earlier [19]. The synthesis process of PEN (HQ/PPL) is schematically illustrated in Scheme 1.
2.2 The silanization of MWCNTs
The silanization of MWCNTs was carried out according to the previous research [20] with minor modification: 2.0 g of MWCNTs and 300 ml of NMP were added into a three-necked round-bottom flask equipped with a mechanical stirrer and refluxing condenser, ultrasounding for 0.5 h. For silanization, 7.5 ml of 1.0 wt% A1100 solution in toluene was added in the three-necked bottle and stirred for 6 h at 60–65 °C. Then, 30 ml of anhydrous ethanol was gradually added into the bottle to remove the unreacted APTES. The production was washed by deionized water and ethanol. Finally, the silanizated MWCNTs were obtained after being subjected to heat treatment at 80 °C for 12 h.
2.3 The forming of reactive MWCNTs
Phenolphthalin (PPL) was prefabricated by the phenolphthalein, NaOH and Zn. The reacted production of MWCNTs and phenolphthalin, which be called reactive MWCNTs (PPL–MWCNTs), was synthesized via in situ fabrication by consulting previous work [21, 22]. A certain amount of phenolphthalin, MWCNTs, CaCl2, TPP and Py were mixed in 100 ml three-necked flask with 45 ml NMP and 20 ml toluene, and heated continuously for 8 h under an atmosphere of nitrogen. The product of reaction was washed several times by deionized water and absolute alcohol to remove the unreacted organics. The synthetic route of reactive MWCNTs monomer in detail is shown in Scheme 2.
2.4 Preparation of reactive MWCNTs/PEN nanocomposites
The reactive MWCNTs/PEN nanocomposites were prepared by solution-casting method. A certain amount of reactive MWCNTs powder and NMP were sonicated for 1 h before being added into the PEN solution to make sure that the powder could disperse better. PEN and NMP were added into 100 ml three-neck round bottle flask equipped with mechanical stirrer and refluxing condenser to dissolve. After the powder sonicated, reactive MWCNTs powder were added into PEN solution refluxed at the stirring speed of 1,200 rpm until the PEN completely dissolved. Then the reactive MWCNTs and PEN solution were sonicated for 0.5 h in a low-power ultrasonic bath. The mixture was cast on a clean and horizontal glass plate, and dried in an oven at a temperature of 80, 100, 120 °C each for 1 h, 160, 180, 200 °C for 2 h, respectively. After cooled to room temperature gradually, the reactive MWCNTs/PEN nanocomposites were obtained, which the reactive MWCNTs powder loadings were 0, 1, 3, 5, 6, and 7 wt%. Besides, purified MWCNTs/PEN nanocomposites were also prepared to compare.
2.5 Characterization
The silanization of MWCNTs and the reactive MWCNTs powder were characterized by Fourier transform infrared spectroscopy (FTIR, 200SXV, Nicolet, USA). The Fourier transform infrared spectra were obtained in the transmission mode on pressed MWCNTs and the reactive MWCNTs powder mixed with KBr (mass ratio 1:200), respectively. The cross-sectional micromorphologies of the films were observed with scanning electron microscope (SEM, JEOL, JSM-5900 LV). Before examining, the samples were brittle fractured in liquid nitrogen and then coated with a thin layer of gold. Thermogravimetric analysis (TGA) was conducted on a TA instrument Q50 series analyzer system under nitrogen atmosphere (sample purge flow 60 ml/min at a heating rate of 20 °C/min) from room temperature to 800 °C. Differential scanning calorimetry (DSC) analysis was carried out on a TA Instrument DSC Q100 under nitrogen atmosphere (sample purge flow 50 ml/min) at a heating rate of 10 °C/min from room temperature to 350 °C. Mechanical properties were measured by employing a SANS CMT6104 Series Desktop Electromechanical Universal Testing Machine. Dielectric properties of reactive MWCNTs/PEN nanocomposites films were tested by a TH 2819A precision LCR meter (Tong hui Electronic Co., Ltd), which was carried out at different frequencies (50–100 kHz) at room temperature.
3 Results and discussion
3.1 FTIR characterization
To confirm the successful forming of reactive MWCNTs, FTIR spectra were investigated, as shown in Fig. 1. Figure 1a displays the differences of purified MWCNTs and silanized MWCNTs. For the purified MWCNTs, a wide absorption band appears at 3,440 cm−1, which belongs to the characteristic absorption band of –OH on the surface of MWCNTs [23]. A weak absorption band at 1,712 cm−1 can be noted, and this peak corresponds to the characteristic absorption band of carboxyl group [19, 21]. To the silanized MWCNTs, the peak of –OH has no obvious change, bespeaking that the hydroxyl group on the MWCNTs surface are still available after silanization, which is important for the following modification with phenolphthalin. The silanized MWCNTs possess other peaks at 2,930, 2,850 cm−1, which correspond to the stretching of methylene groups from the 3-aminopropyltriethoxysilane molecules. Two absorption bands of 1,633 and 1,394 cm−1 are geared to the bending vibration of –NH and the characteristic absorption of C=O. The appearance of methylene groups and amide bond could better testify that the MWCNTs have coated by the silane functionalization agent. Additionally, at 1,712 cm−1, the peak of –COOH still exists, and the intensity is weaker than that of purified MWCNTs, which can be concluded that the –COOH has taken part in the reaction but hasn’t react completely.
Figure 1b shows the spectrum of reactive MWCNTs. The absorption band at 3,620 cm−1 is attributed to the stretching vibration of dissociatived hydroxyl. The absorption bands at 3,030 and 1,689 cm−1 are attributed to the stretching vibration of C–H on aromatic ring and O=C of amide. In addition, the absorption bands at 1,600, 1,500 and 1,450 cm−1 are characteristic absorption bands of the stretching vibration of benzene ring skeleton. Finally, the peak at 1,240 and 1,888 cm−1 are assigned to the stretching vibration of C–O and carbonyl. To the characteristic absorption bands of –NH and methylene groups may coincide with the vibration of benzene ring skeleton. However, compared with Fig. 1a, b, the hydroxyl on the surface of MWCNTs has disappeared, and the carbonyl group has appeared, which can be explained by the fact that the hydroxyl and carboxyl groups have been bonded. All these results provide powerful evidence to the forming of reactive MWCNTs.
3.2 Thermal analyses
Under nitrogen atmosphere, DSC was investigated to discern the thermal inducted phase transition behavior of the composite films. The thermodynamic states of the MWCNTs/PEN and reactive MWCNTs/PEN nanocomposites are exhibited in Fig. 2. From the illustration, a significant improvement of T g can be detected when comparing the MWCNTs/PEN with reactive MWCNTs/PEN nanocomposites. This may be credited to the thermal cross-link behavior of the carboxyl group in PEN chain and the hydroxyl in phenolphthalin, restricting segmental mobility of the polymer chain [24]. Moreover, the cross-link behavior and the reaction of hydroxyl and carboxyl group can reduce free volume and segmental mobility, which also can improve T g . However, from the Fig. 2b, the T g of reactive MWCNTs nanocomposites has slight and unobvious increase (227, 228, 229, 229, 230 °C, respectively) as the increasing reactive MWCNTs contents. This may be attributed to the fact that the variety of reactive MWCNTs content is small and not enough to cause the obvious change of Tg, and the change of Tg is also in the error range. Besides, whether it is MWCNTs/PEN or reactive MWCNTs nanocomposites, the values of Tg are all over 225 °C, suggesting that the nanocomposites have excellent thermal properties, which are important to use in aggressive environment. It is mainly attributed to the well thermal stability of PEN matrix. To further compare the variation of T g directly through the senses, Fig. 2c was provided to confirm the increase of T g after fabrication, indicating the thermal stability has been significantly reinforced by the reactive MWCNTs.
In addition, Fig. 3 shows the 5 % weight loss temperature (T 5%) and the maximum decomposition rate temperatures (T max ) of nanocomposites. The T max of reactive MWCNTs/PEN nanocomposites are all higher than 490 °C, further attesting that the production of cross-link behavior and group reaction has no destruction to the polymer matrix and still inherit better thermal stability, which can fully satisfy the demand of practical application. In addition, In Fig. 3a, there is obvious weight loss at about 250 °C, while for the reactive MWCNTs/PEN nanocomposites, the weight loss progress disappeared. This may be caused by the decomposition of phenolphthalin, which is one monomer involved in PEN–COOH synthesis. When added the reactive MWCNTs, the cross-link behavior of carboxyl and hydroxyl will also occur. Therefore, the thermal stabilities of reactive MWCNTs/PEN composite films become more stable with the thermal cross-link.
3.3 Morphological properties
To explore the dispersion and compatibility of the fillers and PEN matrix, the morphology properties of the nanocomposites with loading of 3 and 5 wt% MWCNTs at the time of 40,000 were investigated, shown in Fig. 4. According to the comparison with them, the reactive MWCNTs/PEN nanocomposites possess better dispersion and compatibility with the matrix, which are the prerequisites for stable interfacial interaction. Interestingly, after the reactive MWCNTs was added in PEN polymer matrix, the homogeneous morphology was observed without any obvious aggregation (Fig. 4c), even a large MWCNTs loading (5 wt%) was used (see Fig. 4d).
The improved dispersion of reactive MWCNTs in PEN polymer matrix should be attributed to two factors: (1) the effect of cross-link behavior of reactive MWCNTs and PEN matrix, which can restrict the free motion of molecule chain, (2) hinder the direct contact of MWCNTs to a certain extent. These also contribute to the enhanced interfacial interaction with PEN polymer main chain, which is the prerequisite for better electric performances of nanocomposites. Besides, as an organic compound, the compatibility and connectivity of phenolphthalin with matrix are better than inorganic fillers. This may be another factor for the better compatibility of reactive MWCNTs/PEN nanocomposite.
3.4 Dielectric properties
Figure 5 shows the dielectric constant versus contents of MWCNTs and reactive MWCNTs fillers in the MWCNTs/PEN and reactive MWCNTs/PEN nanocomposites at the room temperature. It can be seen that both the nanocomposites exhibit percolation behavior and have the same percolation threshold (6 wt%), implying that various forms of MWCNTs are efficient conductive filler. In addition, from Fig. 5a, b, the dielectric constants decrease with the scope of frequencies, which may be caused by the effect of polarization and construction of the conductive network in the nanocomposites. The comparison of these nanocomposites with the varying fillers loading at the 1 kHz is shown in Fig. 5c, and the dielectric constant of reactive MWCNTs filler is higher than that of purified MWCNTs filler. This is mainly attributed to the connection and dispersion between polymer and filler verified by the SEM images, declaring that the processed MWCNTs could enhance the dielectric constant of the PEN nanocomposites.
The dielectric loss versus frequency of the MWCNTs/PEN and reactive MWCNTs/PEN nanocomposites is depicted in Fig. 6a, b. In the alternating electric field, the dielectric loss is related to polarization relaxation and conductivity. The appearance of leakage current caused by existing vacancies also can affect the dielectric loss [25]. As the applied frequency increases, the decrease trend of loss reflects that the polarization of particles cannot row down the change of frequency. Figure 6c presents the comparison of these nanocomposites with the varying fillers loading at the 1 kHz. The loss of reactive MWCNTs/PEN nanocomposites is lower than that of MWCNTs/PEN, especially for the nanocomposites containing 5 wt% MWCNTs, suggesting that the reactive MWCNTs/PEN nanocomposites have less free vacancies and consume less energy. Thanks to the cross-link behavior and bond of hydroxyl and carboxyl group, the free volume and segmental mobility reduce and further cause the low loss in accordance with the conclusion of thermal property. That is to say, in this study, the reactive MWCNTs prepared via in situ fabrication could ameliorate the dielectric loss and improve dielectric constant of PEN nanocomposites simultaneously.
3.5 Mechanical properties
In most cases, the mechanical properties of composites depend strongly on the amount of fillers and the level of molecular orientation [26, 27]. Figure 7 shows the tensile strengths and modulus of MWCNTs/PEN and reactive MWCNTs/PEN nanocomposites at varying filler loadings. In the case of these nanocomposites, the tensile strengths and modulus are all increased until the loading is up to 5 wt%, then a slight decrease occurs while still higher than 1 wt% MWCNTs loading. This is attributed to the fact that MWCNTs can improve the mechanical performance of these nanocomposites within the certain scope of loading. However, when the dose of MWCNTs exceeds the scope, the agglomeration phenomenon of MWCNTs will become more and more serious as the increasing MWCNTs content. Besides, the physical entanglement of PEN macromolecular chains and MWCNTs will also be weaker. All these will lead to the decrease of the tensile strengths and modulus of nanocomposites. Compared with Fig. 7a, b, the mechanical properties of reactive MWCNTs/PEN nanocomposites are better than that of the simple purified MWCNTs. From another angle, it is reflect that the interfacial interaction between reactive MWCNTs and PEN is stronger than that of MWCNTs and matrix, which is consistent with the results of morphological properties. This condition can be ascribed to the decrease of polar group, resulting in the reduction of the filler polarity and repulsive force, subsequently enhance the interfacial compatibility and mechanical properties. Furthermore, the reduced free volume may cause bind more tightly between macromolecular chains and MWCNTs, which also could improve the tensile strengths and modulus of the nanocomposites. Therefore, to some extent, the reactive MWCNTs also could reinforce the strength of PEN nanocomposites, which can be adapt to the industrial application as engineering plastic.
4 Conclusions
Nanocomposites based on PEN and different types of MWCNTs could successfully be prepared by solution-casting method and the reactive MWCNTs were prepared via in situ fabrication. A homogenous distribution and compatibility of the nanotubes with polymer could be achieved, which can be proved by the observation of SEM images. The FTIR also confirmed that the MWCNTs have been bonded with phenolphthalin and silane functionalization agent, and this may be the major reason for the better connectivity of filler and polymer matrix. Furthermore, the dielectric constant of reactive MWCNTs/PEN nanocomposites was higher than that of acid purified MWCNTs and the dielectric loss was lower, suggesting that the reactive MWCNTs optimized the dielectric performance of PEN nanocomposites. The results of thermal and mechanical performance tests provided additional evidences that the MWCNTs synthesized via in situ fabrication can better reinforce PEN nanocomposites.
References
K. Babak, V.H. Michelle, L. Zhang, J.B. Michael, M. Harish, B. Behnam, NeuroImage 37, S9 (2007)
X. Zhang, G. Wang, W. Zhang, W. Yan, B. Fang, Biosens. Bioelectron. 24, 3395 (2009)
S. Thanyarat, C. Anon, S.K. Chaiyuth, P. Sirapat, T. Gamolwan, Diam. Relat. Mater. 18, 524 (2009)
P. Calvert, Nature 399, 210 (1999)
M. Feng, X. Huang, H. Tang, Z. Pu, X. Liu, J. Mate. Sci. Mater. Electron. 24, 3652 (2013)
E.T. Thostenson, Z.F. Ren, T.W. Chou, Nanocomposites Sci. Technol. 61, 1899 (2001)
Y. Rao, C. Wong, J. Appl. Polym. Sci. 92, 2228 (2004)
H. Wang, W. Zhong, P. Xu, Q. Du, Macromol. Mater. Eng. 289, 793 (2004)
L. Liu, Q. Lu, J. Yin, Z. Zhu, D. Pan, Z. Wang, Mater. Sci. Eng. C 22, 61 (2002)
X. Huang, Z. Pu, L. Tong, Z. Wang, X. Liu, J. Mater. Sci. Mater. Electron. 23, 2089 (2012)
L. Liu, J.C. Grunlan, Adv. Funct. Mater. 17, 2343 (2007)
P.M. Ajayan, L.S. Schadler, C. Giannaris, A. Rubio, Adv. Mater. 12, 750 (2000)
B. Yang, K.P. Pramoda, G. Xu, S.H. Goh, Adv. Funct. Mater. 17, 2062 (2007)
X. Liu, S. Long, D. Luo, W. Chen, G. Cao, Mater. Lett. 62, 19 (2008)
S. Ganguli, H. Aglan, P. Denning, G. Irvin, J. Reinf. Plast. Compos. 25, 175 (2006)
H. Xie, H. Lee, W. Youn, M. Choi, J. Appl. Phys. 94, 4967 (2003)
Y. Zhan, R. Zhao, Y. Lei, F. Meng, J. Zhong, Liu XB. J. Magn. Magn. Mater. 323, 1006 (2011)
M. Pumera, B. Smíd, K. Veltruská, J. Nanosci. Nanotechnol. 9, 2671 (2009)
H. Tang, J. Yang, J. Zhong, R. Zhao, X. Liu, Mater. Lett. 65, 2758 (2011)
P. Ma, J.K. Kim, B. Tang, Carbon 44, 3232 (2006)
H. Tang, J. Yang, J. Zhong, R. Zhao, X. Liu, Mater. Lett. 65, 1703 (2011)
J. Yang, H. Tang, Y. Zhan, H. Guo, R. Zhao, X. Liu, Mater. Lett. 72, 42 (2012)
M. Feng, X. Huang, H. Tang, X. Liu, Colloids Surf. A Physicochem. Eng. Asp. 441, 556 (2014)
Y. Zhan, F. Meng, X. Yang, X. Liu, Colloids Surf. A Physicochem. Eng. Asp. 390, 112 (2011)
T. Nakinpong, S. Bualeklimcharoen, A. Bhutton, O. Aungsupravate, T. Amorn-sakchai, J. Appl. Polym. Sci. 84, 561 (2002)
X. Huang, Z. Pu, M. Feng, L. Tong, X. Liu, Mater. Lett. 96, 139 (2013)
A. Ajji, J. Brisson, Y. Qu, J. Polym. Sci. Part B Polym. Phys. 30, 505 (1992)
Acknowledgments
The authors wish to thank for financial support of this work from the National Natural Science Foundation (Nos. 51173021, 51373028, 51403029) and “863” National Major Program of High Technology (2012AA03A212).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Feng, M., Jin, F., Huang, X. et al. In situ fabrication of MWCNTs reinforce dielectric performances of polyarylene ether nitrile nanocomposite. J Mater Sci: Mater Electron 26, 1–10 (2015). https://doi.org/10.1007/s10854-014-2355-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2355-7