Abstract
The characteristics of an auditory steady-state response (ASSR) signal can be affected by the pathophysiological statuses of the left and right ears, such as a smeared sensation by native spectral smearing owing to sensorineural hearing impairment, because they can affect the perception of the stimulus, the degree of concentration on the stimulus and comfort in concentration. However, to date, few studies have examined the effects of such smeared sensations on the amplitude of the evoked ASSR signal. In this study, we synthesized various auditory stimuli with different degrees of spectral smearing using a hearing loss simulator to match the age of participant groups with different degrees of spectral smearing. We then performed three subjective tests, representing symmetric and asymmetric bilateral spectral smearing, with 16 normal-hearing individuals to observe the effects of the severity and symmetricity of bilateral spectral smearing, the value of the carrier frequency of auditory stimuli, and the sex of the individual on the amplitude in evoked ASSR signals. The experimental results demonstrated the following: (1) the application of spectral smearing to normal sounds may result in amplitude-reduced ASSR signals, (2) the effect of spectral smearing on the amplitude of the ASSR signals is most significant when the degrees of bilateral spectral smearing are asymmetric, (3) the selection of carrier frequency in an auditory stimulus can affect the amplitude of evoked ASSR signals regardless of the degree of spectral smearing, and (4) the sex of the individual can affect the amplitude of the evoked ASSR signal in various test conditions. The results of this study can help estimate the effects of smeared sensation by spectral smearing owing to sensorineural hearing impairment on the amplitude of evoked ASSR signals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The auditory steady-state response (ASSR) is a complex superimposed brain response evoked by auditory stimuli and whose responses have short interstimulus intervals (Kim and Lim 2017). The ASSR is generally measured by a scalp electroencephalogram (EEG) in the vicinity of the primary auditory cortex (PAC) or the fronto-central locations according to the device settings (Tanaka et al. 2013; Schwarz and Taylor 2005). The ASSR signal has been primarily utilized in the fields of auditory brain–computer interface (BCI), audiometric assessment for patients with autism or infants, and neuropsychiatry as a biomarker for cortical N-methyl-d-aspartate receptors and other neuropsychiatric disorders (Hosseinabadi and Jafarzadeh 2015; Swanepoel et al. 2004; Maanen and Stapells 2010; Lin et al. 2009; Sivarao et al. 2016; Wang et al. 2018; Melynyte et al. 2018).
In many previous studies related to ASSR-based auditory BCI, the authors considered several systemic and environmental factors (e.g., frequencies of modulated stimuli, contact/environmental noises, patterns of auditory stimuli, and arrangements of audio sources) to improve the performance of their implemented algorithm (Matsumoto et al. 2012; Nakamura et al. 2013). However, the characteristics of the evoked ASSR signal can also be affected by the pathophysiological statuses of the left and right ear (i.e., the severity and clinical symptoms of hearing impairment) and the sex of the subject because they can affect the perception of the stimulus, the degree of concentration on the stimulus, comfort in concentration, hormonal production, hormone levels, anatomical factors, and the size of the PAC (Voicikas et al. 2016; Zakaria et al. 2016; Melynyte et al. 2018). Therefore, to more effectively personalize the ASSR-based BCI algorithm according to the native hearing ability of an individual to improve system performance, the effects of the pathophysiological status of the ears and the sex of the subjects on the evoked ASSR signal characteristics—especially variations in the ASSR amplitude to a specific auditory stimulus—require investigation.
However, the number of studies discussing the effects of native hearing ability and sex on ASSR amplitude is relatively low. For example, Wilding et al. investigated the repeatability of the ASSR amplitude using 20 participants with normal-hearing (NH) ability and 11 individuals with hearing impairment (HI); however, they divided the participants into only two groups (NH and HI) and did not further divide the HI group into various sub-groups according to the degree and pattern of hearing impairment (Wilding et al. 2012). In addition, they did not match the ages of the NH (18–49 years) and HI (60–77 years) groups because they could not recruit age-matched participants with HI and did not investigate the effect of sex in the HI group. The researchers recruited only two female individuals with HI. Zakaria et al. investigated the effects of the sex of subjects and the modulation frequency of auditory stimuli on the ASSR threshold using 28 participants (14 females of 23.7 ± 2.1 years and 14 males of 22.6 ± 2.6 years); however, they did not investigate the effects of such factors on the ASSR amplitude (Zakaria et al. 2016). Melynyte et al. investigated the effects of the handedness and sex of subjects on the phase-locking and strength of 40 Hz ASSRs using 44 participants (11 right-handed females, 11 left-handed females, 11 right-handed male, and 11 left-handed males; right-handed = 22 ± 1.8 years and left-handed = 23 ± 3.7 years). However, the researchers recruited only NH subjects (Melynyte et al. 2018).
In this study, to obtain more detailed information about the effects of native hearing impairment on the evoked ASSR amplitude, we focused on the smeared sensation owing to spectral smearing. When the auditory filter bandwidth of the native inner ear becomes widened owing to the sensorineural HI, the effect of an auditory stimulus on a specific hair cell is transmitted to the surrounding hair cells, which results in deteriorated spectral selectivity. This phenomenon is called spectral smearing. Further, the effect of a deteriorated hearing threshold, which is also a major clinical symptom of sensorineural HI, can be avoided in real cases by selecting proper frequencies of auditory stimuli and adjusting the stimuli volume to a comfortable level for the individual. In our previous study, we demonstrated that the application of spectral smearing to auditory stimuli can decrease the performance of ASSR-based BCI algorithms. To do this, we used an eight-channel vocoder and 10 individuals with NH (Hwang et al. 2017). However, in that study, we divided the severity of spectral smearing into only three classes (normal, moderate, and severe) and did not investigate the effect of sex, because we recruited only three female participants. Furthermore, supplying identical sound to both ears was not investigated in our previous study because it was aimed only to verify the effect of spectral smearing on the accuracy of a binary BCI algorithm.
In this study, to further understand the effect of spectral smearing on the characteristics of an evoked ASSR signal, we further divide the degree of spectral smearing into four classes using a hearing loss simulator. Then, we perform three subjective tests with 16 participants with NH (eight males and eight females) to see the effects of the spectral smearing degree, symmetricity of bilateral spectral smearing, carrier frequency of auditory stimuli, and the sex of the individual on the variations in ASSR amplitude without an age-matching problem between groups.
Materials and methods
Synthesizing spectral-smearing-reflected stimuli
In this study, to match the ages of the participant groups with different degrees of HI, a hearing loss simulator (Cochlear Implant and Hearing Loss Simulator Version 1.08.01; TigerSpeech Technology, Shanghai, China) was utilized for synthesizing spectral smearing-reflected auditory stimuli. To verify the feasibility of the utilized simulator, first, we applied three linear audiogram patterns representing moderate hearing loss (ML), moderately-severe hearing loss (MSL), and severe hearing loss (SL) to the simulator (Fig. 1a). Then, we drew the patterns of the simulator output for white noise input. As shown in Fig. 1b–d, the simulator output (solid lines) showed descending patterns similar to the applied audiograms (dashed lines). Second, we already verified the effect of variations in the smearing parameter of the simulator in a previous report, which showed that the auditory filter bandwidth of the simulator output widens as the value of the smearing parameter increases (Hwang et al. 2015). To find the relevant values of the smearing parameter for each HI setup, we referred to a report of Glasberg and Moore (1986) that demonstrated that the equivalent rectangular bandwidth increased monotonically as the absolute threshold increased, as in our recent report (Hwang et al. 2017). In the Glasberg study, the ratios of the equivalent rectangular bandwidth (ERBR) between HI and NH for a 1000 Hz pure-tone input signal were approximately 1.43, 2.31, and 3.18 for ML, MSL, and SL individuals, respectively. Based on this report, we varied the smearing parameter from 0.0 to 3.0 (step = 0.5) and calculated the ERBRs for a 1000 Hz pure-tone input signal. Based on those measurements, we set the values of the smearing parameter as 0.5 (ERBR = 1.43), 1.0 (ERBR = 2.15), and 1.5 (ERBR = 3.28) for the ML, MSL, and SL setups, respectively. Then, after verifying the relevance of the utilized simulator, first, two sinusoidal amplitude-modulated stimuli—S500{37} and S2000{43} (also denoted by S500{37}NH and S2000{43}NH)—were synthesized with 100% modulation depth, where 500/2000 represented the carrier frequency in Hz and 37/43 represented the modulation frequency in Hz, respectively (Hwang et al. 2017; Kim et al. 2011). Next, to synthesize the spectral smearing-reflected stimuli, S500{37}NH and S2000{43}NH were individually applied to the simulator with ML, MSL and SL setups. Then, the output of the simulator was high-pass filtered using a cut-off frequency of 250 Hz and normalized [denoted by S500{37}ML, S500{37}MSL, S500{37}SL, S2000{43}ML, S2000{43}MSL, and S2000{43}SL] (Fig. 2).
Preparations for ASSR measurement
Sixteen individuals with NH ability participated in this study (eight males and eight females, age range 21–29 years, median age 24 years, average age 24.5 years). All individuals had no history of neurological or psychiatric diseases or illness and passed a pure-tone audiometry test before participating in the experiment. The experimental protocols were approved by the Institutional Review Board of Hanyang University, and written informed consent was obtained from all participants, who were compensated for their participation. During the data recording, each participant sat on a chair in the center of an electrically isolated cage (200 × 120 × 190 cm), with three electrodes for EEG measurement attached to the head via a headset (MM50 iP; Sennheiser Electronic GmbH, Wedemark, Germany) that provided auditory stimuli. The EEG signals were measured using a commercial device (MOBIlab+ with Sahara box; g.tec Medical Engineering GmbH, Schiedlberg, Austria) (Rutkowski and Mori 2015). A dry electrode (g.SAHARAelectrode with 7 mm g.SAHARAclip; g.tec) at the CZ position was used for signal measurement, and two hydraulic electrodes (g.SAHARAclipREF/g.SAHARAclipGND; g.tec) at the left/right ear mastoid were used for the reference and ground. Before the main experiments, S500{37}NH was presented in both ears via the headset, and the participants were asked to concentrate on the sound for 15 s. Then, the recorded EEG signals were FFT-processed to determine whether the ASSR pattern was observed. If the pattern was not observed, the recording setups were repeatedly tuned (e.g., hair was removed, the electrode tip wiped clean, electrodes reattached to reduce skin–electrode impedance, and the lines were rearranged to eliminate line noises) until the signal quality became satisfactory.
Protocols for subjective tests
To simulate various real-world HI conditions, we performed the following three subjective evaluations: (1) SYM-I test: symmetric smearing and identical stimulus to both ears (no BCI setting), (2) SYM-II test: symmetric smearing and different stimuli to each ear (binary BCI setting), and (3) ASYM test: asymmetric smearing and different stimuli to each ear (binary BCI setting). In all testing, five training trials were performed before the main experiments to familiarize the participants with the 15 s concentration/5 s rest protocol. The volume of each stimulus was fixed at 65 dB SPL using a commercial sound level meter (SC-30; CESVA, Barcelona, Spain) to eliminate the effect of hearing threshold variations between the HI setups. To minimize participant fatigue and aid in concentration, intermediate rest periods (< 2 min) were provided after five successive trials, and the total elapsed time per measurement was limited to less than 20 min per day.
In the SYM-I test, (1) training trials were performed, and (2) one of eight stimuli—S500{37}NH, S500{37}ML, S500{37}MSL, S500{37}SL, S2000{43}NH, S2000{43}ML, S2000{43}MSL, and S2000{43}SL—was presented to both ears for 15 s. The participant was asked to concentrate on the stimulus. Step 2 was performed 40 times (8 stimuli × 5 repetitions; the order of the test conditions was randomized). In the SYM-II test, (1) training trials were performed, and (2) one of four stimuli sets—[S500{37}NH, S2000{43}NH], [S500{37}ML, S2000{43}ML], [S500{37}MSL, S2000{43}MSL], and [S500{37}SL, S2000{43}SL]—was presented for 15 s. The participant was asked to concentrate on a randomly instructed stimulus (one of the four stimuli sets was randomly presented, and the participant was asked to concentrate on the S500{37}NH/ML/MSL/SL). Step 2 was performed 20 times (4 smearing levels × 5 repetitions; order randomized). In the ASYM test, (1) training trials were performed, (2) one of two stimuli sets—[S500{37}NH, S2000{43}MSL], and [S500{37}ML, S2000{43}SL]; two-level difference—was presented for 15 s, and the participant was asked to concentrate on a randomly instructed stimulus. Step 2 was performed 20 times (2 stimuli sets × 2 directions × 5 repetitions; order and direction randomized).
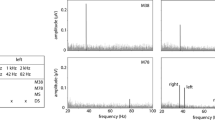
After completing the experiments, from each EEG measurement, the ratio between the spectral power at the modulation frequency and the average spectral power around the modulation frequency (± 5 bins from the modulation frequency in FFT) was calculated. In this study, we assumed that if the ratio was ≥ 2, the ASSR was generated, and if the ratio was < 2, the ASSR was not generated. Based on these criteria, only the trials that contained the proper ASSR signal were utilized for post-experimental statistical investigation (Hwang et al. 2017). In addition, the ASSR phase was neglected during the investigation based on a report of Gander et al., which demonstrated that attention had no detectable effect on the ASSR phase (Gander et al. 2010).
Results
Tables 1 and 2 list the amplitudes of the evoked ASSR signals in the SYM-I, SYM-II, and ASYM tests.
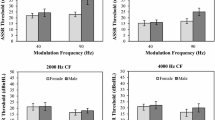
Figure 3 illustrates the ASSR amplitudes in the SYM-I test. When considering all participants (Fig. 3a), there were no statistically significant differences in the Kruskal–Wallis test between the four spectral smearing levels when the carrier frequency was the same [S500{37}NH/ML/MSL/SL and S2000{43}NH/ML/MSL/SL]. By contrast, the non-parametric Mann–Whitney test between each pair with identical spectral smearing levels and different carrier frequencies [(S500{37}NH, S2000{43}NH) (S500{37}ML, S2000{43}ML) (S500{37}MSL, S2000{43}MSL), and (S500{37}SL, S2000{43}SL)] showed significant differences between all setups (p < 0.05). When considering only the female participants (Fig. 3b), a significant difference was observed in ML setups between the two carrier frequencies. When considering only the male participants (Fig. 3c), significant differences were observed between the two carrier frequencies for all setups, as well as between the NH and MSL, NH and SL, NH and HI (average of ML, MSL, and SL), and ML and SL setups when the carrier frequency was 2000 Hz.
Figure 4 illustrates the ASSR amplitudes in the SYM-II test. When considering all individuals (Fig. 4a), significant differences were observed between the NH and ML, NH and SL, and NH and HI setups. However, no significant differences were observed when considering only the female individuals (Fig. 4b) and only the male individuals (Fig. 4c).
Figure 5 illustrates the ASSR amplitudes in the ASYM test. Commonly, significant differences were observed between the NH/MSL and ML/SL setups when considering all (Fig. 5a), female (Fig. 5b), and male (Fig. 5c) participants.
Results of ASYM test (mean and standard deviation). In figures, NH/NH and ML/ML represent results of SYM-II test (equivalent to NH and ML in Fig. 4). a Entire group, b female group, c male group
Effects of degree of spectral smearing
As shown in Tables 1 and 2, the average ASSR amplitudes in the NH cases decreased by 10% (between S500{37}NH and S500{37}HI) and 25% (between S2000{43}NH and S2000{43}HI) versus HI cases in the entire group during the SYM-I test (Fig. 3a). NH cases decreased by 10% versus HI cases in the entire group during the SYM-II test (Fig. 4a), and NH/MSL cases decreased by 25% versus ML/SL cases in the entire group during the ASYM test (Fig. 5a). These results imply that the application of spectral smearing to normal sounds may result in amplitude-diminished ASSR signals, although in the current study these differences were not statistically significant.
Effects of symmetricity of bilateral spectral smearing
There were no significant differences in ASSR amplitude between the ML, MSL, and SL cases for either 500 Hz or 2000 Hz carrier frequencies in the entire group during the SYM-I test (Fig. 3a). There were no significant differences in ASSR amplitude between the ML, MSL, and SL cases in the entire group during the SYM-II test (Fig. 4a). Finally, a significant difference in ASSR amplitude was observed between the NH/MSL and ML/SL cases in the entire group during the ASYM test (Fig. 5a). These results imply that the effect of spectral smearing on the amplitude of the ASSR signals is most significant when the degrees of bilateral spectral smearing are asymmetric.
Effects of carrier frequencies of auditory stimuli
In the SYM-I test, there were no significant differences in ASSR amplitude between the NH, ML, MSL, SL, and HI cases in the entire group when the carrier frequency was identical (Fig. 3a). In addition, when comparing cases with the same smearing level and different carrier frequencies, significant differences were observed between the two carrier frequencies for all setups in the entire group (Fig. 3a). These results imply that the selection of carrier frequency in an auditory stimulus can affect the amplitude of the induced ASSR signal regardless of the degree of spectral smearing.
Effects of sex of individual
During the SYM-I test, in the female group, there were no significant differences in ASSR amplitude between the NH, ML, MSL, SL, and HI cases when the carrier frequency was fixed (Fig. 3b). By contrast, in the male group, the same phenomenon was observed only when the carrier frequency was 500 Hz, and there were significant differences between the NH and MSL, NH and SL, NH and HI, and ML and SL cases when the carrier frequency was 2000 Hz (Fig. 3c). When comparing cases with the same smearing level and different carrier frequencies, a significant difference in ASSR amplitude between the two carrier frequencies was observed only in the ML case of the female group (Fig. 3b). By contrast, significant differences in ASSR amplitude were observed between the two carrier frequencies for all setups in the male group (Fig. 3c). There were no significant differences in the ASSR amplitude between the ML, MSL, and SL cases in either the male or female groups during the SYM-II test (Fig. 4b, c). Significant differences in the ASSR amplitude between the NH/MSL and ML/SL cases were commonly observed in both the male and female groups during the ASYM test (Fig. 5b, c). In addition, a significant difference in ASSR amplitude was observed between the ML/ML (in SYM-II test) and ML/SL (in ASYM test) cases in the female group (Fig. 5b). However, no significant difference in ASSR amplitude was observed between the same cases of the male group (Fig. 5c). These results imply that the sex of the individual can affect the amplitude of the evoked ASSR signal in various conditions.
Discussion
When comparing the current simulation data with that of previous reports, Wilding et al. reported that the average ASSR amplitude in NH cases was higher than in HI cases when an S2000{95} stimulus was applied to both ears simultaneously, which is consistent with the results for S2000{43} in the SYM-I test (Wilding et al. 2012). Ross et al. reported that the ASSR amplitude monotonically decreased as the carrier frequency increased from 250 to 4000 Hz in NH individuals, which is consistent with the results of the SYM-I test (Ross et al. 2000). In addition, the results of the SYM-I test demonstrated that this trend was maintained in all smearing-reflected setups. Papakonstantinou et al. used two different stimuli, S1000{38} for probe stimulus and S2000{42} for the interferer, on 20 NH individuals and reported that the ASSR amplitude was reduced owing to the existence of the interferer. This is consistent with the results of our study (Papakonstantinou et al. 2013). More specifically, the average ASSR amplitudes for S500{37} in the SYM-I test were reduced during the SYM-II test in all setups and were also reduced during the ASYM test in the NH/MSL and ML/SL setups. Picton et al. demonstrated the difference in ASSR amplitude between female and male groups; more specifically, the ASSR amplitude of the female NH group was higher than that of the male NH group (Picton et al. 2009). In our study, the average ASSR amplitudes of the male group were 3, 7, 13, and 6% lower than those of the female group at S500{37}NH/ML/MSL/HI in the SYM-I test, and 44, 11, 53, 48, and 38% lower than those of the female group at S2000{43}NH/ML/MSL/SL/HI in the SYM-II test, respectively. In the Mann–Whitney test, the average ASSR amplitudes of the male and female groups for S2000{43}MSL/SL/HI showed a statistically significant difference. By contrast, in the ASYM test, the average ASSR amplitudes of the female group were 3% and 13% lower than those of the male group for the NH/MSL and ML/SL setups, although there were no significant differences. These values coincide with previous reports and demonstrate the validity of the current simulation study. Further, Güntekin et al. reported that females had higher connectivity between different parts of the brain than males during emotional processes. This is another case that demonstrates the difference in EEG parameters between female and male groups as in our current study (Güntekin et al. 2017). Among the previous reports, Ross et al., Papakonstantinou et al., and Picton et al. recruited only NH individuals and did not consider HI cases (Ross et al. 2000; Picton et al. 2009; Papakonstantinou et al. 2013). The reports of Hosseinabadi and Jafarzadeh, Swanepoel et al., Maanen and Stapells, and Lin et al. focused only on the relationship between the ASSR threshold and the degree of hearing loss, not the ASSR amplitude, because their primary concern was the development of a new methodology of EEG-based audiometric assessment for infants or patients with autism (Hosseinabadi and Jafarzadeh 2015; Swanepoel et al. 2004; Maanen and Stapells 2010; Lin et al. 2009). In addition, Wilding et al. divided the participants into only two (NH and HI) groups, did not match the ages of the NH and HI groups, and did not investigate the effect of sex in the HI group (Wilding et al. 2012). In this study, we performed the following steps: (1) we divided the degree of spectral smearing into ML, MSL, and SL as well as NH and HI, (2) we matched the ages of the participant groups with different degrees of spectral smearing using a hearing loss simulator (i.e., we minimized the effects of other hearing loss symptoms vs. the degree of spectral smearing), (3) we investigated the effect of the symmetricity of bilateral spectral smearing, (4) we investigated the effect of the carrier frequency of the auditory stimulus, and (5) we investigated the effect of sex between participant groups. These are the key differences of the current study versus previous reports.
Deteriorated hearing thresholds and reduced spectral selectivity owing to spectral smearing are the most representative clinical symptoms of sensorineural HI. As we mentioned in the Introduction, the effect of the former is not serious in BCI applications because it can be avoided by selecting proper frequencies of auditory stimuli and adjusting the stimuli volume to a comfortable level for the individual; however, avoiding the effect of the latter is not a simple problem. As we demonstrated in our previous report, the degree and pattern of spectral smearing can affect the performance of an ASSR-based BCI system because they can affect the perception of the stimulus, the degree of concentration on the stimulus, and comfort in concentration (Hwang et al. 2017). For this reason, to improve the clinical usability of the ASSR-based BCI system for persons with sensorineural HI, it is important to reflect the degree and pattern of spectral smearing of the individual in the parameter fine-tuning.
Although we focused on the ASSR-based auditory BCI in this study, there are several auditory BCI algorithms that depend on other EEG parameters such as P300. In previous reports, Arslan et al. reported that the cognitive function can affect the amplitude and latency of P300, Huang et al. reported that the amplitude of auditory P300 changes in accordance with variations in patterns in an auditory stimulus, and Li et al. reported that the degree of auditory attention can affect the characteristics of the N1 signal in an EEG signal (Arslan et al. 2018; Huang et al. 2016; Li et al. 2017). As the degree and pattern of spectral smearing can affect the perception of and concentration on the auditory stimulus, it is expected that the spectral smearing may also affect the auditory BCI algorithms based on P300 and N1 as well as ASSR.
As the average life expectancy increases, it is expected that the number of individuals with neurological disease and sensorineural HI will consistently increase as time goes on. The National Institute on Deafness and Other Communication Disorders (NIDCD) reported that 30% of American adults aged 65–74 and 47% of adults aged over 75 had hearing problems owing to aging in 2006 (NIDCD 2006). In addition, Feder et al. reported that 7.7% of Canadian youth aged 6–19 had hearing impairments, less than 2.2% of Canadian youth aged 6–19 had sensorineural HI, and less than 3.5% of Canadian youth aged 6–19 had conductive HI (Feder et al. 2017). In addition, in a report of Tufatulin et al., 72% of hearing-impaired adults had sensorineural HI, and among them, 47% had asymmetric HI (Tufatulin et al. 2018). These surveillance data demonstrate the importance of considering the native hearing ability of individuals during the design of auditory BCI systems, as we discussed in the current study.
The current study had several limitations. First, we utilized a hearing loss simulator to prevent age mismatches between participant groups. Although the performances of the utilized simulator in simulating deterioration in the hearing threshold and deterioration in the auditory filter bandwidth were evaluated in a verification study (Fig. 1) (Hwang et al. 2015), in real-world situations, various clinical symptoms are observed in patients with sensorineural HI that are not covered by the simulator. These symptoms include loudness recruitment, dead zones, and tinnitus. Second, we used linear audiogram patterns to set the hearing thresholds of the simulator (Fig. 1a). However, in real-world situations, the patterns are generally non-linear, and the hearing threshold value at a specific frequency is not unique for a certain degree of HI. Third, we recruited only young volunteers with NH ability; therefore, the effects of alterations in morphology, structure, connectivity, and function of intracranial auditory processing parts owing to long-term sensorineural HI are not covered in this study (Emmorey et al. 2003; Smith et al. 2011; Shibata 2007; Li et al. 2015).
Conclusion
In this study, we investigated the effect of smearing sensation by spectral smearing owing to sensorineural HI on the amplitude of evoked ASSR signals using the aspects of severity, symmetricity, carrier frequency, and sex of the individual without an age-mismatching problem between groups using a hearing loss simulator. The results of this study can aid in estimating the effect of decreased auditory function on the characteristics of the evoked ASSR signal.
References
Arslan F, Tasdemir S, Durmaz A, Tosun F (2018) The effect of nasal polyposis related nasal obstruction on cognitive functions. Cognit Neurodyn 12(4):385–390
Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H (2003) A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci USA 100(17):10049–10054
Feder KP, Michaud D, McNamee J, Fitzpatrick E, Ramage-Morin P, Beauregard Y (2017) Prevalence of hearing loss among a representative sample of Canadian children and adolescents, 3 to 19 years of age. Ear Hear 38(1):7–20
Gander PE, Bosnyak DJ, Roberts LE (2010) Acoustic experience but not attention modifies neural population phase expressed in human primary auditory cortex. Hear Res 269(1–2):81–94
Glasberg BR, Moore BC (1986) Auditory filter shapes in subjects with unilateral and bilateral cochlear impairments. J Acoust Soc Am 79(4):1020–1033
Güntekin B, Femir B, Gölbaşı BT, Tülay E, Başar E (2017) Affective pictures processing is reflected by an increased long-distance EEG connectivity. Cognit Neurodyn 11(4):355–367
Hosseinabadi R, Jafarzadeh S (2015) Auditory steady-state response thresholds in adults with conductive and mild to moderate sensorineural hearing loss. Iran Red Crescent Med J 17(1):e18029
Huang M, Daly I, Jin J, Zhang Y, Wang X, Cichocki A (2016) An exploration of spatial auditory BCI paradigms with different sounds: music notes versus beeps. Cognit Neurodyn 10(3):201–209
Hwang JH, Nam KW, Yoon SH, Kim J, Yook S, Hong SH, Jang DP, Kim IY (2015) Effects of the simultaneous application of nonlinear frequency compression and dichotic hearing on the speech recognition of severely hearing-impaired subjects: simulation test. Clin Exp Otorhinolaryngol 8(2):102–110
Hwang JH, Nam KW, Jang DP, Kim IY (2017) Effects of spectral smearing of stimuli on the performance of auditory steady-state response-based brain–computer interface. Cognit Neurodyn 11(6):515–527
Kim SY, Lim W (2017) Dynamical responses to external stimuli for both cases of excitatory and inhibitory synchronization in a complex neuronal network. Cognit Neurodyn 11(5):395–413
Kim DW, Cho JH, Hwang HJ, Lim JH, Im CH (2011) A vision-free brain–computer interface (BCI) paradigm based on auditory selective attention. Conf Proc IEEE Eng Med Biol Soc 2011:3684–3687
Li Z, Zhu Q, Geng Z, Song Z, Wang L, Wang Y (2015) Study of functional connectivity in patients with sensorineural hearing loss by using resting-state fMRI. Int J Clin Exp Med 8(1):569–578
Li X, Zhang Y, Li L, Zhao H, Du X (2017) Attention is shaped by semantic level of event-structure during speech comprehension: an electroencephalogram study. Cognit Neurodyn 11(5):467–481
Lin YH, Ho HC, Wu HP (2009) Comparison of auditory steady-state responses and auditory brainstem responses in audiometric assessment of adults with sensorineural hearing loss. Auris Nasus Larynx 36(2):140–145
Maanen AV, Stapells DR (2010) Multiple-ASSR thresholds in infants and young children with hearing loss. J Am Acad Audiol 21(8):535–545
Matsumoto Y, Nishikawa N, Makino S, Yamada T, Rutkowski TM (2012) Auditory steady-state response stimuli based BCI application-the optimization of the stimuli types and lengths. In: APSIPA ASC 2012
Melynyte S, Pipinis E, Genyte V, Voicikas A, Rihs T, Griskova-Bulanova I (2018) 40 Hz auditory steady-state response: the impact of handedness and gender. Brain Topogr 31(3):419–429
Nakamura T, Namba H, Matsumoto T (2013) Classification of auditory steady-state responses to speech data. Conf Proc IEEE EMBS Neural Eng 2013:1025–1028
National Institute on Deafness and Other Communication Disorders (2006) Strategic plan FY 2006-2008, p 3
Papakonstantinou A, Kollmeier B, Riedel H (2013) Ipsi- and contralateral interaction in the 40 Hz auditory steady state responses (ASSRs) with two carriers at 60 dB SPL. Int J Audiol 52(9):626–635
Picton TW, van Roon P, John MS (2009) Multiple auditory steady state responses (80–101 Hz): effects of ear, gender, handedness, intensity and modulation rate. Ear Hear 30(1):100–109
Ross B, Borgmann C, Draganova R, Roberts LE, Pantev C (2000) A high-precision magnetoencephalographic study of human auditory steady-state responses to amplitude-modulated tones. J Acoust Soc Am 108(2):679–691
Rutkowski TM, Mori H (2015) Tactile and bone-conduction auditory brain computer interface for vision and hearing impaired users. J Neurosci Methods 244:45–51
Schwarz DWF, Taylor P (2005) Human auditory steady state responses to binaural and monaural beats. Clin Neurophysiol 116(3):658–668
Shibata DK (2007) Differences in brain structure in deaf persons on MR imaging studied with voxel-based morphometry. Am J Neuroradiol 28:243–249
Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y, Whiterock V, Li YW, Ahlijanian MK (2016) 40 Hz auditory steady-state response is a pharmacodynamic biomarker for cortical NMDA receptors. Neuropsychopharmacology 41(9):2232–2240
Smith KM, Mecoli MD, Altaye M, Komlos M, Maitra R, Eaton KP, Egelhoff JC, Holland SK (2011) Morphometric differences in the Heschl’s gyrus of hearing impaired and normal hearing infants. Cereb Cortex 21(5):991–998. https://doi.org/10.1093/cercor/bhq164
Swanepoel DW, Hugo R, Roode R (2004) Auditory steady-state responses for children with severe to profound hearing loss. Arch Otolaryngol Head Neck Surg 130(5):531–535
Tanaka K, Kuriki S, Nemoto I, Uchikawa Y (2013) Auditory steady-state responses in magnetoencephalogram and electroencephalogram: phenomena, mechanisms, and applications. Adv Biomed Eng 2:55–62
Tufatulin GS, Boboshko MY, Artyushkin SA (2018) Asymmetric sensorineural hearing impairment in the adult population. Vestn Otorinolaringol 83(3):20–24
Voicikas A, Niciute I, Ruksenas O, Griskova-Bulanova I (2016) Effect of attention on 40 Hz auditory steady-state response depends on the stimulation type: flutter amplitude modulated tones versus clicks. Neurosci Lett 629:215–220. https://doi.org/10.1016/j.neulet.2016.07.019
Wang Y, Ma L, Wang X, Qin L (2018) Differential modulation of the auditory steady state response and inhibitory gating by chloral hydrate anesthesia. Sci Rep 8(1):3683
Wilding TS, McKay CM, Baker RJ, Kluk K (2012) Auditory steady state responses in normal-hearing and hearing-impaired adults: an analysis of between-session amplitude and latency repeatability, test time, and F ratio detection paradigms. Ear Hear 33(2):267–278
Zakaria MN, Jalaei B, Wahab NA (2016) Gender and modulation frequency effects on auditory steady state response (ASSR) thresholds. Eur Arch Otorhinolaryngol 73(2):349–354
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2017M3A9E1064781), by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2017R1D1A1B03028811), and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI17C2397).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hwang, J.H., Nam, K.W., Jang, D.P. et al. Effects of degree and symmetricity of bilateral spectral smearing, carrier frequency, and subject sex on amplitude of evoked auditory steady-state response signal. Cogn Neurodyn 13, 151–160 (2019). https://doi.org/10.1007/s11571-018-9512-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-018-9512-2