Abstract
Fast oxide-ion conductors La2Mo2-xWxO9 (x = 0–1) have been prepared using mechanochemical activation (MA) of starting oxides in a high-power planetary ball mill. Studies of La2Mo2-xWxO9 genesis and structural properties using thermal analysis, XRD, SEM, IR, and Raman spectroscopy have revealed that MA results in the formation of an amorphous precursor, while the cubic β-phase is formed after calcination at 700–900 °C. Due to a high dispersion of powders, high-density pellets of W-LAMOX ceramics have been obtained already after sintering at 950 °C. Their electrical conductivity measured by the impedance spectroscopy depends on the W concentration being sufficiently high (up to 5.6∙10−3 S/cm at 630 °C) at temperatures below 650 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family of fast oxide-ion conductors based on La2Mo2O9 (so-called LAMOX) has attracted a considerable interest due to their possible application in solid oxide fuel cells operating at intermediate temperatures [1–5]. Their high anionic conductivity is provided by a high concentration of intrinsic oxygen vacancies in the crystal lattice required for the fast oxygen ion diffusion. However, for parent La2Mo2O9, a phase transition from a cubic high-temperature β form to a monoclinic low-temperature α phase observed at about 580 °C leads to an abrupt decrease of conductivity by almost two orders of magnitude [1–3, 6]. Another problem hampering LAMOX application is their poor phase stability at low oxygen concentrations or in reducing conditions [3–5]. Doping of La2Mo2O9 with different cations in La sites (alkaline [7], alkaline-earth [8, 9], rare-earth [10, 11], Bi [12]) was shown to stabilize a highly conductive β phase to room temperature while substitution of Mo with W not only stabilizes β phase but considerably improves LAMOX stability under reducing conditions as well [13–23].
W-doped LAMOX oxides are usually prepared by the solid-state method, which requires prolonged heating at high temperatures with the intermediate homogenization of the powder precursors to obtain a target phase and dense ceramics [13–17]. Besides, such procedures cause losses of Mo and W due to a high volatility of their oxides that result in formation of multiphase products. There are only several works in the literature devoted to W-LAMOX synthesis by microwave method [18], Pechini method [19], freeze drying [20, 21], and spray pyrolysis [22]. These methods usually involve several steps which take many hours to achieve the complete mixed oxide phase formation or require special complex equipment. Besides, productivity of “wet” methods including Pechini route is limited by a low solubility of raw tungsten salts [24].
The alternative route to avoid these problems is using high-energy milling of starting oxides [25–27]. During milling, a high-energy input into the reaction zone generates defects in solids up to their amorphization and transfer of compounds into so-called «atom-ionic state» that greatly accelerate the chemical interaction between solids. The raw compounds can form target products directly during milling or they can be transformed into precursors. In the first case, the term “mechanochemical synthesis” (MS) is usually used while the term “mechanochemical activation” (MA) can be used if only precursors are formed. MS(MA) is an environmentally friendly method due to absence of any wastes. Such approach was proved to be very efficient in synthesis of complex oxides of different types: perovskites, fluorites, pyrochlores, apatites, etc. [25–27]. Among LAMOX, only the undoped La2Mo2O9 was obtained by MS after milling of La2O3 and MoO3 mixture during 12 h [28]. For W-doped LAMOX, ball milling has been used only for production of powders to prepare dense ceramics from La2−xRxMo2−yWyO9 (R: Nd, Gd, Y) samples obtained by solid-state reaction [29].
In this work, La2Mo2-xWxO9 (x = 0–1) oxides have been prepared using high-energy milling (mechanochemical activation) of starting oxides in a high-energy planetary ball mill AGO-2. The results of the study of LAMOX genesis during milling and subsequent annealing of its products as well as the effect of the partial substitutions of Mo6+ by W6+ cations in La2Mo2O9 on structural and electrical properties are presented.
Experimental
Synthesis
To synthesize La2Mo2-xWxO9 (y = 0–1), the mechanical milling of La, Mo, and W oxides was used. Lanthanum oxide contained admixtures of lanthanum hydroxide and oxycarbonate as revealed by XRD analysis. Mo and W oxides were obtained via calcination of (NH4)6Mo7O24·6H2O and (NH4)6W7O24·6H2O mixture at 270 °C for 4 h to prevent evaporation of molybdenum oxide [24]. To provide a target stoichiometry of samples, corresponding amounts of starting compounds were taken on the basis of their thermal analysis. For La2O3, the analysis was conducted up to 1200 °C that ensured decomposition of oxycarbonate and removal of hydroxyls.
Stoichiometric mixtures of oxides were milled in the high-energy planetary ball mill AGO-2 with two steel drums (150 ml volume) at 1200 rpm rotation rate. The steel ball diameter was 8 mm, and ball-to-sample mass ratio was 20. The time of milling was 1–30 min in the study of La2MoWO9 genesis during mechanical activation (MA), and it was 15 min for synthesis of two other samples. In each experiment of MA genesis study, the same fresh portion of a given starting mixture was milled for respective time period (1 or 5, 10, 15, 20, 30 min). Before each synthesis, a preliminary treatment of steel drums and balls with the corresponding mixture was performed to minimize contamination of samples with Fe. As a result, the surface of drums and balls was covered by a layer of the initial mixture. Such procedure was earlier shown to decrease the amount of Fe in MA products below 0.05 ± 0.01 wt% [30]. For the study of LAMOX formation under annealing, the starting mixture of oxides corresponding to La2MoWO9 was milled during 15 min and the MA products were calcined at 550–950 °C. The products obtained after each experiment (MA or annealing) were studied by XRD, IR, and Raman spectroscopy.
The chemical composition of all samples and their abbreviations are presented in Table I.
Characterization
The genesis of prepared LAMOX and their structural properties were studied by thermal analysis, XRD, SEM, IR, and Raman spectroscopy. X-ray diffraction patterns were recorded at room temperature with a D8 Advance diffractometer (Bruker, Germany) using CuKα radiation in 2θ scanning range 15–80° with step 0.05°. Qualitative phase analysis has been carried out by using PDF-2–ICDD files and ICSD/retrieve database. Thermal analysis (DTA/TG) of LAMOX was carried out with a Simultaneous DTA-TG Apparatus DTG-60H SHIMADZU in the flow of air (30 ml/min) at a heating rate of 10 °C/min. Particle size distribution of powdered samples calcined at 700 °C was determined using laser diffraction particle size distribution analyzer SALD 2101 (Shimadzu Corp., Japan) (measuring range: 0.03–1000 μm) equipped with a batch cell. Data processing was carried out using the standard data processing program “Wing-1.” For analysis, the suspension of the sample in Na-H.M.P. solution was stirred with a magnetic stirrer for 5 min and then subjected to ultrasonic treatment at 35 kHz frequency for 3 min. Microstructural characterization and EDX analysis of powdered samples and sintered pellets were carried out with Tesla BS-350 scanning electron microscope.
FTIR spectra were recorded in the range of 200–4000 cm−1 using a BOMEM MB-102 FT-IR spectrometer. The samples were pre-treated by a standard method that involved pelletizing the powder with CsI (2 mg of a sample and 500 mg of CsI). FT-Raman spectra (3600–100 cm−1, 300 scans, resolution 4 cm−1, 180° geometry) were recorded using a RFS 100/S Bruker spectrometer. Excitation of the 1064-nm line was provided by a Nd:YAG laser (100 mW power output).
For electrical characterization, LAMOX powders calcined at 700 °C were pressed into pellets (diameter 15 mm, thickness 1 mm) under ~150 kgf/cm2 and sintered at 950 °C for 6 h. The impedance spectroscopy was used to study a total conductivity of the sintered pellets. Both of their sides were coated with silver paste to form electrodes. The measurements were carried out at 250 to 650 °С in air using a MNIPI Е7–25 Meter at potential value of 10 mV in the frequency range 25 Hz–1 MHz. The specific conductivities (σ) were calculated by simulating the experimental impedance spectra using the equivalent electric circuit method [20].
Results and discussion
Genesis of phase composition
The genesis of La2Mo2-xWxO9 at different synthesis stages (during mechanical activation and subsequent calcinations) has been studied in detail for the sample with x = 1.
XRD patterns of starting lanthanum oxide and mechanical activation products are presented in Fig. 1. According to XRD data, starting lanthanum oxide contains admixtures of hexagonal La(OH)3 and La2O2CO3. XRD patterns of MA products show a gradual interaction of starting oxides during activation. Thus, after 5 min, a halo in the 2θ range of 20–35° corresponding to amorphous phase appears. Simultaneously, hydration of lanthanum oxide with formation of hydroxide occurs. There is only one weak reflection of molybdenum oxide at 2θ ~23.37° in the pattern while reflections of tungsten oxide are absent. At increasing milling time, intensity of lanthanum hydroxide diffraction peaks declines and peak corresponding to molybdenum oxide disappears, while halo of the amorphous phase rises by intensity. Note that after 30 min of activation, reflections of lanthanum hydroxide and oxycarbonate are still observed.
Infrared and Raman spectra give additional data on formation of amorphous phase and its nature (Fig. 2). The IR spectrum of the starting lanthanum oxide shows a broad intense band at 400–600 cm−1 corresponding to vibrations of La-O in La2O3 [31], while the bands at 645 and 3610 cm−1 are related to the bending and stretching OH-group vibrations of lanthanum hydroxide [32]. The narrow bands at 746, 853, 1089 and a broad band in the range of 1280–1650 cm−1 with the maximum at 1464 cm−1 correspond to stretching and bending vibrations of carbonate ion in La2O2CO3 [31, 33]. The presence of lanthanum hydroxide and oxycarbonate in the starting material is explained by the fact that the sesquioxides of rare earth elements and their hydroxides are strongly subjected to hydration and carbonization being exposed to atmospheric CO2 and H2O at room temperature and standard pressure [34, 35].
After 1 min milling, the band corresponding to La2O3 disappears in the IR spectrum of products while the intensity of bands at 645 and 3610 cm−1 related to bending and stretching vibrations of OH-groups in La(OH)3 increases as a result of lanthanum oxide hydration (Fig. 2, left). The bands at 995, 880, and 820 cm−1 correspond to stretching vibrations of different Mo(W)-O bonds in starting oxides [36–39]. The bands at 1495, 1465, 1365, 1085 (stretching vibrations), and 855 cm−1 (bending vibrations ν4) correspond to carbonate ion in La2O2CO3. The bands at 3400 and 1625 cm−1 are assigned to stretching and bending vibrations of OH group in water. Besides, the bands at 3190, 3030, 2850 (stretching vibrations ν3), and 1415 cm−1 (bending vibrations ν4) of NH4 + remained from precursors salts are also observed.
At further milling for 5–30 min, the intensity of bands corresponding to La(OH)3 decreases, showing its partial consumption, while the bands assigned to Mo(W)-O vibrations in initial oxides also reduce in intensity and disappear after milling for 13 min. Simultaneously, bands at 910, 800, 740, and 640 cm−1 corresponding to molybdates and tungstates [40, 41] appear evidencing formation of a new compound. The bands assigned to carbonate ion in crystalline La2O2CO3 are reduced by intensity during milling, but their presence even after 30 min of treatment shows, in agreement with XRD data, only partial decomposition of lanthanum oxycarbonate with simultaneous formation of surface carbonates characterized by the broad bands at ~1410 and 1495 cm−1.
Raman spectra widely used for studies of Mo(W)-based compounds [36–44] allow to explore, in more details, transformation of products during mechanical activation. In the Raman spectra of the sample milled for 1 min, two groups of bands are observed. The bands between 150 and 400 cm−1 (at 290, 245, and 155 cm−1) are associated with Mo(W)-O bending vibrations, while the high frequency bands at 995, 820, and 665 cm−1 correspond to stretching vibrations of Mo(W)-O in Mo(W)O3 [36–39, 41–44]. The bands of a low intensity at 975, 795, and 350 cm−1 corresponding to molybdates and tungstates [40, 41] are also observed in the spectrum, probably, due to incomplete decomposition of these starting compounds after calcination at 270 °C. The intensity of starting oxides bands decreases after milling for 5–10 min, and they disappear after milling for 15 min. Simultaneously, the intensity of molybdates (tungstates) bands at 330–360 cm−1 (bending vibrations of Mo(W)-O bond (ν2 and ν4)) and 795–810 cm−1 (stretching vibrations (ν3)) increases, and the band at 915–920 см−1 (asymmetric stretching vibrations (ν1) [41–44]) appears after 10 min milling. The changes in the intensity of ν1 and ν3 bands could be due to interaction of components in the course of milling and formation of mixed lanthanum molybdate–tungstate oxide. The exact assignment of bands to either molybdate or tungstate is difficult due to their large width and close position of individual bands [40, 44, 45].
Formation of La2MoWO9 during annealing of the amorphous precursor obtained after 15 min milling has been studied by XRD, IR, and Raman spectroscopy. According to XRD data (Fig. 3), calcination of milling products at 400 °C leads to disappearance of remaining unreacted La(OH)3, retaining of the amorphous phase and lanthanum oxycarbonate, and appearance of a weak reflection at 2θ~27.86°. After annealing at 500 °C, the halo of the amorphous phase disappears while the intensity of the reflection at 2θ~27.86° increases and a number of additional reflections emerges. In general, these reflections correspond to the pattern of La2Mo3O12 (JCPDS 26–0821), though some shifts of several peaks are observed due to formation of La2(MoW)3O12 mixed oxide. The formation of La2Mo3O12 as an intermediate compound during synthesis of La2Mo2O9 has been observed in previous work [46]. At 600 °C, weak reflections of La2MoWO9 additionally appear. After calcination at 700 °C, the main phase is the target LAMOX with the admixture of La2 (MoW)3O12 provided by interaction between La2(MoW)3O12 and remaining lanthanum oxycarbonate. Increasing annealing temperature up to 900 °C results in formation of single-phase La2MoWO9 sample.
Infra-red spectra (Fig. 4, left) agree with X-ray diffraction data and add information on the genesis of target LAMOX during annealing of the amorphous precursor. Thus, after calcination at 400 °C, the bands at 645 and 3610 cm−1 related to δ(La-OH) and ν(OH) vibrations in La(OH)3 [31, 32] disappear while the bands at 1495, 1465, and 1365 cm−1 show the presence of La2O2CO3 [33–35] in agreement with XRD data. The bands in the range of 600–900 cm−1 (732, 820, 933 cm−1) corresponding to amorphous mixed molybdate-tungstate [40, 44, 45] are slightly shifted with changing their relative intensities, thus evidencing distortion of Mo(W)-Ox polyhedra. These bands disappear after calcination at 500 °C, showing complete transformation of the amorphous phase into a new phase characterized by a number of bands in both low and high frequency regions, the most intensive ones being situated at 915, 810, 715, 625, 520, and 405 cm−1. On the whole, the spectra are similar to that of La2Mo3O12 phase [47]; however, some differences in the bands intensity and positions are observed due to formation of a mixed La2(MoW)3O12 oxide. Annealing at 600 °C leads to a slight shifting of bands, their narrowing, and increase in intensity along with a higher splitting, thus evidencing perfection of the structure. After calcination at 700 °C, the bands of lanthanum oxycarbonate completely disappear while the band alterations and appearance of new bands in the region corresponding to vibrations of Mo(W)-O polyhedra show partial decomposition of La2(MoW)3O12 along with formation of target La2MoWO9 phase. Annealing at 900 °C results in disappearance of bands at 719, 750, 773, and 838 cm−1 assigned to La2(MoW)3O12 [47], and the spectrum showing a.b. at 520, 615, 715, 795 cm−1 becomes close to those typical for La2Mo2O9 [48].
Raman spectra show evolution of amorphous mixed molybdate–tungstate during annealing (Fig. 4, right). The spectrum of sample calcined at 400 °C is a broad band due to a distortion of Mo(W)-Ox polyhedra as agrees with IR data. After calcination at 500 °C, a number of bands with a low intensity appear in the low and high frequency regions corresponding to bending and stretching vibrations of Mo(W)-O bonds close to the ones in La2Mo3O12. Annealing at 600–700 °C leads to pronounced splitting of the bands that is a characteristic feature of monoclinic La2Mo3O12 [49]. Simultaneously, some changes in position and intensity of the bands evidence the starting formation of the target La2MoWO9. After calcinations at 800–900 °C, bands splitting disappears showing complete consumption of La2(MoW)3O12, and the spectra become close to that for La2Mo2O9 [18]. A reliable interpretation of bands in the spectra of La2(MoW)3O12 and La2Mo2O9 is difficult due to strong couplings of different polyhedral vibrations in the lattice as considered in [17, 18].
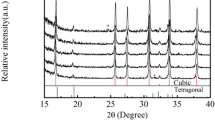
Thus, our results have shown that though the target tungsten doped molybdates are not formed directly during mechanical activation, it facilitates formation of the single phase LAMOX. The XRD patterns of La2Mo2O9 and La2Mo2-xWxO9 pellets obtained after calcination of MA products at 950 °C (Fig. 5) show only reflections of LAMOX.
Structural properties
It is known that La2Mo2O9 crystallizes in two different forms: a low-temperature α phase and a high-temperature β-form. The high-temperature β form has a cubic structure (space group P213), and the α-phase is considered as a slight monoclinic distortion of the β-form [1–6]. The differences in the XRD patterns of these two phases are minimal, and the monoclinic distortion results in the presence of a few extra peaks with a low intensity and in the splitting of some pseudo-cubic (111), (201), (211) and (321) reflections. The most sensitive to a monoclinic distortion of the β-phase is the (321) reflection in the 2θ range of 47–48°. Therefore, the analysis of its shape could be used for the preliminary determination of the doped LAMOX structure and estimation of the cubic phase stabilization at room temperature by various dopants.
The enlarged (321) reflections for pellets calcined at 950 °C are shown in Fig. 5. In the case of undoped La2Mo2O9, the (321) reflection is a broad line with some splitting that is a characteristic feature of monoclinic α-phase. The (321) reflection of W-doped LAMOX is a sufficiently narrow line which is typical for high-temperature cubic β-phase evidencing its retention at room temperature.
Additionally, the thermal analysis of samples calcined at 900 °C has been conducted. The DTA thermogram of undoped La2Mo2O9 (Fig. 6) shows an endothermic peak at about 570 °C corresponding to the structural α → β transition [1–6], while any endothermic peaks are absent for W-doped sample. Thus, the data of the thermal analysis also confirm stabilization of cubic structure in W-doped LAMOX. Stabilization of the cubic phase is also confirmed by TEM data (Fig. 7). In the electron diffraction pattern of undoped La2Mo2O9, positions of reflections and interplanar spaces correspond to monoclinic α-La2Mo2O9 (ICSD 172479) [13]. Electron diffraction patterns of LMW show reflections and interplanar spaces close to those for cubic β-La2Mo2O9 phase (ICSD 98871) [6, 12].
The unit cell parameter of La2MoWO9 estimated from XRD data is a little larger as compared with that for undoped La2Mo2O9 (0.7156(9) and 0.7149(3) nm, respectively) due to a slightly larger ionic radius of W6+ (0.6 Å) versus that of Mo6+ cation (0.59 Å) in agreement with the literature data [13, 14, 16].
The structure of β-LAMOX is considered to be a rigid framework built up by [O1(La)3Mo] tetrahedra surrounded by partially occupied O2 and O3 oxygen sites [2, 3, 6]. According to structural studies of La2Mo2O9 by neutron powder diffraction, there is a substantial disorder in the oxygen sublattice of β–phase, which is considered to determine a high-oxygen mobility [2, 3, 6]. Doping La2Mo2O9 with tungsten, along with a slight increase of the unit cell, results in alteration of O2 and O3 site occupation leading to a higher disordering [13, 16].
SEM analysis
The SEM image and particle size distribution for the powder of LaMoWO9 calcined at 700 °C are presented in Fig. 8. The SEM image shows presence of separate particles of a small size along with large aggregates. According to the analysis of the particles size distribution, it is in the range of 0.35–15 μm. The histogram passes through the two maxima at 0.54 and 2.4 μm. EDX analysis of the powdered LaMoWO9 and the pellet of La2Mo1.5W0.5O9 sintered at 950 °C shows sufficiently uniform distribution of La, Mo, and W in both samples (Fig. 9-10).
SEM image of unpolished surface of the La2Mo1.5W0.5O9 pellet annealed at 950 °C. EDX analysis was carried out at the points 1–4 (see Fig. 10, right)
The SEM images of La2Mo2O9 and LaMoWO9 pellets sintered at 950 °C are presented in Fig. 11 and show a low porosity with a high (>95 % ) apparent density and well-connected grains. Besides, the size of grains for LaMoWO9 is noticeably smaller as compared with those for undoped La2Mo2O9. Thus, in agreement with previous works [14, 49], W doping hampers the grain growth of LAMOX.
Conductivity
The conductivity measurements were carried out for pellets sintered at 950 °C. The impedance spectra of sintered samples are typical for ionic conductors (Fig. 12). All Z” = f (Z’) dependences may be satisfactorily described by an equivalent scheme which includes the bulk and electrode impedances connected in series (Fig. 12). The bulk impedance includes two elements: the bulk resistance R b and the constant phase element (CPE)b; the electrode impedance is observed at high temperatures and comprises only the constant phase element (CPE)e. The total conductivity values were calculated from the R b values taking into account geometric parameters of the sample (1):
where σ is the total conductivity (S/cm), R b is the bulk resistance, t is the pellet thickness, and S is the electrode surface area.
Temperature dependences of the total conductivity for La2Mo2O9 and La2Mo1-xWxO9 samples are shown in Fig. 13. Conductivity of La2Mo2O9 shows a sharp increase at around 570 °C due to the structural α → β phase transition [1–6], whereas no abrupt conductivity changes are observed for W-doped samples showing stabilization of the cubic phase in agreement with the structural and thermal analysis data. Besides, at temperatures above 400 °C, their conductivity exceeds that of pure La2Mo2O9. In general, the conductivity values of samples obtained in this work (4.2–5.6⋅10−3 S/cm at 630 °C) are close to those reported earlier in the literature [13–23, 49]. For instance, conductivity of La2MoWO9 (5.6∙10−3 S/cm at 630 °C) is close to the value of ~5∙10−3 S/cm reported for a similar system by Ge et al. [49].
Usually, two regions of the temperature dependence of conductivity are considered: below and above ~450 °C [2, 3]. The conductivity of samples at low temperatures can be satisfactorily fitted by the conventional Arrhenius-type equation (2):
\( \sigma =\frac{A}{T} \exp \left(-\frac{E_a}{kT}\right) \) (2),
where A and E a are the pre-exponential factor and the activation energy, respectively. The activation energy E a for W-doped LAMOX is higher as compared with pure La2Mo2O9 (Table 1). Its values (1.5 and 1.1 eV, respectively) are close to those obtained in Ref. [16]: 1.4–1.7 eV for La2Mo1-xWxO9 and 1.11 and 0.9 eV for α- and β-La2Mo2O9. The conduction mechanism in the low temperature region is considered as due to the thermally activated oxide ions mobility [2, 3].
At temperatures above 400 °C, a deviation from the linear Arrhenius dependences is observed, presumably caused by the change in the conduction mechanism [13–18]. In this temperature range, the conductivity is satisfactorily described by the empirical Vogel-Tammann-Fulcher (VTF) equation (3) [2, 3, 6, 12]:
\( \sigma =\frac{A}{T} \exp \left(-\frac{E_{VTF}}{k\Big(T-{T}_0}\right) \) (3),
where A, E VTF, and T 0 are some parameters determined by fitting the experimental data. In such a VTF-type model for LAMOX, the oxide ion migration is assumed to be thermally assisted by the vibration of the cation framework, in which [O1(La)3Mo] tetrahedra are able to tilt and rotate with respect to each other, thus opening up a new conduction paths [12, 50]. This leads to a decrease of the activation energy (Table 1) and increase of conductivity due to migration of oxygen from fully occupied O1 sites toward partially occupied O2 and O3 oxygen sites which increase the overall charge carrier concentration.
Conclusion
La2Mo2-xWxO9 (x = 0.5–1) samples were synthesized using mechanochemical activation (MA) of starting oxides in a high-power planetary ball mill followed by calcination at 700–900 °C. The study of W-doped LAMOX samples genesis during MA has revealed the formation of mixed lanthanum tungstates-molybdates amorphous precursors. Their calcination at 500° leads to disappearance of amorphous precursors and formation of intermediate La2(MoW)3O12 structures. After annealing at 600°, La2Mo2-xWxO9 appears while a pure cubic phase is formed at 800–900 °C. These results have shown that though the target W-doped molybdates are not formed directly during mechanical activation, its application facilitates formation of the single phase LAMOX. The cubic β-phase formed from MA products after calcination at 700–950 °C was shown to be stabilized at room temperature.
High-density pellets of WLAMOX ceramics have been obtained already after sintering at 950 °C. W-doped LAMOX possess a higher electrical conductivity as compared with pure La2Mo2O9 at temperatures above 400 °C (i.e., 4.7–5.6∙10−3 S/cm at 630 °C).
References
Lacorre P, Goutenoire F, Bohnke O, Retoux R, Laligant Y (2000) Designing fast oxide-ion conductors based on La2Mo2O9. Nature 404:856–858
Georges S, Goutenoire F, Bohnke O, Steil MC, Skinner SJ, Wiemhufer HD, Lacorre P (2004) The LAMOX family of fast oxide-ion conductors: overview and recent results. J New Mat Electrochem Systems 7:51–57
Malavasi L, Fisher C.A J and Islam MS (2010) Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem Soc Rev 39:4370–4387
Jacquens J, Farrusseng D, Georges S, Viricelle JP, Gaudillere C, Corbel G, Lacorre P (2010) Tests for the use of La2Mo2O9-based oxides as multipurpose SOFC Core materials. Fuel Cells 10:433–439
Lo JC, Tsai DS, Chen YC, Le MV, Chung WH (2011) La2Mo2O9-based electrolyte: ion conductivity and anode-supported cell under single chamber conditions. J Am Ceram Soc 94:806–811
Malavasi L, Kim HJ, Billinge SJL, Proffen T, Tealdi C, Flor G (2007) Nature of the monoclinic to cubic phase transition in the fast oxygen ion conductor La2Mo2O9 (LAMOX). J Am Chem Soc 129:6903–6907
Tealdi C, Chiodelli G, Malavasi L, Flor G (2004) Effect of alkaline-doping on the properties of La2Mo2O9 fast oxygen ion conductor. J Mater Chem 14:3553–3557
Subasri R, Matusch D, Nafe H, Aldinger F (2004) Synthesis and characterization of (La1-xMx)2Mo2O9-δ; M = Ca2+, Sr2+ or Ba2+. J Eur Ceram Soc 24(1):129–137
Marrero-Lopez D, Perez-Coll D, Ruiz-Morales J, Canales-Vazquez J, Martin-Sedeno MC, Nunez P (2007) Synthesis and transport properties in La2−xAxMo2O9−δ (A = Ca2+, Sr2+, Ba2+, K+) series. Electrochim Acta 52:5219–5231
Georges S, Goutenoire F, Altorfer F, Sheptyakov D, Fauth F, Suard E, Lacorre P (2003) Thermal, structural and transport properties of the fast oxide-ion conductors La2-xRxMo2O9 (R = Nd, Gd, Y). Solid State Ionics 161:231–241
Tsai DS, Hsieh MJ, Tseng JC, Lee HY (2005) Ionic conductivities and phase transitions of lanthanide rare-earth substituted La2Mo2O9. J Eur Ceram Soc 25:481–487
Corbel G, Suard E, Lacorre P (2011) Structural key of the thermal expansion and the oxide ionic conduction in derivatives of La2Mo2O9: a temperature-controlled neutron diffraction study of β-La1.7Bi0.3Mo2O9. Chem Mater 23:1288–1298
Corbel G, Laligant Y, Goutenoire F, Suard E, Lacorre P (2005) Effects of partial substitution of Mo6+ by Cr6+ and W6+ on the crystal structure of the fast oxide-ion conductor structural effects of W6+. Chem Mater 17:4678–4684
Jin TY, Madhava Rao MV, Cheng CL, Tsai DS, Hung MH (2007) Structural stability and ion conductivity of the Dy and W substituted La2Mo2O9. Solid State Ionics 178:367–374
Georges S, Goutenoire F, Laligant Y, Lacorre P (2003) Reducibility of fast oxide-ion conductors La2-xRxMo2-yWyO9 (R ~ Nd, Gd). J Mater Chem 13:2317–2321
Georges S, Bohnke O, Goutenoire F, Laligant Y, Fouletier J, Lacorre P (2006) Effects of tungsten substitution on the transport properties and mechanism of fast oxide-ion conduction in La2Mo2O9. Solid State Ionics 177:1715–1720
Collado JA, Aranda MAG, Cabeza A, Olivera-Pastor P, Bruque S (2002) Synthesis, structures and thermal expansion of the La2Mo2−xWxO9 series. J Solid State Chem 167:80–85
Tealdi C, Chiodelli G, Flor G, Leonardi S (2010) Electrode stability and electrochemical performance of LAMOX electrolytes under fuel cell conditions. Solid State Ionics 181:1456–1461
Chiodelli G, Malavasi L (2013) Electrochemical open circuit voltage (OCV) characterization of SOFC materials. Ionics 19:1135–1144
Marrero-Lopez D, Canales-Vazquez J, Ruiz-Morales J, Irvine J, Nunez P (2005) Electrical conductivity and redox stability of La2Mo2 − xWxO9 materials. Electrochem Acta 50:4385–4395
Marrero-Lopez D, Canales-Vazquez J, Zhou W, Irvine JTS, Nunez P (2006) Structural studies on W6+ and Nd3+ substituted La2Mo2O9 materials. J Solid State Chem 179:278–288
Baque L, Vega-Castillo J, Georges S, Caneiro A, Djurado E (2013) Microstructural and electrical characterizations of tungsten-doped La2Mo2O9 prepared by spray pyrolysis. Ionics 19:1761–1774
Vega-Castillo JE, Ravell UK, Corbel G, Lacorrec P, Caneiro A (2014) Thermodynamic stability of La2Mo2−yWyO9, La2Mo2−yWyO8.96+0.02y and La7Mo7(2−y)/2W7y/2O30 (y = 0, 0.5 and 1.0). Dalton Trans 43:2661–2669
Haynes WM (ed) (2012-2013) CRC Handbook of Chemistry and Physics. 93rd Edition CRC Press. https://books.google.ru/books
Rojac T, Kosec M (2010) High-energy ball milling. In: Sopicka-Lizer M (ed) Mechanochemical processing of nanopowders, Woodhead Publishing Limited, pp 111–141
Sepelak V, Duvel A, Wilkening M, Beckerb KD, Heitjans P (2013) Mechanochemical reactions and syntheses of oxide. Chem Soc Rev 42:7507–7520
Zyryanov VV (2008) Mechanochemical synthesis of complex oxides. Russ Chem Rev 77:105–135
Lacorre P, Retoux R (1997) First direct synthesis by high energy ball milling of a new lanthanum molybdate. J. Solid State Chem 132:443–446
Georges S, Goutenoire F, Lacorre P, Steil MC (2005) Sintering and electrical conductivity in fast oxide ion conductors La2−xRxMo2−yWyO9 (R: Nd, Gd, Y). J Eur Ceram Soc 25:3619–3627
Kharlamova T, Pavlova S, Sadykov V, Chaikina M, Kriger T, Lapina O, Khabibulin J, Uvarov N, Frade J, Argirusis C (2007) Low-temperature synthesis methods of doped apatite-type lanthanum silicates. J Chem Eng Jap 40:1187–1191
Salavati-Niasari M, Hosseinzadeh G, Davar F (2011) Synthesis of lanthanum hydroxide and lanthanum oxide nanoparticles by sonochemical method. J Alloys Compd 509:4098–4103
Bernal S, Djerdj I, Garnweitner G, Su DS, Niederberger M (2007) Morphology-controlled nonaqueous synthesis of anisotropic lanthanum hydroxide nanoparticles. J Solid State Chem 180:2154–2165
Le Van T, Che M, Tatibouet JM, Kermarec M (1993) Infrared study of the formation and stability of La2O2CO3 during oxidative coupling of methane on La2O3. J Catal 142:18–26
Adachi G, Imanaka N (1998) The binary rare earth oxides. Chem Rev 98:1479–1514
Bernal S, Botana FJ, Garcia R, Rodriguez-Izquierdo JM (1987) Behavior of rare earth sesquioxides exposed to atmospheric carbon dioxide and water. React Solids 423:23–40
Segun L, Figlaz M, Cavagnat R, Lassegues JC (1995) Infrared and Raman spectra of MO3 molybdenum trioxides and MO3⋅xH,O molybdenum trioxide hydrates. Spectr Acta A 51:1323–1344
Krishna G, Ravikumar RVSN, Vijaya Kumar T, Ephraim SD, Ranjith B, Pranoy M, Dola S (2016) Investigation and comparison of optical and Raman bands of mechanically synthesised MoO3 Nano powders. Materials Today: Proceedings 3:4–63
Ingham B, Chong SV, Tallon JL (2005) Layered tungsten oxide-based organic-inorganic hybrid materials: an infrared and Raman study. J Phys Chem B 109:4936–4940
Sungpanich J, Thongtem T, Thongtem S (2012) Large-scale synthesis of WO3 nanoplates by a microwave-hydrothermal method. Ceram Inter 38:1051–1055
Saraiva GD, Luz-Lima C, Freire PTC, Ramiro de Castro AJ, de Sousa PF, Melo EA, Silva JH, Mendes Filho J (2013) Vibrational and structural properties in the dihydrate sodium tungstate and in the dihydrate sodium molybdate crystals. J Mol Struc 1033:154–161
Mirgorodsky A, Colas M, Smirnov M, Merle-Mejean T, El-Mallawany R, Thomas P (2012) Structural peculiarities and Raman spectra of TeO2/WO3-based glasses: a fresh look at the problem. J Solid State Chem 190:45–51
McEvoy TM, Stevenson KJ (2005) Spatially-resolved imaging of inhomogeneous ion/charge transfer behavior in polymorphous MoO3. I Conductivity and Chemical Mapping using Conductive Probe AFM and Raman Microscopy Langmuir 21:3521–3528
Diaz-Droguetta DE, El Fara R, Fuenzalida VM, Cabrera AL (2012) In situ-Raman studies on thermally induced structural changes of porous MoO3 prepared in vapor phase under He and H2. Mater Chem Phys 134:631–638
Meng L, Han H, Zhou D, Xia Y, Wang Z, Meng J (2016) Synthesis and luminescence properties of three dimensional architectures of nanostructural WO3. Optik 127:3454–3458
Pless JD, Kim HS, Smit JP, Wang X, Stair PC, Poeppelmeier KR (2006) Structure of Mg2.56V1.12W0.88O8 and vibrational Raman spectra of Mg2.5VWO8 and Mg2.5VMoO8. Inorg Chem 45:514–520
Sellemi H, Coste S, Barre M, Retoux R, Ali AB, Lacorre P (2015) Synthesis by the polyol process and ionic conductivity of nanostructured La2Mo2O9 powders. J All Compd 653:422–433
del Viola C M, Sangra AM, Pedregosa JC (1993) Vibrational spectroscopic characterization of lanthanide molybdates. J Mat Sci 28:6587–6590
Hayward SA, Redfern SAT (2004) Thermodynamic nature of and spontaneous strain below the cubic–monoclinic phase transition in La2Mo2O9. J Phys Condens Matter 16:3571–3583
Ge L, Guo K, Guo L (2015) Sinterability, reducibility, and electrical conductivity of fast oxide-ion conductors La1.8R0.2MoWO9 (R = Pr, Nd, Gd and Y). Ceram Int 41:10208–10215
Lacorre P, Selmi A, Corbel G, Boulard B (2006) On the flexibility of the structural framework of cubic LAMOX compounds in relationship with their anionic conduction properties. Inorg Chem 45:627–635
Acknowledgments
Support by RSF (Russian Science Foundation) 16-13-00112 Project, Federal Agency of Scientific Organizations (V.45.3.8 Project) and the Russian Ministry of Education and Science is gratefully acknowledged. We are very much thankful to Dr. Kharlamova T. for assistance in samples preparation and Dr. Chesalov Y. for recording IR and Raman spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlova, S., Bespalko, Y., Krieger, T. et al. Genesis, structural, and transport properties of La2Mo2-xWxO9 prepared via mechanochemical activation. Ionics 23, 877–887 (2017). https://doi.org/10.1007/s11581-016-1869-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1869-9