Abstract
Several specimens of putative Lentinus pilososquamulosus (including the type specimen) and Le. martianoffianus from Siberia and the Russian Far East associated with different hosts and collected during the past two decades were studied. Morphological examination of the studied specimens showed a close similarity to specimens of Lignomyces vetlinianus, a species originally described from Central Europe, but later discovered in European Russia, the Caucasus (Abkhazia), and the Urals. Cultures of Li. vetlinianus were characterized by growth and morphology, and their adaptation to various temperatures was evaluated. Growth rate of the strains at 25 °C varied between 1.2 and 3.1 mm/day; the majority of them could survive freezing at −20 °C and grew at temperature ranging from 5 to 35 °C. Comparative culture characters, mating compatibility, and ITS sequencing revealed that the specimens earlier identified as Le. pilososquamulosus or Far East Russian Le. martianoffianus (misapplied name) were identical to Li. vetlinianus. It was shown that the distribution area of Li. vetlinianus extends from Central Europe to the South (Caucasus) and through Western Siberia to the Russian Far East. Since several attempts of Le. pilososquamulosus holotype sequencing were unsuccessful, an epitype of this taxon, represented by a successfully sequenced old topotype specimen, was proposed. Le. pilososquamulosus is considered as synonym of Li. vetlinianus, following the principle of priority. A molecular study of true Le. martianoffianus (type specimen) supported its conspecificity with Panus lecomtei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For more than 40 years, a pleurotoid wood-decaying fungus described in 1964 as Pleurotus vetlinianus Domański was not observed outside Central Europe, although it was known from several locations in Poland and Croatia (Red book, 2008). In 2008, it was discovered in European Russia, and the species epithet was recombined as Lignomyces vetlinianus (Domański) R.H. Petersen & Zmitr. (Petersen et al. 2015). Later the fungus was found on the Ural Mountains (Stavishenko and Zmitrovich 2017) and Western Caucasus (Abkhazia). Separately, over the past few decades, several specimens identified as Lentinus pilososquamulosus and Le. martianoffianus were collected from different hosts in Siberia and the Far East (Russia).
Lentinus pilososquamulosus Lj. N. Vassiljeva was described in 1973 with the following diagnosis: “Pileo laterali, pallido, piloso-squamuloso, 10–18 cm lato, carnoso; lamellis confertis, salmoneo-cremeis, 6–7 mm latis, cum lamellulis; sporidia alba; sporis hyalinis, ellipsoideis, 6–8 × 2.5–4.5 μm, basidiis 18–20 × 5–6 μm.” The fungus had been found in 1946 in the Far East of Russia (Primorsky Krai, Ussuriysk State Nature Reserve) on unidentified rotten wood. Any comparison with similar species was absent, but in the extended Russian description, it was indicated that the cap had a lateral, hairy-scaly attachment, exhibited close salmon-cream gills of different ranks, white spore powder, and ellipsoid, colorless spores. The hyphal system of the carpophore was not described (Vassilieva 1973).
Later, these characters were sometimes associated with Le. martianoffianus Kalchbr. (Bulakh et al. 2010; Bulakh 2015), a species known from a single type specimen belonging presumably to the genus Panus (Pegler 1983; Zmitrovich et al. 2018). A comparison of these two species was based on the absence of a stipe in both species, their pubescent caps, and ellipsoid spores, comparable in size. However, Le. martianoffianus was reported as exhibiting a dimitic hyphal construction and thick-walled cheilocystidia (Pegler 1983), which were not indicated in the original description of Le. pilososquamulosus.
Initially, we assumed that specimens under these two names were identical to those of Lignomyces vetlinianus. To verify this, we conducted a study of type specimens of Le. pilososquamulosus and Le. martianoffianus together with several specimens from Siberia and the Far East misidentified as Le. martianoffianus and specimens of Li. vetlinianus from European Russia. The aims of the study were (1) to examine the relationships among these three species (i.e., to test the hypothesis that Le. pilososquamulosus and Li. vetlinianus are conspecific and to confirm the misapplication of Le. martianoffianus to Le. pilososquamulosus in a literature) and (2) to discuss the distribution of this taxon and characterize it using an integrated approach including comparative morphological, cultural, mating, and molecular analyses of herbarium and cultured material. Misapplied taxon’s names are given in inverted commas.
Materials and methods
Material examined
In the text below, Le. = Lentinus; Li. = Lignomyces.
Preserved type and ancillary specimens of Le. pilososquamulosus and Le. martianoffianus were studied. Cultures associated with Le. martianoffianus and Lignomyces vetlinianus were also studied. The basidioma collected in Bryansk region was originally identified as ‘Lentinus strigosus’ (current name Panus lecomtei), but a colony mat of the strain resembled those of Lignomyces vetlinianus. For this reason, the putative ‘Lentinus strigosus’ strain was included in this study. An herbarium specimen of Panus lecomtei was also included in the investigation. Examined specimens, their herbarium and strain numbers, and information about their origin are presented in Table 1. Herbarium specimens are preserved in the Mycological Herbarium LE of the Komarov Botanical Institute RAS (St. Petersburg, Russia) and in the herbarium VLA of the FSC of the Far East Asia Terrestrial Biodiversity, Far Eastern Branch of RAS (Vladivostok, Russia). The type specimen of Le. martianoffianus is preserved in the Herbarium of the Royal Botanic Gardens, Kew (England). Cultures are maintained in the Komarov Botanical Institute Basidiomycetes Culture Collection using traditional preservation methods (Psurtseva 2010) and cryopreservation at −80 °C (10% glycerol; freezing rate 1 °C min−1).
Morphological analysis of herbarium specimens
Microscopic preparations from dried material were mounted in Melzer’s solution, 10% ammoniacal Congo Red or 5% aqueous solution of KOH, using a LOMO Micmed-6 (St. Petersburg, Russia) light microscope. The hyphal system was observed and described according to Zmitrovich et al. (2009). The size of mature spores was measured on 30 spores in distilled water and Melzer’s solution.
Cultural study
Inoculum plugs (7 mm in diameter) were placed mycelium-side down on the edge of 90-mm Petri plates containing malt extract agar (MEA) (50 g/L, Oxoid, England) and potato dextrose agar (PDA) (39 g/L, Panreac, Germany) and incubated for 8 weeks in growth chambers in the dark at 25 °C to evaluate the growth rate of the strains. On the center of the plates containing beer-wort agar (BWA) (beer-wort 4%, brewery “Severnie pivovarni,” Russia; agar 20 g/L, Difco, USA), inoculum plugs were incubated in the dark at 5, 10, 25, 30, and 35 °C to study a temperature growth range of the strains and at −20 °C for 2 weeks to study the viability of the strains after freezing. Three replicates on each medium were incubated for each strain in the experiments. The growth rate was recorded every other day as linear mycelium extension in millimeters (n = 3), and the standard deviation was estimated using the MS Excel statistics tool. The advancing zone was studied after 2 weeks and colony morphology at weeks 4 and 6. Micromorphology was studied under Axio Imager A1, Axio Scope A1, and Stemi 2000-CS (Zeiss, Germany) using transmitted light.
Fruiting in culture
To obtain single spore isolates (SSIs) for mating studies, all strains were fruited in culture. To induce fruiting, the strains were grown on sawdust. Betula (birch) sawdust and wheat bran (3:1, respectively) were mixed, and boiling water was added until the substrate became moistened. Glass beakers were filled with the prepared substrate, covered with aluminum foil, and autoclaved. The beakers were inoculated with diced strains grown in Petri plates on MEA, incubated in darkness at room temperature for 3 weeks, and then transferred to a Sanyo growth chamber (20 °C, 2000 lх, 90% humidity). The covers were removed when the culture began to form visible basidiomata.
Single spore isolation
Isolation of single spore isolates (SSIs) from fruited basidiomata was performed as follows: a slice of lamella was imbedded in a small aliquot of petroleum jelly on the inner side of the lid of a MEA Petri plate. The plate was tilted on edge overnight. From spores dropped on the medium surface, germlings were excised manually and individually onto separate plates. Between 16 and 20 SSIs of each strain were obtained. Resulting colonies were checked for the presence of clamp connections to exclude dikaryon isolates.
Molecular analyses
DNA was isolated from original herbarium specimens and from dikaryon cultures. A small fragment (100 mg) of a dried herbarium specimen was incubated in 400 μL of 2% CTAB for 3 days, then homogenized for 3 min using TissueLyser LT (Qiagen, Germany), and centrifuged (10 min, 10,000g). The supernatant was used for DNA extraction. Cultures were grown in baby-food jars on liquid malt extract media (15 g/L malt extract; Conda, Spain). After 4 weeks of cultivation in the dark at 25 °C, mycelia were separated from the substrates using a sieve, washed with distilled water, blotted with filter papers, and utilized for DNA extraction.
DNA extraction was performed using the protocol of the kit NucleoSpin Plant II (Macherey-Nagel, Germany). DNA extraction from the Kew authentic material Lentinus martianoffianus KM179349 and Panus lecomtei LE5834 was conducted using the E.Z.D.N.A Forensic Kit as described in Seelan et al. (2015). PCR amplification of the nuclear ITS rDNA region (fungal bar code) used the primers ITS1F and ITS4B (Gardes and Bruns 1993) and iQ Supermix (Bio-Rad). These primers were also used for dideoxy Sanger sequencing. PCR products were purified using the AxyPrep PCR Cleanup Kit (Axygen Biosciences, CA, USA). Sequencing was performed with an ABI model 3130 genetic analyzer (Applied Biosystems) using the BigDye™ Terminator Cycle Sequencing Ready Reaction Kit (AB). Raw data were processed using MEGA 7 and MEGA X; the final sequences were manually corrected using the same programs (Kumar et al. 2016, 2018). Complementary forward and reverse reads were assembled to obtain clean consensus sequences; ambiguous edges were trimmed. A BLAST algorithm was used for searching for closely related sequences from NCBI public database (https://www.ncbi.nlm.nih.gov). The newly obtained ITS sequences, some sequences from closely related taxa, and sequences of diverse species with pleurotoid basidiomata were combined in multiple alignment in MEGA X (Kumar et al. 2018), re-aligned using MAFFT online service (Katoh et al. 2019) with iterative refinement E-INS-I option, and corrected manually. Evolutionary divergence analyses between sequences were conducted in MEGA X using the maximum composite likelihood model (Tamura et al. 2004).
Phylogenetic analysis at the nrITS resolution inferred by using the maximum likelihood method (PhyML) was conducted in IQ-TREE 1.6.12 (Nguyen et al. 2015) by running at IQ-TREE web server (Trifinopoulos et al. 2016) with model parameters TIM2+F+I+G4 that were selected using the ModelFinder tool (Kalyaanamoorthy et al. 2017). To obtain confidence values for the branches, 1000 ultrafast bootstrap replicates were performed. Bayesian analysis (BA) was also performed on the same dataset using MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001) with GTR+I+G model for 10 million generations (sampling every 1000), four chains, and two independent runs. The quality of chains was estimated using Tracer 1.7.1 (Rambaut et al. 2018), according to which the first 10% of sampled trees were discarded for burn-in; the convergence of Markov chain Monte Carlo (MCMC) analyses was checked; and the posterior distribution of parameter values was estimated. The results, with the effective sample size (ESS) > 9000 and the potential scale reduction factor (PSRF) = 1, were accepted.
Sequences generated as part of this project are presented in the Supplementary Information (SI) (Online Resource 1). They were deposited in GenBank NCBI (https://www.ncbi.nlm.nih.gov/nuccore/). Their NCBI accession numbers marked with an asterisk are presented in Table 1. Alignment made in MAFFT and manually corrected, together with BA tree, was deposited in TreeBASE (TB2:S27931).
Results
Morphological study of herbarium material
Characteristics of type specimen of Le. pilososquamulosus, some Far Eastern material of putative Le. martianoffianus, and basidiomes of Li. vetlinianus obtained in culture on sawdust substrate are presented below.
Type specimen of Lentinus pilososquamulosus (dried herbarium material)
The sole pileus initially 18 × 10 cm divided into several pieces. Basidiomata crepidotoid, with subdorsal attachment, broadly cuneiform with slightly inrolled margin, arising deep within the substratum. Pileus surface clearly pubescent, hispid near the base with piles reaching 2-mm long, the color near “light ochraceous buff” with grayish tint in several areas. Context cream with several gelatinized isabelline streaks; gelatinized layer juxtaposed to lamellae, 1–1.5 mm thick, dark ochraceous. Lamellae of 1–3 ranks, 1–3 mm broad, thin, dark isabelline, entire, gradually narrowing downward on stipe, convergent on upper stipe, in herbarium crispate and of corneous consistency, exhibiting a glassy gelatinized trama. Partial veil absent. Stipe-like base hard-spongy, narrowing from the broadly cuneiform pileus, macroscopically homogeneous, of the same color as the context. Odor rather pleasant, fungous, not strong.
Hyphal system monomitic, with ubiquitous clamp connections. In pileus context, hyphae 2.2–12.0 μm wide (ampullate inflations up to 45 μm), with prominent wall, covered with resinous or crystalline incrustation. In gelatinized layers of pileus context, hyphae with thick deliquescent wall, 6–17 μm wide. In lamellar mediostratum, hyphae deliquescent, 1.7–3.5(7) μm. In subhymenium, hyphae frequently branched, intricate, 2–2.5 μm wide. Cystidia of subhymenial origin, of two types: (1) pleurocystidia 50–100 × 5.5–10.5 (modal limits 39–65 × 6–9) μm, rare, fusoid or cylindrical and somewhat flexuose, thin-walled, hyaline, or with yellowish contents, protruding from hymenium 0–35 μm; and (2) cheilocystidia 50–80 × 5–8 μm, numerous, more or less cylindrical, thin-walled, hyaline, protruding from the hymenium 10–20 μm. Basidia clavate, 25–45 × 5.5–8 μm, 4-spored, with basal clamp. Basidiospores (6.5)7–9(9.5) × 3.5–4.5 μm, ellipsoid-cylindrical, in some projections phaseoliform or subreniform, thin-walled, inamyloid, cyanophilous, smooth, or with adhering lipid globules.
The specimen was collected on unidentified frondose wood 26.06.1946 (coll. Lj. Vassiljeva) in the Komarovka River valley in Ussuriysk State Nature Reserve, Primorsky Krai (Fig. 1a–d).
Holotype (a–d) and epitype (e, f) material on Lentinus pilososquamulosus: a VLA herbarium label of L. pilososquamulosus with misapplied Notae criticae referring to L. martianoffianus; b dried holotype material; c drawing of fresh fruit body of L. pilososquamulosus type according to Vassilieva (1973, Pl. I, 2); d D. Pegler’s handwritten note devoted to microstructure analysis of L. pilososquamulosus holotype; e epitype of L. pilososquamulosus (dry fruit body underside); f epitype of L. pilososquamulosus (dry fruit body upperside). Scale bars b, e = 1 cm
Epitypification of Lentinus pilososquamulosus
Several attempts to isolate DNA from L.N. Vassilyeva’s holotype were unsuccessful, but we had at our disposal one more recent specimen from the same region, from which the nucleotide signature was obtained (KY349097; KY349096). Despite the fact that originally the name Le. martianoffianus was misapplied to this collection, formally, this misapplication can only be proven in the course of type studies. A Le. pilososquamulosus epitype from the topotype locality and morphologically congruent with the type was selected in order to its molecular comparison both with Li. vetlinianus (a priori conspecific), and with the Le. martianoffianus holotype (Kew Herbarium). Also, a priori, we understood that true Le. martianoffianus was a Panus representative, but no formal proof had yet been undertaken till now.
Dried herbarium specimen is demonstrated in Fig. 1e, f and described below.
The material presented by sole inrolled pileus 4.5 × 2.5 cm. Basidiome conchate, with sublateral attachment, inrolled, arising deep within the substratum. Pileus surface hispid, pale with grayish tint; margin tomentose, cream. Context cream with several gelatinized brownish streaks; gelatinized layer juxtaposed to lamellae, ca. 1 mm thick, dark ochraceous. Lamellae of 3 ranks, 1–2 mm broad, thin, dark melleous, entire, gradually narrowing downward on stipe, of corneous consistency, exhibiting a glassy gelatinized trama. Partial veil absent. Stipe-like base hard, of the same color as the context. Odor pleasant, fungous, or rather strong.
Hyphal system monomitic, with ubiquitous clamp connections. In pileus context, hyphae 2.0–11.0 μm wide, with prominent wall. In gelatinized layers of pileus context, hyphae with thick deliquescent wall, 6–20 μm wide. In lamellar mediostratum, hyphae deliquescent, 1.5–5.5 μm. In subhymenium, hyphae frequently branched, intricate, 2–3.0 μm wide. Cystidia of subhymenial origin, of two types: (1) pleurocystidia 45–85 × 5.0–11.5 μm, rare, fusoid, or cylindrical; and (2) cheilocystidia 45–75 × 5–7.5 μm. Basidia clavate, 23–45 × 5.0–8 μm, 4-spored, with basal clamp. Basidiospores (5)7–9 × 3.5–4.5 μm, ellipsoid-cylindrical, thin-walled, inamyloid, cyanophilous, smooth.
The fungus was collected on Betula sp. 27.07.2010 (coll. E.M. Bulakh) in a broadleaf forest (Muravyov-Amurskiy Peninsula, Primorsky Krai) and successfully cultured (LE-BIN 3649).
What is the Lentinus martianoffianus type?
This taxon was described by Thümen (1877) in his “Contribution to the fungal flora of Siberia” published in the Bulletin of the Moscow Society of Naturalists (punctuation and italics are original): “72. Lentinus Martianoffianus Kalchbr. Nov. spec. – L. pileo laterali, subsessile, membranaceo, coriaceo, pertenui, lobato, in disco verruculoso, ad limbum lineatocostato, molliter velutino, rufo-alutaceo; lamellis perangustis, confertissimis dentatis, pallidis, exsiccando rufescentibus; caro alba.—A proximo Lentino castreo Fr. omino differe videtur.—“Speciem distinctam censeo—sed specimennimis mancum.” Kalchbrenner. In trunco Populi balsamiferae Lin., pr. Minussinsk. – (no. 2.)”. Specimens of this species collected by Martianoff near Minussinsk (Russia) were distributed within exsiccate series by Thümen in 1880 (Fung. Exot. Dec. 21). Now the authentic material of the species is kept in the Herbarium of Kew. The specimen was studied by Pegler, who described its macromorphology and micromorphology in more detail indicating, in particular, a dimitic hyphal system with generative and skeletal hyphae (Pegler 1983). The type material exhibited similar morphology as Panus lecomtei (old name L. strigosus). The authentic material includes a strigose pileus with hairy margin that resembles that of basidiomata of Panus lecomtei. The presence of cheilocystidia, metuloids, and skeletal hyphae was similar as in Panus lecomtei (L. strigosus). The nrITS sequence of L. martianoffianus (type material) is clustered together with Panus lecomtei (see “Molecular analysis” below).
Morphology of Lignomyces vetlinianus/‘Lentinus martianoffianus’ basidiomata obtained in pure culture
Basidiomata were obtained by culturing on sawdust substrate of strains obtained in Siberia (LE-BIN 2612, Khanty-Mansiysk) and European Russia (LE-BIN 2335 and LE-BIN 2339, Moscow region; LE-BIN 3253 and LE-BIN 3668, Novgorod region). An example of natural basidiomata from the Khanty-Mansiysk region in comparison with cultured basidiomata of the same collection is illustrated in Fig. 2.
Basidiomata mainly 2.5–3.5 cm in largest dimension and gregarious on resupinate patches. In contrast to native basidiomata, which have root-like subdorsal stem, the basidiomes obtained in culture are resupinate without any discrete root; margin more crispate and undulating and on lateral orientation of substrate give rise to elongated Phyllotus-like processes. Pileus surface is ivory-white or cream, subtomentose (less strigose than in native basidiomata). Lamellae often sterile and the same color as upperside, but by maturity become honey or coffee-milk colored, on the manner of Panellus stypticus. In culture, LE-BIN 2335 maturation ceased at a stage with a pronounced peach shade and resembled the hymenophore of Phyllotopsis nidulans.
The microcharacters vary within limits characteristic to the species: hyphae 2.2–15.6μm wide (ampullate inflations up to 18 μm wide), pleurocystidia 45–67 × 6–9.5 μm, cheilocystidia 45–77 × 5.5–8 μm, basidia clavate 25–45 × 5.2–7.5 μm, and basidiospores 7.2–9.2 × 3.4–4.6 μm.
The morphological study of preserved and cultured basidiomata indicated a close correspondence of all the main macromorphological and micromorphological characteristics of Le. pilososquamulosus type and the modern description of Li. vetlinianus (Petersen et al. 2015).
Physiological and molecular studies
Growth and morphology
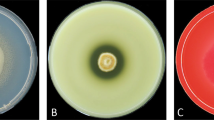
Culture growth and morphology of Lignomyces vetlinianus strains LE-BIN 2335, LE-BIN 2339, and LE-BIN 3253 from Moscow and Novgorod regions have already been described previously (Petersen et al. 2015; Sazanova et al. 2018). A comparative cultural study of European, Siberian, and Russian Far Eastern strains showed their ability to grow in temperature diapason 5–35 °C. Optimum growth temperature was 25–30 °C, depending on the strain (Fig. 3). The Caucasian strain LE-BIN 3907 exhibited growth at 15–30 °C. At 5 and 35°C, only inoculum plugs covered with short erect hypha, without growing on fresh substrate. After 2 weeks of incubation at these extreme temperatures, the strain recovered at 25 °C faster after incubation at 5 °C than at 35 °C. The diameter on MEA at 25°C for the 11-day colonies was 45.3 ± 3.5 mm after 5 °C and 25.3 ± 2.4 mm after 35 °C. Growth rate of the studied strains at 25 °C varied from 1.2 ± 0.1 mm/day (LE-BIN 2335) to 3.1 ± 0.1 mm/day (LE-BIN 3253) on MEA and from 1.2 ± 0.2 mm/day (LE-BIN 2335 and LE-BIN 3907) to 2.5 ± 0.1 mm/day (LE-BIN 3592) on PDA (Fig. 4). The majority of strains survived after freezing inoculum plugs in Petri plates at −20 °C for 2 weeks. The strain LE-BIN 3907 was the only one that did not recover after freezing.
The variety of colony morphologies of the examined strains at 4 weeks on MEA is given in Figs. 5 and 6. On MEA, the advancing zone was even, with hyphae surface or submerged. Aerial mycelium was mostly zonal, with thin white or almost transparent more or less radially oriented hyphae, forming fibrous bands. By 4 weeks, strains formed basidiomata (LE-BIN 3253, LE-BIN 3592, and LE-BIN 3668) or primordia (LE-BIN 2339, LE-BIN 2612, and LE-BIN 3649). On PDA, strains formed two types of colonies: slow-growing, surface, thin matte hyphae (LE-BIN 2339 and LE-BIN 2612) and others faster growing, with white radial fibrous hyphal bands. Strains LE-BIN 3253 and LE-BIN 3649 formed distinctive plumose colonies. The advancing zone was even, hyphae mostly submerged. By 4 weeks, basidiomata did not appear on PDA; primordia were observed only in plates of LE-BIN 3253 (Fig. 6). Moreover, some strains, especially LE-BIN 2335, LE-BIN 2339, and LE-BIN 3592, produced on MEA and PDA the same agglomerates of crystals that previously were observed (Petersen et al. 2015) and later were identified as phthalides (Sazanova et al. 2018). It was noted that the LE-BIN 3907 strain, incubated for 2 weeks at 35 °C and growing afterwards at 25 °C, produced the phthalides crystals more intensively than without pre-incubation at high temperature.
Micromorphology of studied strains did not differ significantly. On both media, hyphae of the advancing zone were thin-walled, branched, 1.5–4.5(5) μm wide, with almost universal clamps. Aerial hyphae were also branched, 1.5–5.0 μm wide; with regular clamps, anastomoses, hyphal rings, encrusted hyphae, and terminal and intercalary swellings 9.0–12.5 × 10.0–13.5 μm in diameter were observed. Substrate hyphae were the same in diameter, but shorter than aerial, also branched, with clamps, short brush-like hyphal tufts, and regularly terminal and intercalary swellings presented. Numerous crystals were observed under the microscope in all strains.
Mating study
A tetrapolar mating system was previously determined for Lignomyces vetlinianus (Petersen et al. 2015). In this study, four SSIs obtained from basidiomata of each strain cultured on sawdust were chosen randomly for the mating experiment. The SSIs were paired in all combination. The experiment revealed 100% compatibility, indicating that all studied strains (i.e., Li. vetlinianus LE-BIN 2335, LE-BIN 2339, LE-BIN 3253, and LE-BIN 3668; ‘Lentinus strigosus’ LE-BIN 3592; and ‘Le. martianoffianus’ LE-BIN 2612 and LE-BIN 3649) belonged to the same biological species (Fig. 7). There were no SSIs of putative Le. pilososquamulosus involved in the mating experiment since the species was represented in our study only by the herbarium type specimen.
Molecular analysis
Sequences of the ITS (ITS1–5.8S–ITS2) region of the rDNA were obtained in this study for five strains: LE-BIN 2612 from Siberia, LE-BIN 3592 from Bryansk region, LE-BIN 3649 from the Far East, LE-BIN 3668 from Novgorod region, and LE-BIN 3907 from Western Caucasus. The strains of Li. vetlinianus from Novgorod region LE-BIN 2335, LE-BIN 2339, and LE-BIN 3253 were sequenced earlier (Petersen et al. 2015). Four of the studied herbarium specimens were also successfully sequenced. Extraction of DNA from the type specimen of Le. pilososquamulosus was not successful, probably due to the long storage in the herbarium and chemical treatment of the specimen. At the same time, sequencing of the type herbarium specimen of Le. martianoffianus KM179349 (Kew Herbarium) originated from Russia (vic. Minusinsk, Krasnoyarsk Krai) was successful together with a specimen of Panus lecomtei LE5834 (Bashkortostan Republic, Russia). A total of 11 ITS sequences generated in this study were used for analysis. The sequences from herbarium and culture material were aligned to evaluate their identity. The alignment showed two sets with high homology—Panus group (type Le. martianoffianus and Panus lecomtei) and Lignomyces group (Li. vetlinianus, ‘Le. martianoffianus’, and ‘Le. strigosus’).
A BLAST search for closely related sequences from the NCBI public database showed query cover 99–100% and a high percent identity (98.39–100%) of the majority of specimens sequenced in the present research and with Lignomyces vetlinianus strains LE-BIN 2335 and LE-BIN 2339, and their voucher herbarium specimens sequenced in the previous study (Petersen et al. 2015). The specimen VLAM-19125 showed a query cover 98–99% and 97.61–98.56% identity with Lignomyces specimens examined in this study.
The nrITS phylogeny contains diverse species with pleurotoid basidiomata and species potentially related with Lignomyces (Pleurotus, Hohenbuehelia, Neonothopanus, Phyllotopsis, Resupinatus, Panus, and Lentinus). Plicaturopsis crispa as a pleurotoid representative of a basal lineage to the considered agaricomycete crown group (Agaricales and Polyporales) was selected as an outgroup. The alignment used for PhyML had 45 sequences with 741 nucleotide sites, 529 distinct patterns, 411 parsimony-informative, and 277 constant sites. The PhyML consensus tree constructed from 1000 bootstrap trees with the highest log likelihood (−8401.5) is shown in Fig. 8. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site.
PhyML analysis based on the ribosomal ITS region. Support values to the left of each node are posterior probabilities from Bayesian analysis > 0.95 and ML bootstrap values > 80% based on 1000 replicates. Boldface lines indicate bootstrap support greater than 90%. Asterisks indicate sequences generated as part of this study. Inverted commas indicate misapplied names. Position in classification of submitted taxa: I, Agaricales; II, Polyporales; and III, Amylocorticiales
The pairwise distance analysis on the maximum composite likelihood model showing the number of base substitutions per site from between sequences revealed 0.0017 distance between sequences in the Panus lecomtei clade. In the Lignomyces clade, the greatest distance between ITS sequence of the herbarium specimen from the Far East VLAM-19125 and sequences of the other specimens did not exceed 0.012. Distances between sequences of the other studied Lignomyces-like specimens varied between 0.0016 and 0.0082. The results on evolutionary divergence between Lignomyces sequences are presented in the SI (Online Resource 2).
Discussion
Initially, the taxon under discussion was described by S. Domański under the name Pleurotus vetlinianus (Domański 1964) and later transferred to the genus Resupinatus (Moser 1979). The decision of Moser seems unusual only at first glance: despite the fact that this species is characterized by rather large, fleshy basidiomes (while the most well-known representative of the genus, Resupinatus applicatus, produce reduced basidiomata), all the microstructures of “Pleurotus vetlinianus” correspond to those tropical representatives of the genus Resupinatus, characterized by ellipsoidal-cylindrical spores, whereas temporal representatives have subglobose spores (Thorn, Barron 1986). Until now, the systematic position of “P. vetlinianus” remained uncertain. Comparative studies of the region of the ribosomal DNA cluster, in general, confirmed the affiliation of Li. vetlinianus to the phylogenetic radiation of Resupinatus (Resupinataceae, Agaricales), although they allowed for the monotypic genus Lignomyces (Petersen et al. 2015).
Morphological examination of Le. pilososquamulosus type specimen revealed identity with other studied Far Eastern specimens, and one of which was chosen as an epitype of Le. pilososquamulosus. Since the complex investigation of specimens from Eastern Siberia and the Russian Far East, collected as Le. martianoffianus, and the specimen from Bryansk region, previously identified as Le. strigosus, showed that they were the same organism as specimens of Li. vetlinianus from Moscow and Novgorod regions, association of these specimens with a name other than Li. vetlinianus must be considered as misidentification. Results obtained in this investigation lead us to conclusion that synonymization would be the best solution for the two names. Considering later description of Lentinus pilososquamulosus (Vassilieva 1973) than Pleurotus vetlinianus (Domański 1964) as well as further taxonomic revision of Petersen and co-authors, the correct combination for this taxon should remain Lignomyces vetlinianus (Domański) R.H. Petersen & Zmitr. (Petersen et al. 2015), and Lentinus pilososquamulosus Lj. N. Vassiljeva would go under synonymy.
Concerning true Le. martianoffianus, analysis of Pegler’s detailed description (Pegler 1983) let Zmitrovich with co-authors assume the close relationships of Lentinus martianoffianus with Panus lecomtei (Zmitrovich et al. 2018). Malkovský (1932) considered Le. martianoffianus as a synonym of Panus rudis, whereas Pegler (1983) abstained from synonymization. Thus, this taxon, even basing on morphological characters, was assigned to the genus Panus, although there have been attempts to misapply this name to Le. pilososquamulosus (Bulakh et al. 2010; Bulakh 2015). Our molecular analysis confirmed the position of Le. martianoffianus in a P. lecomtei clade (Fig. 8).
Our cultural study showed that all strains involved in the experiment, excluding one—LE-BIN 3907—could survive prolonged freezing (−20°C) and grow in the relatively wide (5–35 °C) temperature range (Fig. 3), which is typical for the representatives of the temperate zone and agrees with the strains’ geographical origins: (1) area of Atlantic and the continental influence, southern taiga (LE-BIN 2335, LE-BIN 2339, LE-BIN 3253, LE-BIN 3592, LE-BIN 3668); (2) continental West Siberian (forest) area, taiga (LE-BIN 2612), and monsoon, the continental influence in the winter and Pacific in the summer, taiga (LE-BIN 3649). Nevertheless, Li. vetlinianus strain LE-BIN 3907 found in the subtropical zone in Abkhazia grew at 15–30 °C, survived at 5 and 35 °C recovering the growth activity after replacing at 25 °C, and lost its viability after freezing at −20 °C. Probably the reason of such sensitivity of this strain to freezing and extreme temperature values could be its Caucasian origination—the geographical zone with wet climate and mild winters.
The studied Li. vetlinianus strains appeared to be rather slowly growing cultures with growth rate range 1.2–3.1 mm per day (Fig. 4) and some difference in macromorphology within the intraspecific strain diversity and depending on the medium used (Figs. 5 and 6). However, the strains had common colony characters—thin spread on the surface zonate mycelium with more or less expressed radial fibrous hyphal bands, abundant fruiting in culture, and visible crystal conglomerates appearing on the colonies with age. The micromorphology of all studied strains was identical, without difference on employed media and species-specific characters—branched, 1.5- to 5-μm wide hyphae, regular clamps, and numerous crystals.
Biological conspecificity was confirmed by universal sexual compatibility in a mating experiment (Fig. 7) and by high percent homology of the ITS sequences (97.6–100%). Phylogenetic analysis indicated the clustering of all studied Lignomyces-like specimens as a monophyletic taxon with a 100% bootstrap support (Fig. 8).
Thus, it can be concluded that authentic Le. martianoffianus closely relates to Le. strigosus and P. lecomtei and was associated with Lignomyces by mistake. Originally misidentified specimens and strains should be relabeled as Lignomyces vetlinianus. The Far Eastern Le. pilososquamulosus and the European Li. vetlinianus represent the same species and should be synonymized with priority name Li. vetlinianus [=Le. pilososquamulosus]. The present study broadens our understanding of the biology of Li. vetlinianus and shows that the geographic distribution of this species extends from Europe south to the Abkhazia on Western Caucasus and through Western Siberia to the Russian Far East.
Data availability
A data availability statement is present within the text. The datasets generated as part of this project are presented in the Supplement Information (SI) (Online Resource 1); generated sequences were deposited to the GenBank (NCBI). The results on evolutionary divergence between Lignomyces sequences are presented in the SI (Online Resource 2). Alignment and BA tree were deposited in TreeBASE: http://purl.org/phylo/treebase/phylows/study/TB2:S27931.
References
Bulakh EM (2015) Fungi of the forests of Far East of Russia. Dalnauka, Vladivostok [In Russian]
Bulakh EM, Vassiljeva NV, Erofeeva EA (2010) The first data about fungi basidiomycetes of the “Bureinskiy” nature reserve. Mikologiya i fitopatologiya 44:89–98 [In Russian]
Domański S (1964) Pleurotus vetlinianus sp. nov. Acta Societatis Botanicorum Poloniae 33:243–246
Gardes M, Bruns TD (1993) ITS primers with enhanced specification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Kalyaanamoorthy S, Minh BQ, Thomas KF, Wong TKF, Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20:1160–1166. https://doi.org/10.1093/bib/bbx108
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Malkovský KM (1932) Über die europäischen Arten der Gattung Panus. Annales Mycologici 30:10–80
Moser M (1979) Über einige neue oder seltene Agaricales-Arten aus dem Pieniny und aus Biesczciade, Polen. Sydowia Beiheft 8:268–275
Nguyen L-T, Schmidt HA, Arndt von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Pegler DN (1983) The genus Lentinus: a world monograph. Kew Bulletin additional series X. L.:1–281
Petersen RH, Psurtseva NV, Zmitrovich IV, Chachuła P, Arslanov S, Hughes KW (2015) Lignomyces, a new genus of pleurotoid Agaricomycetes. Mycologia 107:1045–1054. https://doi.org/10.3852/14-355
Psurtseva NV (2010) Conservation of medicinal mushrooms in the LE (BIN) culture collection. Int J Med Mushrooms 12:193–199. https://doi.org/10.1615/IntJMedMushr.v12.i2.100
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology. syy032. https://doi.org/10.1093/sysbio/syy032
Red book of Croatian fungi / Ed Tkalčec Z, Mešić A (2008) Tiskara Zelina, Zagreb
Sazanova KV, Psurtseva NV, Shavarda AL (2018) Cultural and metabolomic studies of a new phthalides producer Lignomyces vetlinianus. Int J Med Mushrooms 20:1031–1045. https://doi.org/10.1615/IntJMedMushrooms.2018028687
Seelan JSS, Justo A, Nagy LG, Grand EA, Redhead SA, Hibbett DS (2015) Phylogenetic relationships and morphological evolution in Lentinus, Polyporellus and Neofavolus, emphasizing southeastern Asian taxa. Mycologia 107:460–474. https://doi.org/10.3852/14-084
Stavishenko IV, Zmitrovich IV (2017) The first find of Lignomyces vetlinianus (Tricholomataceae, Agaricomycetes) in the Urals. Mikologiya i fitopatologiya 51:60–63
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA) 101:11030-11035
Thorn RG, Barron GL (1986) Nematoctonus and the tribe Resupinateae in Ontario, Canada. Mycotaxon 25:321–454
Thümen F (1877) Beiträge zur Pilze-Flora Sibiriens. Byulleten Moskovskogo obshchestva ispytateley prirody 54:128–152
Trifinopoulos JL-T, Nguyen A, von Haeseler BQ, Minh (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. https://doi.org/10.1093/nar/gkw256
Vassilieva LN (1973) Agarikowie shlyapochnye griby (poryadok Agaricales) Primorskogo kraya [Die Blätterpilze und Röhrlinge (Agaricales) von Primorye Region]. Nauka, Leningrad [In Russian]
Zmitrovich IV, Malysheva VF, Malysheva EF (2009) The hyphal types of polyporoid and pleurotoid fungi: a terminology revision. Ukrainian botanical journal 66:71–87 [in Russian]
Zmitrovich IV, Bondartseva MA, Perevedentseva LG, Myasnikov AG, Kovalenko AE (2018) The Meruliaceae of Russia. II. Panus. Turczaninowia 21:29–44
Acknowledgements
The study was performed within the framework of the institutional (BIN RAS) research project “Biodiversity, ecology, structural and functional features of fungi and fungus-like protists” АААА-А19-119020890079-6, using equipment at The Core Facility Centre at the Komarov Botanical Institute RAS. The authors thank Vera Malysheva and Ilya Prikhod’ko for helpful advices on using online service programs for phylogenetic analysis, and anonymous reviewers for useful comments and suggestions on the manuscript improvement.
Funding
This work was partially funded by the Russian Foundation for Basic Research (project no. 15-04-06211).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Nadezhda Psurtseva, Ivan Zmitrovich, Eugenia Bulakh, and Jaya Seelan; analysis and discussions were performed by Nadezhda Psurtseva, Ivan Zmitrovich, Roland Petersen, and Karen Hughes. The first draft of the manuscript was written by Nadezhda Psurtseva and Ivan Zmitrovich and revised by Ronald Petersen and Karen Hughes. All authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Zhu-Liang Yang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Psurtseva, N.V., Zmitrovich, I.V., Seelan, J.S.S. et al. New data on morphology, physiology, and geographical distribution of Lignomyces vetlinianus, its identity with Lentinus pilososquamulosus, and sufficient phylogenetic distance from Le. martianoffianus. Mycol Progress 20, 809–821 (2021). https://doi.org/10.1007/s11557-021-01701-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-021-01701-z