Abstract
Fungi belonging to the genus Induratia are endophytes that have received considerable attention because of the production of natural bioactive secondary metabolites, such as volatile compounds, with antimicrobial activity. In this study, we distinguished I. coffeana and Induratia sp. isolated from Coffea arabica in Brazil based on three-loci phylogeny, ITS, RPB2, and TUB2. The Induratia isolates showed high morphological plasticity and produced different volatile organic compounds, indicating that these compounds might not be correlated with their phylogenetic assignment. However, PLS-DA was able to discriminate Induratia isolates into three different clusters: one associated with I. coffeana and two with Induratia sp. Induratia isolates showed biofumigant activity against Botrytis cinerea with emphasis on I. coffeana (CML 4019), which inhibited the pathogenic fungus in postharvest strawberries. Nematicidal activity against Meloidogyne incognita was also observed in filtrates and volatile compounds produced by Induratia isolates. Moreover, we observed that I. coffeana (CML 4019) metabolites showed antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, and E. faecium and that extracts of seven other Induratia isolates reduced the pre-formed biofilm of Staphylococcus aureus and Staphylococcus epidermidis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the diverse microbial communities that inhabit plants internally, endophytic fungi interact with host plants, increasing their productivity and resistance to biotic and abiotic stresses. These microorganisms can use different mechanisms during symbiotic interactions, including the synthesis of a large number of specific secondary metabolites that play many different physicochemical and biological roles, such as enzymes, antibiotics, toxins, soluble compounds, and volatile organic compounds (VOCs). Some of these compounds are used for the development of pharmaceuticals and agrochemicals, while others as mycotoxins tend to be more problematic because of their widespread occurrence as contaminants of food for humans and livestock, as well as mold-contaminated indoor environments (Hardoim et al. 2015; Lata et al. 2018; Hyde et al. 2019; Yadav 2019). Three complementary reviews cover a good part of literature about the breadth of fungal secondary metabolite diversity, with information of the biosynthesis of the most important fungus-derived metabolites including those produced from Xylariales, their biological activities, and recent developments that have contributed to human health and agriculture (Bills and Gloer 2016; Helaly et al. 2018; Becker and Stadler 2021).
The genus Muscodor, an important endophyte previously accommodated in the order Xylariales, has been reported as an efficient biocontrol agent for the synthesis of VOCs. Muscodor albus, strain cz620 isolated from Cinnamomum zeylanicum, showed production of VOCs with broad antimicrobial activity, and it was the first biological control agent commercially available and effective in controlling fungal decay in apples and peaches, green mold, and sour rot of stored lemon and damping-off disease of sugar beet by mycofumigation (Strobel et al. 2001; Worapong et al. 2001; Worapong et al. 2002; Mercier and Jiménez 2004; Mercier and Smilanick 2005; Strobel and Ezra 2005; Grimme et al. 2007; Hutchings et al. 2017).
Since the description of M. albus by Worapong et al. (2001), 26 Muscodor species from different hosts and ecological niches have been reported. However, the identification of most species was based on cultural morphology, VOC profiles, and molecular phylogenetic analyses using the ITS rRNA gene, which is highly questionable. Stadler et al. (2013) reviewed the nomenclatural changes for taxonomy of Xylariaceae after the introduction of the One Fungus-One Name (1F1N) concept and suggested the abandonment of some ill-defined anamorph genera, such as Muscodor. The authors presented confident arguments to do not integrate Muscodor into the Xylariaceae. More recently, Chen et al. (2019) concluded that the single ITS region cannot resolve Muscodor species and described a new species named Muscodor yunnanensis using four loci (ITS rRNA, 28S rRNA, RPB2, and TUB1) in the phylogenetic analyses. Certainly, incongruities and apparent mismatches between phenotypes and genotypes observed when ITS is used as the primary barcode for some families (Xylariaceae) and genera of fungi result from the number of copies intragenomic of rDNA genes and differences in nucleotide identity (Stadler et al. 2020). Thus, these authors propose that additional genomes should be checked for such ITS polymorphisms to reassess the validity of this non-coding part of the fungal DNA for molecular identification. Finally, Samarakoon et al. (2020) used ITS, LSU, RPB2, and TUB2 DNA sequences of 89 isolates, transferred all Muscodor species to Induratia, and proposed the new family Induratiaceae. They also reported some probable structures of sexual states as apiospores and described the new species I. ziziphi, isolated from a dead branch of Ziziphus sp., and I. thailandica from a dead unknown host wood.
Until then, in addition to molecular identification, the Muscodor species discrimination included morphological characteristics and VOC profiles assessed by gas chromatography coupled with mass spectrometry. However, the quality of data obtained appears highly questionable, as few concise study comparatives included type and authentic isolates of Xylariales, which were originally used to erect the genus Muscodor (Stadler et al. 2013). Samarakoon et al. (2020) reported for the first time the production of conidiophores, and apiosporous ascospores to Induratia species, which are morphologically distinct from the Xylariales. In the same study, the authors reported that secondary metabolite profiles can be valuable for chemotaxonomic purposes, but the data require a high degree of standardization once secondary metabolite production is dependent on the culture medium and the growth phase and inclusion of a significant number of species.
In Brazil, Induratia coffeana, Induratia yucatanensis, and Induratia vitigena have been isolated from the stems and leaves of Coffea arabica (Hongsanan et al. 2015; Monteiro et al. 2017), and Induratia braziliensis has been isolated from the leaves of Schinus terebinthifolius, a Brazilian medicinal plant (Pena et al. 2019). These species produce VOCs with antifungal activity against Rhizoctonia solani, Fusarium oxysporum, Phoma sp., Botrytis cinerea, Fusarium solani, Fusarium verticillioides, Cercospora coffeicola, Pestalotia longisetula, Aspergillus ochraceus (Monteiro et al. 2017), and Penicillium digitatum (Pena et al. 2019). Induratia species also reduced the symptoms of disease caused by three phytopathogens, Colletotrichum lindemuthianum, Sclerotinia sclerotiorum, and Pseudocercospora griseola, when re-inoculated in common bean seedlings (Mota et al. 2021). In addition, these fungi produce non-volatile secondary metabolites, including extracellular amylase, cellulase, lipase, pectinase, phytase, protease, endo β-1,4 glucanase, and exo β-1,4 glucanase, and molecules that modulate enzymes that act in human hemostasis (Bastos et al. 2020; Monteiro et al. 2020).
Here, we report the identification based on the phylogeny of three loci (ITS, RPB2, and TUB2), morphological characterization, and VOC analysis of 12 Induratia isolates obtained from coffee plants growing spontaneously in a secondary forest in Brazil. We also show the antifungal, antibacterial, and anti-nematode activities of VOCs and non-volatile metabolites produced by these endophytic fungi.
Material and methods
Fungal isolates
In this study, we evaluated twelve endophytic Induratia isolates, deposited in the Coleção Micológica de Lavras (CML) at the Departamento de Fitopatologia at the Universidade Federal de Lavras (UFLA), Brazil (Table 1). They were isolated from fresh and healthy leaves and stems of coffee plants (Coffea arabica) growing spontaneously in a secondary forest in Mata do Paraíso (20° 48′ 03.4″ S, 42° 51′ 42.6″ W), Zona da Mata region, Viçosa, Minas Gerais, Brazil.
DNA extraction, amplification, and sequencing
Fresh mycelia scraped from the margin of colonies grown on 2% malt extract broth (Himedia Laboratories, Mumbai, India) was used for DNA extraction using the Wizard® Genomic DNA Purification Kit (Promega, São Paulo, Brazil) according to the manufacturer’s protocol. The ITS was amplified using the primers ITS5 and ITS4 (White et al. 1990), and the following PCR conditions were used: initial denaturation at 95 °C for 2 min; 35 cycles of 95 °C for 1 min, 50 °C for 1 min, and 72 °C for 1 min; and a final extension for 10 min at 72 °C. A portion of the second largest subunit of RNA polymerase II (RPB2) was amplified using the primers 5F2 and 7cR (Liu et al. 1999) with the following cycling conditions: 95 °C for 2 min; 30 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min; and a final extension for 10 min at 72 °C. β-tubulin (TUB2) was amplified using the primers T1 and T22 (O’Donnell and Cigelnik 1997) under the following cycling conditions: 95 °C for 2 min; 30 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min; and a final extension for 10 min at 72 °C. PCR reactions were performed using the GoTaq® Colorless Master Mix kit (Promega, São Paulo, Brazil) in a MyCycler thermal cycler (Bio-Rad, Hercules, USA). The amplified fragments were purified using the Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, USA). Bidirectional DNA sequences for each region were generated by Macrogen (Rockville, Maryland, USA), using the Sanger method using the same primers used for PCR amplification.
Phylogenetic analyses
Consensus sequences were obtained from a bidirectional DNA sequence using the SeqAssem ver. 07/2008 (Hepperle 2004). Sequences from the type and reference of Induratia species available in GenBank were added to the analyses (Table 1). Multiple sequence alignments were performed using Clustal W, as implemented in MEGA X (Kumar et al. 2018). Phylogenetic analyses of each gene separately were carried out to verify which of the gene regions would be more informative for the delimitation of Induratia species, as well as for the combined dataset (ITS-RPB2-TUB2). Analyses were performed with maximum parsimony (MP) and maximum likelihood (ML) using Mega X with 1000 bootstrap replications. The most suitable substitution model was determined based on the lowest Bayesian information criterion (BIC) using Mega X, and the parameters of the matrices used to generate phylogenies are shown in Online Resource 1. Emarcea castanopsidicola and E. eucalyptigena were used as outgroup taxa. The alignments were deposited in TreeBASE (www.treebase.org; study number: S28186). URL:http://purl.org/phylo/treebase/phylows/study/TB2:S28186?x-access- code = 19e96d3d50543e82ca8d617a8e8334a5&format = html.
Morphological characterization
Induratia isolates were incubated on potato dextrose agar (PDA) medium at 25 °C for 7 days to assess the differences in growth rates and colony morphologies. Then, mycelial discs (5 mm diameter) were excised from Induratia colonies and transferred to Petri dishes containing one of the following media: PDA, synthetic nutrient-poor agar (SNA), cornmeal agar (CMA), and water agar (WA) at 25 °C for 15 days. The isolates were also inoculated on the center of a Petri dish containing PDA and incubated in darkness at 15, 20, 25, and 30 °C. After 15 days of incubation, colony characteristics were observed, and their diameters were measured. The growth rate was determined by measuring the colony area in three replicates. The growth rates and colony morphology were also evaluated at 25 °C for 40 days in Petri dishes containing PDA, in addition to odor production and coil presence. The coils and hyphae were also measured. The characteristics of colonies were analyzed by visual inspection, and the morphology was microscopically analyzed using the Induratia isolates cultivated on PDA and SNA media. Light microscopy was performed with the Zeiss observer Z.1 Epifluorescence Microscope with Apotome System and Zeiss Axion Vision software at the Laboratório de Microscopia Eletrônica e Análise Ultraestrutural at the Universidade Federal de Lavras, Brazil. Images were processed using Corel Draw software.
For electron microscopy (SEM), discs of 10-day-old PDA and SNA cultures grown at 25 °C were fixed in Karnovsky solution (2.5% glutaraldehyde and 2.5% paraformaldehyde in 0.05 M sodium cacodylate buffer, CaCl2 0.001 M, pH 7.2) for 24 h. The samples were dehydrated in an ascending series of acetone solutions (25%, 50%, 75%, 90%, and 100% in triplicate) for 10 min at each step. The samples were brought to a critical point, dried, and coated with gold. The structures were observed using a LEO EVO 40 scanning electron microscope at the Laboratório de Microscopia Eletrônica e Análise Ultraestrutural at the Universidade Federal de Lavras, Brazil. The images were digitally generated and recorded at a working voltage of 20 kV and a working distance of 9 mm. Images were processed using Corel Draw software.
Characterization of VOCs
The VOC analyses of Induratia isolates were conducted at the Centro de Análises e Prospeção Química (CAPQ) of the Universidade Federal de Lavras, Brazil. The fungi were inoculated in duplicates in 20 mL SPME vials containing PDA medium, and after 7 days, the VOCs were extracted by headspace solid-phase microextraction (SPME) (Arthur and Pawliszyn 1990). The SPME extraction was performed as follows: divinylbenzene, carboxen, and polydimethylsiloxane (DVB/CAR/PDMS) fiber, extraction temperature of 55 °C at 250 rpm, extraction time of 35 min, and desorption time of 2 min in the GC injector. A GC-MS QP 2010 Ultra (Shimadzu, Japan) gas chromatograph coupled with a mass spectrometer equipped with an AOC-5000 (Shimadzu, Japan) automatic injector for liquids and gases and an HP-5 (5% phenyl-95% dimethylsiloxane) 30 m × 0.25 mm × 0.25 μm column was used to separate the VOCs. The injector, interface, and ion detector temperatures were 250 °C, 240 °C, and 200 °C, respectively. The injector was operated in splitless mode. The carrier gas was grade 5.0 He with a flow of 1.0 mL min−1. The GC oven temperature was increased at a rate of 3 °C min−1 from 40 to 160 °C and then at 10 °C min−1 to 240 °C. The VOCs were identified by comparing the mass spectra obtained via the Automated Mass Spectral Deconvolution and Identification System (AMDIS) v. 2.6 software to those in the NIST library using the Mass Spectral Search Program v. 1.7 (NIST, Washington DC, USA) software (https://webbook.nist.gov/chemistry/). For comparison of the mass spectra, only spectra with a similarity greater than 80% were considered. To improve identification, experimental retention indices (RIExp) were obtained by injecting a homologous series of alkanes and comparing them with those reported in the literature (RI Lit) (Adams 2007).
Partial least squares: discriminant analysis
The total ion chromatograms of the VOCs were arranged in a matrix with 24 samples × 4922 chromatographic signals and then subjected to partial least squares discriminant analysis (PLS-DA) against the classes (I. coffeana and Induratia sp.) from the maximum likelihood phylogenetic tree based on concatenated ITS-RPB2-TUB2 sequences. The proper number of latent variables (LV) was determined by leave-one-out cross-validation. All calculations and graphs were performed using the Chemoface software version 1.64.
Activity VOCs of Induratia isolates against Botrytis cinerea
The effect of VOCs produced by Induratia isolates on the growth of B. cinerea was determined using bipartite Petri dishes containing PDA medium. A mycelial disc of each endophytic strain (5 mm diameter) was cultivated for 7 days at 25 °C on one side of the plate. After this period, the pathogen was placed on the other side of the plate. The effect of volatile compounds was evaluated by measuring the average diameter of pathogen growth after 7 days of exposure to volatile compounds. The experiment was carried out in a randomized design with 13 treatments and three replicates each. The negative control contained only a pathogen plug. The fungistatic or fungicidal activity of the VOCs produced was evaluated by aseptically transferring conidia of B. cinerea from bipartide Petri dishes to new PDA medium.
Botrytis cinerea was cultivated on PDA medium for 7 days at 25 °C. A suspension of 1.0 × 105 conidia/mL was inoculated into the strawberries. Two inoculated strawberries were deposited in a polyethylene terephthalate box containing an open Petri dish with an Induratia strain previously cultivated on PDA medium for 7 days. The boxes were sealed with a plastic film and incubated at 25 °C. After 72 h, the effect of the volatile compounds produced was evaluated based on the presence or absence of pathogen growth on the inoculated strawberries. The negative control consisted of boxes with strawberries inoculated with B. cinerea without endophytic fungi. The experiment was carried out in a randomized design with 13 treatments, each with three replicates.
Activity of metabolites of Induratia isolates against pathogenic bacteria
The fungi were grown in plates containing PDA medium for 7 days at 25 °C. After growth, 5 discs approximately 5 mm in diameter from the fungal colonies were transferred to 1 L of PD medium and incubated at 25 °C in the dark at 125 rpm for 12 days. The supernatants were separated from the mycelia by vacuum filtration. The extraction was carried out with the addition of ethyl acetate to the supernatants, in the proportion of 1:0.5 (supernatant/ethyl acetate), followed by removal of the solvent by rotary evaporation.
Extracts of Induratia isolates were tested against the following bacterial strains of clinical importance to determine their antibacterial activity: Staphylococcus epidermidis ATCC 35984, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, E. faecium ATCC 700221, Klebsiella pneumoniae ATCC 700603, Escherichia coli ATCC 25922, Acinetobacter baumannii ATCC 19606, and Pseudomonas aeruginosa ATCC 27853.
Each extract was diluted in 100% DMSO and filtered through a 0.22 μm filter to prepare a 100 × concentrated stock solution to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). This solution was diluted 1:100 in Cation Adjusted Mueller Hinton broth (CAMHb) (BBL Mueller–Hinton II broth; Becton, USA) to reach a final concentration of 1% DMSO according to CLSI (2015), followed by two-fold serial dilutions in 1% DMSO CAMHb to reach a range of 512 to 0.06 μg/mL in the 96 wells microplates. Bacteria colonies grown in CAMHb for 24 h were added to fresh media in tubes to adjust the suspension to 0.5 McFarland, followed by a 1:10 dilution and distribution of 5 μL pipetted in each microplate well. The tests were performed in duplicate. The results were observed after 24 h of incubation at 37 °C, and the MIC was considered the concentration at which microorganism growth inhibition could be visually observed. No extract was added to 1% DMSO CAMHb as a positive control for bacterial growth. As a negative control, only the broth was incubated to rule out any contamination.
After visual reading of the MIC, the MBC was determined using 100 μL of the following wells inoculated as a single drop on a CAMH-Agar plate: the well containing one dilution above the MIC, the well containing the MIC, and two wells containing concentrations below the MIC. The plate was incubated at 37 °C for 24 h prior to the visual observation. MBC had the lowest concentration, with no bacterial growth. The MBC/MIC ratio was calculated to classify the activity as bactericidal or bacteriostatic. Compounds with a rate less than or equal to four were considered bactericidal; rates above four indicated bacteriostatic activity (Pankey and Sabath 2004).
The biofilm eradication abilities of the extracts of Induratia species were evaluated as previously described by Qin et al. (2014) with some modifications. Briefly, the biofilm producers S. epidermidis ATCC 35984 and S. aureus ATCC 8095 were cultured for 18 h in brain heart infusion broth (BHI; Kasvi, Curitiba, Brazil) supplemented with 0.75% (w/v) glucose. The bacterial suspension was adjusted to an OD600 of 1 and diluted to 1:40 in the same broth. Next, 200 μL of the bacterial dilution was added to the wells of a 96-well plate and incubated for 24 h at 37 °C. After bacterial adhesion, the plate was washed three times in PBS (pH 7.4), and the pre-formed biofilm was incubated with either fresh media (biofilm growth control) or with 512 μg/mL of the extracts, at 37 °C for 24 h. The wells were then washed with PBS before staining with crystal violet (0.2% w/v), and the extract activity was evaluated at 595 nm using a microplate reader (Polaris, Celer, Brazil). Twelve experimental replicates were performed for each extract, and the standard error of the mean was calculated. We considered biofilm reduction percentage any positive value obtained from the following formula: reduction (%) = 100 − (100 × AbsT/AbsGC), where AbsGC is the mean of the absorbance at DO595 of the biofilm growth control and AbsT is the mean of absorbance at DO595 of the biofilm under the fungal extract test. One-way ANOVA was used to compare the absorbance values of the extract treatments with the biofilm growth control (p < 0.05) was considered statistically significant.
Activity of metabolites and VOCs of Induratia isolates against Meloidogyne incognita
The effects of metabolites and VOCs of Induratia isolates on second-stage juveniles (J2) of M. incognita were evaluated. Meloidogyne incognita eggs were obtained from tomato roots according to the technique described by Bonetti and Ferraz (1981). To obtain J2 of M. incognita, a hatching chamber consisting of a 500-mesh sieve (mm) was placed in a glass funnel. Only J2 that hatched after 72 h was used in the experiments.
To prepare the filtrates, three discs (5 mm) collected from 7-day-old PDA Induratia colonies were inoculated in 50 mL potato dextrose (PD) medium at 25 °C and 160 rpm for 10 days. After centrifugation of the culture at 5000×g for 10 min, the supernatant was filtered through a 0.22 μm Durapore® membrane. The fungal filtrates were undiluted (100%) and diluted in sterile water (80%, 60%, and 40%). One hundred microliters of the filtrate was added to a 96-well microplate, and a 20 μL aliquot containing 25 individuals J2 was added to each well. Sterile water and the active ingredient of the nematicide Furadan (2,3-dihydro-2,2-dimethyl-7-benzofuranol, N-methyl-carbamate) at 200 μg/mL were used as controls. The analyses were performed by counting the percentage of immobile J2 after 24 h of incubation and after of 48 h incubation when 50 μL of NaOH (1 M) was added to each well to assess the mortality of J2 (Chen and Dickson 2000). J2 was considered dead if the mobility did not recover. Nematicidal activity was considered when all J2 of M. incognita remained immobile after 48 h.
To select the Induratia isolate producing VOCs toxic to J2 of M. incognita, discs of 5 mm were collected from 7-day-old PDA Induratia colonies, inoculated on one side of split Petri dishes containing media PDA or yeast extract supplemented (YES) incubated at 25 °C for 10 days. The J2 were placed on the other side of the split Petri dish, sealed with plastic film, and incubated at 25 °C in the dark. After 72 h, the percentage of mobile J2 cells was determined. Culture medium without endophytic fungi was used as a control. The Induratia isolate producer of VOCs, which caused greater immobility in J2 of M. incognita, was selected for the next experiment.
The selected Induratia isolate was cultivated in the selected medium for 6, 9, and 12 days at 25 °C. The J2 of M. incognita was exposed to VOCs produced by the Induratia isolate for 72 h. The mobility percentage of J2 was then determined. The J2 exposure to VOCs was removed from the plate dish and inoculated into 30-day-old tomato seedlings in 75 cm3 cell seeding trays containing substrate Plantmax® substrate. The trays were kept in a greenhouse, and the inoculated seedlings were sprayed manually whenever necessary. The number of eggs and galls per root system was quantified after 45 days, as described by Bonetti and Ferraz (1981). The control was a culture medium without I. Coffeana (CML 4011).
The assay was performed in five replicates for each sample. The treatments were subjected to analysis of variance using the R Statistics software, and the means were compared using the Scott and Knott test (1974), with p < 0.05. Data transformation (in the square root of X + 1) was used to minimize the lack of normality of the biological data.
Results
Induratia species phylogeny
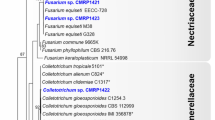
The ITS tree grouped the 12 isolates into three clades, two of which corresponded to the species I. coffeana (n = 7 isolates) and I. yucatanensis (n = 3). The third clade was not well resolved and contained the reference Induratia equiseti, Induratia suturae, I. thailandica, I. vitigena, and I. ziziphi together with Induratia sp. (CML 4013) and Induratia sp. (CML 4015) from this study (Fig. 1). In the analysis of the RPB2 gene (Online Resource 2), Induratia sp. (CML 4013) and Induratia sp. (CML 4015) were phylogenetically related to a clade containing the types of I. thailandica and I. ziziphi. Considering the TUB2 gene tree (Online Resource 3), the Induratia sp. (CML 4015) was phylogenetically more closely related to the types of I. thailandica and I. ziziphi than Induratia sp. (CML 4013). In the combined tree, Induratia sp. (CML 4013) was phylogenetically related to Induratia sp. (CML 4015) (Fig. 2).
Maximum likelihood phylogenetic tree based on ITS sequences showing relationships among Induratia species. Isolates from this study are highlighted in bold. Bootstrap values ≥ 70% (ML/MP) are shown at the internodes. The scale bar represents the expected number of nucleotide substitutions per site. The tree is rooted to Emarcea castanopsidicola and E. eucalyptigena. T = Type
Maximum likelihood phylogenetic tree based on concatenated ITS-RPB2-TUB2 sequences showing relationships among Induratia species. Isolates from this study are highlighted in bold. Bootstrap values ≥ 70 (ML/MP) are shown at the internodes. The scale bar represents the expected number of nucleotide substitutions per site. The tree is rooted to Emarcea castanopsidicola and E. eucalyptigena. T = Type
The other isolates were clustered together with the I. coffeana reference isolate based on the RPB2 gene tree. Using the TUB2 gene and combined dataset trees, these isolates formed a strongly supported clade belonging to I. coffeana and showed polymorphism within the ITS, RPB2, and TUB2 genes, indicating intraspecific variability.
Macromorphology and micromorphology
There was a slight difference in the isolate’s mycelial growth in the different culture media at 25 °C with better growth in PDA medium where colony diameter ranged from 3.2 (I. coffeana CML 4020) to 5.2 cm (Induratia sp. CML 4015). When grown in PDA medium at different temperatures, some of the isolates grew better at 20 °C with colony diameters ranging from 3.4 to 7.2 cm. Induratia coffeana (CML 4009, CML 4012) did not grow at 30 °C, whereas Induratia sp. (CML 4013) had the largest colony diameter at the same temperature (Online Resource 4). Conidia and fruiting bodies were not observed in Induratia species.
The I. coffeana isolates showed high morphological plasticity. Colonies grew slowly on PDA medium, with a linear growth rate of 2.34 to 3.35 mm diameter/day at 25 °C with a 12 h photoperiod (Table 2). The colonies had weak moldy odors, different mycelial growth densities as floccose or cottony surfaces, and color colonies (surface and reverse) ranging from beige to white after 40 days (Fig. 3). The hyaline hyphae were branched, with 2.05 to 3.18 μm diameters, frequently intertwining and forming rope-like strands. Except for I. coffeana (CML 4017), all isolates formed coils with diameters ranging from 24.0 to 31.3 μm. Cauliflower structures were observed in some Induratia isolates.
Morphology of Induratia coffeana. I. coffeana (CML 4012) colony on PDA medium after 15 days, surface (a) and reverse (b) after 45 days surface (c) and reverse (d). I. coffeana (CML 4014) colony on PDA medium after 15 days, surface (e) and reverse (f) after 45 days surface (g) and reverse (h). I. coffeana (CML 4017) colony on PDA medium after 15 days, surface (i) and reverse (j) after 45 days surface (k) and reverse (l). I. coffeana (CML 4018) colony on PDA medium after 15 days, surface (m) and reverse (n) after 45 days surface (o) and reverse (p). I. coffeana (CML 4014) hyphae frequently intertwining and forming rope-like (q, s), coils (r, u), cauliflower structures (t)
The colony of Induratia sp. (CML 4013) grew on PDA medium, with rate of 3.31 mm diameter/day, reaching 68.9 mm diameter in 40 days at 25 °C with a 12-h photoperiod (Table 2). The colony was white on the surface with a soft beige reverse (Fig. 4). Forty-day-old colonies were soft beige in the center and white on the edge. Hyphal growth at the colony surface had a cotton-like pattern, concentric circles, and a weak moldy odor. They had rope-like hyaline hyphae with 1.99 μm diameter with coils and small compacted coil structures measuring 12.1 μm in diameter.
Morphology of Induratia sp. (CML 4013). Colony on PDA medium after 15 days, surface (a) and reverse (b). Colony on PDA medium after 40 days growth, surface (c) and reverse (d). Rope-like with coils and with small and compacts coils structures (i), cauliflower structures (j). Morphology of Induratia sp. (CML 4015). Colony on PDA medium after 15 days growth, surface (e) and reverse (f). Colony on PDA medium after 40 days, surface (g) and reverse (h). Hyphae frequently intertwining (k), and coils appear as fused (l)
Induratia sp. (CML 4015) presented a slow-growth colony on PDA medium, with a daily average of 2.97 mm, reaching a 44.5 mm diameter in 15 days at 25 °C with a 12-h photoperiod (Table 2). The colony was white on the surface with a soft beige reverse (Fig. 4). Forty-day-old colonies were soft beige, with a floccose surface, 2.16 μm diameter hyaline hyphae; frequently intertwined and formed rope-like with fused coils, which measured an 18.5 μm diameter; and produced a weak moldy odor.
VOCs identification
We identified VOCs produced by all samples using SPME and GC-MS. We identified 51 VOCs consisting mainly of terpenes, alcohols, esters, and carboxylic acids, but four peaks remained unidentified sesquiterpenes (Table 3). The mass spectra of these unidentified peaks revealed a fragmentation pattern of non-oxygenated sesquiterpenes, although the correct structure could not be assigned. The production of VOCs of the Induratia isolates were not identical. In samples of I. coffeana (CML 4017), only seven VOCs were detected, while in Induratia sp. (CML 4015) and I. coffeana (CML 4016), 29 were detected.
Partial least squares: discriminant analysis
PLS-DA was able to discriminate Induratia isolates into three different clusters. A cluster of I. coffeana and two clusters of Induratia sp., which were scattered in the scores graph, suggest that these two samples were neither I. coffeana nor of the same species (Fig. 5). The main compounds produced by Induratia isolates were as follows: (4) 1-butanol, 3-methyl- (5) 1-butanol, 2-methyl-, (12) methyl 2-methylpropanoate, (16) methyl 2-methylbutanoate, (38) β-guaiene (cis), (40) β-guaiene (trans), (42) Δ-cadinene, (45) unidentified sesquiterpene, (46) 2-methylpropanoic acid, and (47) 2-methylbutanoic acid. LV1 is classified based on the high production of (46, 47) 2-methylpropanoic and 2-methylbutanoic acids, in addition to their methyl esters, and 2-methyl butanol and 3-methyl butanol. Higher LV1 values indicate high production of carboxylic acids in samples, and lower values of LV1 denote samples with higher production of 2-methyl butanol and 3-methyl butanol (Fig. 5). The LV2 order follows the production of some sesquiterpenes, in which a high value of LV2 indicates high production of these compounds (38, 40, 42, and 45), mainly by Induratia sp. (CML 4015), whereas Induratia sp. (CML 4013) produced more carboxylic acids (compounds 46 and 47) (Fig. 5).
Activity of Induratia species VOC’s in B. cinerea
The isolates I. coffeana (CML 4009, CML 4010, CML 4011, CML 4012, and CML 4019) completely inhibited B. cinerea growth compared to the control, while the other isolates partially inhibited the growth of the pathogen (Table 4). The transfer of pathogen conidia to new PDA media showed fungicidal activity against B. cinerea of I. coffeana isolates (CML 4010, CML 4011, CML 4019). When strawberries were inoculated with B. cinerea, the VOCs produced by I. coffeana (CML 4019) showed an effective biofumigation effect and caused smaller decay of the infected tissues at 25 °C for 3 days compared to the fruits of the control (Fig. 6).
Antibacterial activity of Induratia species extracts
The I. coffeana (CML 4019) extract showed antibacterial activity against S. aureus ATCC 25923, E. faecalis ATCC 29212, and E. faecium ATCC 700221 with MICs of 512 μg/mL. The extracts of other Induratia isolates did not inhibit the tested bacteria at 512 μg/mL. We were only able to determine the MBC of E. faecium ATCC 700221 (512 μg/mL), indicating bactericidal activity of I. coffeana (CML 4019) for this species. We could not identify the extract activity for the other bacteria because the MBC was higher than the highest concentration tested.
We assessed the ability of the Induratia isolates to reduce pre-formed biofilms of S. epidermidis ATCC 35984 and S. aureus ATCC 8095 (Table 4), even with no evidence of antibacterial activity in the planktonic form at 512 μg/mL. Only the I. coffeana (CML 4009) extract eradicated the pre-formed biofilm of S. aureus ATCC 8095 (100% reduction). The extracts produced by I. coffeana (CML 4011, CML 4016, CML 4017) and Induratia sp. (CML 4013, CML 4015) also reduced the pre-formed biofilm of S. aureus ATCC 8095. The pre-formed biofilm of S. epidermidis ATCC 35984 was reduced by extracts of I. coffeana (CML 4009, CML 4011, CML 4012, CML 4017, CML 4020) and Induratia sp. (CML 4013, CML 4015) (Table 4).
Activity of metabolites and VOCs of Induratia species against M. incognita
The metabolites produced by most Induratia isolates caused toxicity in J2 of M. incognita. Except for I. coffeana (CML 4012, CML 4014, CML 4020), nematicidal activity was observed in undiluted filtrates produced by other Induratia isolates, causing 100% J2 immobility after 24 h of exposure and 100% J2 mortality after adding NaOH at 48 h (Table 5). Although the percentage of immobility remained high for most of the filtrates produced by Induratia isolates, the percentage of mortality was reduced with the dilution of the filtrates.
The VOCs produced by Induratia isolates in YES medium were more toxic than those produced in PDA medium because of the highest reduction in the mobility of J2 of M. incognita. The VOCs produced by I. coffeana (CML 4011) showed the highest toxic effect on J2, with a motility of 28% (Table 5). When this isolate was cultivated in YES medium for 6, 9, and 12 days, there was no significant difference in the percentage of mobility of J2 after 72 h of exposure to VOCs, with a reduction of at least 84% in J2 mobility (Table 6). The J2 of M. incognita recovered from exposure to VOCs produced by I. coffeana (CML 4011) lost the ability to infect tomato roots after 45 days and did not form galls in these plants. A significant reduction in the number of eggs of M. incognita was also observed after exposure to VOCs from I. coffeana (CML 4011) cultivated in12 and 9 days. Exposure to VOCs produced after 6 days of fungal culture fully suppressed the development of nematodes without gall and egg formation in tomato plants in vivo.
Discussion
During our investigation of the endophytic fungi in coffee plants grown spontaneously in a secondary forest in Minas Gerais state, Brazil, we found 12 isolates belonging to the genus Induratia identified by molecular phylogeny based on the ITS, RPB2, and TUB2 gene sequences. Although the ITS rDNA gene infers phylogenetic relationships to some Xylariales genera, it provides insufficient resolution for Induratia species (Stadler et al. 2013, 2020; Chen et al. 2019; Samarakoon et al. 2020). The inclusion of the RPB2 and TUB2 sequences provided informative characters, and the three-loci phylogeny consistently distinguished the Induratia species (Chen et al. 2019; Samarakoon et al. 2020). However, there are few RPB2 and TUB2 gene sequences available in GenBank. Induratia isolates (CML 4014, CML 4016, CML 4017) clustered with the I. yucatanensis reference isolate (González et al. 2009) in the ITS tree, as there is only a sequence of this region deposited in GenBank. In the studies by Monteiro et al. (2020), Bastos et al. (2020), and Mota et al. (2021), the same Induratia species isolated from coffee plants were identified using only ITS phylogeny and reported as I. yucatanensis. However, when the RPB2 and TUB2 sequences were used, the Induratia isolates (CML 4014, CML 4016, CML 4017) clustered with I. coffeana. This result can be reforced if we compare the morphologies of these three isolates with the isolate described by Gonzáles et al. (2009). Induratia yucatanensis showed a smaller colony diameter than the three Induratia isolates (CML 4014, CML 4016, and CML 4017). Furthermore, the authors reported a strong odor in colonies, and they did not describe the presence of cauliflower structures that were present in Induratia species (CML 4014, CML 4016, CML 4017). Two Induratia isolates were identified as Induratia sp. (CML 4013 and CML 4015). The alignment without gaps in the nucleotide sequences of the three loci revealed that the Induratia sp. (CML 4013) differs from Induratia sp. (CML 4015) in 7/429 nucleotides in the ITS sequence, 7/865 nucleotides in the RPB2 gene, and 25/698 nucleotides in the TUB2 gene. These two isolates were phylogenetically most closely related to I. equiseti, I. suturae, I. vitigena, I. thailandica, and I. ziziphi based on the ITS tree. However, the sequences of the RPB2 and TUB2 genes of I. equiseti, I. suturae, and I. vitigena are not available in GenBank, preventing species identification of Induratia sp. (CML 4013) and Induratia sp. (CML 4015), which could be putative new Induratia species. Moreover, we observed differences in cultural characteristics and the VOCs produced between these isolates and those species that were phylogenetically most closely related.
The morphological characteristics of Induratia species were based on colony and mycelial characteristics, as reproductive structures have never been observed in any culture medium (Strobel et al. 2001; Zhang et al. 2010; Chen et al. 2019; Mao et al. 2018). However, Samarakoon et al. (2020) found the sexual state of two specimens from Thailand, I. ziziphi and I. thailandica. In our study, the Induratia isolates showed colonies ranging from white to beige, smooth, or flocculated with branched hyphae forming coils and cauliflower structures when cultivated at 25 °C on PDA medium. Induratia sp. (CML 4013 and CML 4015) showed a morphology similar to that of I. coffeana, but with smaller coil structures. In addition, we used other culture media, including CMA, which is known to stimulate sporulation and production of the sexual state. However, none of the isolates produced sexual or asexual spores. The optimal growth temperature range for the tested Induratia isolates was 20–25 °C, with ten isolates grow at 30 °C.
The genus Induratia produces VOCs known for its lethal effects against a wide variety of pathogenic fungi (Strobel 2018; Kaddes et al. 2019). Analysis and comparison of these compounds have been described as important tools for chemotaxonomic characterization (Kudalkar et al. 2012; Suwannarach et al. 2013; Siri-udom et al. 2016; Saxena and Strobel 2020). However, as secondary metabolite profiles may have taxonomic significance, in addition to analyses using gas chromatography coupled with mass spectrometry (GC-MS), it is recommended to include methods such as high-resolution mass spectrometry (HRMS) and nuclear magnetic resonance (NMR) spectroscopy and a series of cultures of isolates in different culture media and the growth phase (Samarakoon et al. 2020). Stadler et al. (2014) showed that Daldinia spp. produce specific secondary metabolites at high concentrations that can be taxonomically significant. In this study, we found that I. coffeana and Induratia sp. produced different VOCs. This indicates that the VOCs might not be correlated with their phylogenetic assignment, at least among these Induratia isolates. However, PLS-DA was able to discriminate Induratia isolates into three different clusters: I. coffeana isolates, Induratia sp. (CML 4013), and Induratia sp. (CML 4015). Some VOCs, such as 2-methylpropanoic acid, have already been identified in other Induratia species that exhibit antifungal and antibacterial activities, while other compounds such as naphthalene and thujopsene produced by Induratia species with anti-insect and antifungal bioactivity were absent in our isolates (Daisy et al. 2002; Kudalkar et al. 2012; Suwannarach et al. 2013).
Induratia isolates obtained from Brazilian coffee plants have already been evaluated against phytopathogenic fungi. Induratia coffeana (CML 4019) inhibited the growth of three pathogenic fungi and reduced the symptoms of anthracnose, white mold, and angular leaf spot when re-inoculated in common bean plants (Mota et al. 2021). We also verified that the VOCs produced by I. coffeana (CML 4019) completely inhibited the growth of B. cinerea in PDA medium and strawberry fruits and reduced fruit decay at room temperature. This pathogen causes gray mold in strawberries, affecting fruit in the field, storage, transport, and market, leading to significant economic losses (Petrasch et al. 2019). Yalage Don et al. (2020) exposed B. cinerea to a mixture of VOCs (ethanol (ethyl alcohol), 2-methyl-1-propanol (1-propanol, 2-methyl-), 3-methyl-1-butanol (1-butanol, 3-methyl), and 2-phenyl ethanol (phenylethyl alcohol) produced by Aureobasidium pullulans. The authors verified the induced electrolyte loss and oxidative stress in this pathogen. The VOCs 3-methyl-1-butanol produced by the isolate Candida intermedia C410 has also been reported to inhibit mycelial growth and conidial germination of B. cinerea (Huang et al. 2011). All these VOCs were produced by I. coffeana (CML 4019), and future investigations could confirm whether the biofumigation was the result of one or more VOCs produced by this isolate. I. coffeana (CML 4019) is a promising candidate for biological control, mainly during the postharvest period.
Because bacterial pathogens require new antibiotics due to multidrug resistance worldwide, we decided to search for new compounds in extracts of Induratia isolates. We used four Gram-negative and four Gram-positive bacteria that belong to the ESKAPE group and are responsible for most healthcare-associated infections world, with multidrug resistance profiles (Santajit and Indrawattana 2016). The inhibition of any of the tested pathogens is significant because of the complications resulting from infections associated with them (Minarini et al. 2020). The I. coffeana (CML 4019) extract showed activity against the Gram-positive bacteria S. aureus, E. faecalis, and E. faecium. Staphylococcus aureus is an opportunistic pathogen that causes a variety of diseases in humans and other animals, and the treatment of these infections is a challenge due to the emergence of multidrug-resistant strains and their ability to escape the host’s immune system attack (Rossi et al. 2014). Enterococci have emerged as the leading cause of antibiotic-resistant infections and are currently ranked among the most common nosocomial pathogens infecting the urinary tract, surgical sites, and the bloodstream, especially in immunocompromised hosts (Richards et al. 2000; Shepard and Gilmore 2002). Despite being commensal, E. faecalis has traits that turn it into an opportunistic pathogen (Shankar et al. 2002).
We also assessed the ability of the extracts to reduce pre-formed biofilms of S. aureus and S. epidermidis. The I. coffeana (CML 4009) extract eradicated the pre-formed biofilm of S. aureus ATCC 8095 (100% reduction) and reduced the biofilm of S. epidermidis ATCC 35984. Some extracts produced by I. coffeana and Induratia sp. also reduced the pre-formed biofilm of two bacteria. These extracts are sources of compounds able, for instance, to inhibit quorum sensing or the production of EPS and disperse the biofilm leading the bacterial cells to a more vulnerable phenotype. Compounds that reduce or disperse biofilms can be used in combination with antibiotics to treat infections (Algburi et al. 2017). Extracts with action in just one species suggest that these mechanisms are more specific, while extracts with action in both likely have a broad mechanism, such as in targets present in different species. Although we have no information regarding the nature of the compounds, this study indicates the presence of antibiotic substances with the potential to be used in the medical field.
Meloidogyne incognita is among the most injurious phytonematodes, particularly tomato crops, causing high harvest losses in Brazil and worldwide (Machado 2014). An alternative control technique is microorganisms that have the capacity to parasitize eggs, juvenile forms, and sometimes even adult nematodes (Lopes et al. 2016). The VOCs produced by Induratia species caused mortality or immobility of J2 of M. incognita, and distinct compounds may have different modes of action on the nematode body. The compound 2-butanone produced by the deep-sea bacterium Virgibacillus dokdonensis MCCC 1A00493 exhibited repellent activity in vitro against M. incognita (Huang et al. 2020). Induratia coffeana (CML 4012) and Induratia sp. (CML 4013) also produced this compound, which likely caused J2 immobility. In previous studies, Grimme et al. (2007) reported that a mixture of the synthetic components of I. albus VOCs (1-butanol, 2-methyl, 1, butanol, 2-methyl acetate, propanoic acid, and 2-methyl propyl ester) protected tomatoes from the root-knot nematode M. incognita. Induratia isolates produced many of these VOCs, which were lethal to M. incognita juveniles after 24 h of exposure. In our study, I. coffeana (CML 4011) showed nematicidal activity against both VOCs and filtrate. VOCs produced by this isolate reduced infectivity and nematode reproduction in tomato plants, and studies are necessary to identify the molecule or a mixture that is toxic to M. incognita.
In conclusion, ten I. coffeana and two Induratia sp. associated with Brazilian coffee showed slight morphological differences and produced VOCs consisting mainly of terpenes, alcohols, esters, and carboxylic acids. These VOCs showed toxicity against B. cinerea and M. incognita, indicating the potential of the isolates as a biofumigant. In addition, it was observed that some Induratia isolates were able to disperse the pre-formed biofilm of S. aureus and S. epidermidis, indicating the production of promising compounds of clinical interest that, once identified, would be worth purifying and checking their activity and selectivity in pure state.
Data availability
The biological reference material was deposited and available in official collections and DNA sequences and alignments at GenBank and TreeBASE, respectively.
Code availability
Not applicable.
References
Adams RP (2007) Identification of essential oil components by gas chromatography /mass spectrometry, 4th edn. Allured Publishing, Corporation, Carol Stream
Algburi A, Comito N, Kashtanov D, Dicks LMT, Chikindas ML (2017) Control of biofilm formation: antibiotics and beyond. Appl Environ Microbiol 83:e02508-e2516. https://doi.org/10.1128/AEM.02508-16
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. https://doi.org/10.1021/ac00218a01
Bastos APPS, Cardoso PG, Santos ÍAFM, Trento MVC, Porto LCJ, Marcussi S (2020) Enzymatic modulators from Induratia spp. Current Microbiol 77:3603–3611. https://doi.org/10.1007/s00284-020-02170-5
Becker K, Stadler M (2021) Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J Antibiot 74:1–23. https://doi.org/10.1038/s41429-020-00376-0
Bills GF, Gloer JB (2016) Biologically active secondary metabolites from the fungi. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0009-2016
Bonetti JI, Ferraz S (1981) Modificações do método de Hussey & Barker para extração de ovos de Meloidogyne exigua em raízes de cafeeiro. Fitopatol Bras 6:553
Chen SY, Dickson DW (2000) A technique for determining live second-stage juveniles of Heterodera glycines. J Nematol 32:117–121
Chen JJ, Feng X, Xia CY, Kong D, Qi ZY, Liu F, Chen D, Lin F, Zhang C (2019) Confirming the phylogenetic position of the genus Muscodor and the description of a new Muscodor species. Mycosphere 10:187–201. https://doi.org/10.5943/mycosphere/10/1/2
Clinical and Laboratory Standards Institute – CLSI (2015) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 10th ed. CLSI document M07-A10 (ISBN 1-56238-987-4). Wayne, Pennsylvania 19087-1898, USA. https://clsi.org/media/1632/m07a10_sample.pdf
Daisy B, Strobel G, Ezra D, Castillo U, Baird G, Hess WM (2002) Muscodor vitigenus anam. sp. nov., an endophyte from Paullinia paullinioides. Mycotaxon 84:39–50
Duong LM, Lumyong S, Hyde KD, Jeewon R (2004) Emarcea castanopsidicola gen. et sp. nov. from Thailand, a new xylariaceous taxon based on morphology and DNA sequences. Stud Mycol 50:253–260
González MC, Anaya AL, Glenn AE, Macías-Rubalcava ML, Hernández- Bautista BE, Hanlin RT (2009) Muscodor yucatanensis, a new endophytic ascomycete from Mexican chakah, Bursera simaruba. Mycotaxon 110:363–372
Grimme E, Zidack NK, Sikora RA, Strobel GA, Jacobsen BJ (2007) Comparison of Muscodor albus volatiles with a biorational mixture for control of seedling diseases of sugar beet and root-knot nematode on tomato. Plant Dis 91:220–225. https://doi.org/10.1094/PDIS-91-2-0220
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79(3):293–320. https://doi.org/10.1128/MMBR.00050-14
Helaly SE, Thongbai B, Stadler M (2018) Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat Prod Rep 35(9):992–1014. https://doi.org/10.1039/c8np00010g
Hepperle D (2004) SeqAssem©. Win32–version. A sequence analysis tool contig assembler and trace data visualization tool for molecular sequences. http://www.sequentix.de
Hongsanan S, Hyde KD, Bahkali AH, Camporesi E, Chomnunti P, Ekanayaka H, Gomes AAM, Hofstetter V, Gareth JEB, Pinho DB, Pereira OL, TianQ WDN, Xu JC, Buyck B (2015) Fungal biodiversity profiles 11–20. Cryptogam Mycol 36:355–380. https://doi.org/10.7872/crym/v36.iss3.2015.355
Huang R, Li GQ, Zhang J, Yang L, Che HJ, Jiang DH, Huang HC (2011) Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 101:859–869. https://doi.org/10.1094/PHYTO-09-10-0255
Huang D, Yu C, Shao Z, Cai M, Li G, Zheng L, Yu Z, Zhang J (2020) Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 25:744. https://doi.org/10.3390/molecules25030744
Hutchings ML, Alpha-Cobb CJ, Hiller DA, Berro J, Strobel SA (2017) Mycofumigation through production of the volatile DNA-methylating agent N-methyl-N-nitrosoisobutyramide by fungi in the genus Muscodor. J Biol Chem 292(18):7358–7371. https://doi.org/10.1074/jbc.M117.779009
Hyde KD, Xu J, Rapior S et al (2019) The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers 97:1. https://doi.org/10.1007/s13225-019-00430-9
Kaddes A, Fauconnier ML, Sassi K, Nasraoui B, Jijakli MH (2019) Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 24:1065. https://doi.org/10.3390/molecules24061065
Kudalkar P, Strobel G, Riyaz-Ul-Hassan S, Geary B, Sears J (2012) Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 53:319–325. https://doi.org/10.1007/S10267-011-0165-9
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lata R, Chowdhury S, Gond S, White JF (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Appl Microbiol 66:268–276. https://doi.org/10.1111/lam.12855
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
Lopes KC, Terra W, Freire ES et al (2016) Analogues of dihydrouracil and 9H-purine control Meloidogyne incognita in tomato plants. Nematropica 46:138–146
Machado ACZ (2014) Current nematode threats to Brazilian agriculture. Curr Agric Sci Technol 20:26–35. https://doi.org/10.18539/CAST.V20I1.3737
Mao LJ, Chen JJ, Xia CY, Feng XX, Kong DD, Qi ZY, Liu F, Chen D, Lin FC, Zhang CL (2018) Identification and characterization of new Muscodor endophytes from gramineous plants in Xishuangbanna, China. Microbiol Open 8:e00666. https://doi.org/10.1002/mbo3.666
Mercier J, Jiménez JI (2004) Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol Technol 31:1–8. https://doi.org/10.1016/j.postharvbio.2003.08.004
Mercier J, Smilanick JL (2005) Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol Control 32:401–407. https://doi.org/10.1016/j.biocontrol.2004.12.002
Meshram V, Kapoor N, Saxena S (2013) Muscodor kashayum sp. nov. a new volatile anti-microbial producing endophytic fungus. Mycology 4:196–204. https://doi.org/10.1080/21501203.2013.877990
Meshram V, Saxena S, Kapoor N (2014) Muscodor strobelii, a new endophytic species from south India. Mycotaxon 128:93–104. https://doi.org/10.5248/128.93
Meshram V, Gupta M, Saxena S (2015) Muscodor ghoomensis and Muscodor indica: new endophytic species based on morphological features, molecular and volatile organic analysis from northeast India. Sydowia 67:133–146. https://doi.org/10.12905/0380.sydowia67-2015-0133
Meshram V, Kapoor N, Chopra G, Saxena S (2017) Muscodor camphora, a new record from Cinnamomum camphora. Mycosphere 8:568–582. https://doi.org/10.5943/mycosphere/8/4/6
Minarini LADR, Andrade LND, de Gregorio E, Grosso F, Naas T, Zarrilli R, Camargo ILBC (2020) Editorial: antimicrobial resistance as a global public health problem: how can we address it? Front Public Health 8:612844. https://doi.org/10.3389/fpubh.2020.612844
Mitchell A, Strobel G, Hess W, Vargas P, Ezra D (2008) Muscodor crispans, a novel endophyte from Ananas ananassoides in the Bolivian Amazon. Fungal Divers 31:37–43
Monteiro MCP, Alves NM, de Queiroz MV, Pinho DB, Pereira OL, de Souza SMC, Cardoso PG (2017) Antimicrobial activity of endophytic fungi from coffee plants. Biosci J. https://doi.org/10.14393/BJ-v33n2-34494
Monteiro MCP, Tavares DG, Nery EM, Queiroz MV, Pereira OL, Cardoso PG (2020) Enzyme production by Induratia spp. isolated from coffee plants in Brazil. Braz Arch Biol Technol 63:e20180673. https://doi.org/10.1590/1678-4324-2020180673
Mota SF, Pádua PF, Ferreira AN, Gomes LBW, Dias MA, Souza EA, Pereira OL, Cardoso PG (2021) Biological control of common bean diseases using endophytic Induratia spp. Biol Control 159(2):104629. https://doi.org/10.1016/j.biocontrol.2021.104629
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116. https://doi.org/10.1006/mpev.1996.0376
Pankey GA, Sabath LD (2004) Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis 38:864–870. https://doi.org/10.1086/381972
Pena LC, Jungklaus GH, Savi DC, Ferreira-Maba L, Servienski A, Maia BHLNS, Annies V, Galli-Terasawa LV, Glienke C, Kava V (2019) Muscodor brasiliensis sp. nov. produces volatile organic compounds with activity against Penicillium digitatum. Microbiol Res 221:28–35. https://doi.org/10.1016/j.micres.2019.01.002
Petrasch S, Knapp SJ, van Kan JAL, Blanco-Ulate B (2019) Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol Plant Pathol 20:877–892. https://doi.org/10.1111/mpp.12794
Qin N, Tan X, Jiao Y, Liu L, Zhao W, Yang S, Jia A (2014) RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci Rep 4:5467. https://doi.org/10.1038/srep05467
Richards MJ, Edwards JR, Culver DH, Gaynes RP (2000) Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 21:510–515. https://doi.org/10.1086/501795
Rossi F, Diaz L, Wollam A, Panesso D, Zhou Y, Rincon S, Narechania A, Xing G, Di Gioia TS, Doi A, Tran TT, Reyes J, Munita JM, Carvajal LP, Hernandez-Roldan A, Brandão D, van der Heijden IM, Murray BE, Planet PJ, Weinstock GM, Arias CA (2014) Transferable vancomycin resistance in a community-associated MRSA lineage. N Engl J Med 370:1524–1531. https://doi.org/10.1056/NEJMoa1303359
Samarakoon MC, Thongbai B, Hyde KD, Brönstrup M, Beutling U, Lambert C, Miller AN, Liu JKJ, Promputtha I, Stadler M (2020) Elucidation of the life cycle of the endophytic genus Muscodor and its transfer to Induratia in Induratiaceae fam. nov., based on a polyphasic taxonomic approach. Fungal Divers 101:177–210. https://doi.org/10.1007/s13225-020-00443-9
Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067. https://doi.org/10.1155/2016/2475067
Saxena S, Strobel GA (2020) Marvellous Muscodor spp.: update on their biology and applications. Microb Ecol 82:5–20. https://doi.org/10.1007/s00248-020-01644-0
Saxena S, Meshram V, Kapoor N (2014) Muscodor darjeelingensis, a new endophytic fungus of Cinnamomum camphora collected from northeastern Himalayas. Sydowia 66:55–67. https://doi.org/10.12905/0380.sydowia66(1)2014-0055
Saxena S, Meshram V, Kapoor N (2015) Muscodor tigerii sp. nov. Volatile antibiotic producing endophytic fungus from the northeastern Himalayas. Ann Microbiol 65:47–57. https://doi.org/10.1007/s13213-014-0834-y
Scott AJ, Knott M (1974) A cluster analysis method for grouping means in the analysis of variance. Biometrics 30(3):507–512
Shankar N, Baghdayan AS, Gilmore MS (2002) Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746–750. https://doi.org/10.1038/nature00802
Shepard BD, Gilmore MS (2002) Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect 4:215–224. https://doi.org/10.1016/s1286-4579(01)01530-1
Siri-udom S, Suwannarach N, Lumyong S (2016) Existence of Muscodor vitigenus, M. equiseti and M. heveae sp. nov. in leaves of the rubber tree (Hevea brasiliensis Müll. Arg.), and their biocontrol potential. Ann Microbiol 66:437–448. https://doi.org/10.1007/s13213-015-1126-x
Stadler M, Kuhnert E, Persŏh D, Fournier J (2013) The Xylariaceae as model example for a unified nomenclature following the “one fungus-one name” (1F1N) concept. Mycology 4:5–21. https://doi.org/10.1080/21501203.2013.782478
Stadler M, Læssøe T, Fournier J, Decock C, Schmieschek B, Tichy HV, Peršoh D (2014) A polyphasic taxonomy of Daldinia (Xylariaceae). Stud Mycol 77:1–143. https://doi.org/10.3114/sim0016
Stadler M, Lambert C, Wibberg D, Kalinowski J, Cox RJ, Kolarik M, Kuhnert E (2020) Intragenomic polymorphisms in the ITS region of high quality genomes of the Hypoxylaceae (Xylariales, Ascomycota). Mycol Prog 19:235–245. https://doi.org/10.1007/s11557-019-01552-9
Strobel G (2018) The emergence of endophytic microbes and their biological promise. J Fungi 4:57. https://doi.org/10.3390/jof4020057
Strobel GA, Dirkse E, Sears J, Markworth C (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943–2950. https://doi.org/10.1099/00221287-147-11-2943
Strobel GA, Ezra D (2005) Application of volatile antibiotics and non-volatile inhibitors from Muscodor spp. to control harmful microbes in human and animal wastes. 6911338 B2. U.S. Patent. 2005 Jun 28. https://patents.google.com/patent/US20040018168
Suwannarach N, Bussaban B, Hyde KD, Lumyong S (2010) Muscodor cinnamomi, a new endophytic species from Cinnamomum bejolghota. Mycotaxon 114:15–23. https://doi.org/10.5248/114.15
Suwannarach N, Kumla J, Bussaban B, Hyde KD, Matsui K (2013) Molecular and morphological evidence support four new species in the genus Muscodor from northern Thailand. Ann Microbiol 63:1341–1351. https://doi.org/10.1007/s13213-012-0593-6
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic Press, London, pp 315–322
Worapong J, Strobel G, Ford EJ, Li JY, Baird G, Hess WM (2001) Muscodor albus anam. gen. et sp nov., an endophyte from Cinnamomum zeylanicum. Mycotaxon 79:67–79
Worapong J, Strobel GA, Daisy B, Castillo UF, Baird G, Hess WM (2002) Muscodor roseus anam. sp. nov., an endophyte from Grevillea pteridifolia. Mycotaxon 81:463–475
Yadav AN (2019) Endophytic fungi for plant growth promotion and adaptation under abiotic stress conditions. Acta Sci Agric 3:91–93
Yalage Don SM, Schmidtke LM, Gambetta JM, Steel CC (2020) Volatile organic compounds produced by Aureobasidium pullulans induce electrolyte loss and oxidative stress in Botrytis cinerea and Alternaria alternata. Res Microbiol 172(1):103788. https://doi.org/10.1016/j.resmic.2020.10.003
Zhang CL, Wang GP, Mao LJ, Komon-Zelazowska M (2010) Muscodor fengyangensis sp. nov. from southeast China: morphology, physiology and production of volatile compounds. Fungal Biol 114:797–808. https://doi.org/10.1016/j.funbio.2010.07.006
Acknowledgements
We would like to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG/CBB-RED-00005-14), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Consórcio Pesquisa Café (ConCafé 10.18.20.047.00.00), and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP #2103/07600-3) for the financial support and scholarships.
Funding
This research was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Consórcio Pesquisa Café, Fundação de Amparo a Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Author information
Authors and Affiliations
Contributions
PGC designed the project, supervised its execution, and wrote, reviewed, and edited the manuscript; OLP, MVdQ, and MCP contributed with collection of material, isolation, identification, and preservation of fungal isolates and reviewed the manuscript; UGdPL and EAG contributed to the sequencing of gene sequences and reviewed the manuscript; DGT contributed with morphological characterization and the assessment of anti-nematodes activity of Induratia isolates; SdSCG contributed with sequence analysis, preparation of phylogenetic trees, and overall analysis of the data and wrote, reviewed, and edited the manuscript; MPP and CAN contributed with characterization of VOCs and reviewed the manuscript; ILBCC, DKRB, BM, and ISeC contributed with the assessment of antibacterial activity of Induratia extracts in bacterial pathogens and reviewed the manuscript. All authors commented on the previous versions of the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Section editor: Marc Stadler
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11557_2021_1743_MOESM1_ESM.docx
Supplementary file1 (DOCX 13 kb) Online Resource 1 Overview of alignment characteristics used for phylogenetic analyses (including outgroups) and best nucleotide substitution models
Supplementary file2
Online Resource 2 Maximum Likelihood phylogenetic tree based on RPB2 sequences showing relationships among Induratia species. The isolates obtained in this study are highlighted in bold. Bootstrap values ≥ 70% (ML/MP) are shown at the internodes. Scale bar represents the expected number of nucleotide substitutions per site. The tree is rooted in Emarcea castanopsidicola and E. eucalyptigena. T =Type (PNG 417 kb)
Supplementary file3
Online Resource 3 Maximum Likelihood phylogenetic tree based on TUB2 sequences showing relationships among Induratia species. The isolates obtained in this study are highlighted in bold. Bootstrap values ≥ 70% (ML/MP) are shown at the internodes. Scale bar represents the expected number of nucleotide substitutions per site. The tree is rooted in Emarcea castanopsidicola and E. eucalyptigena. T =Type (PNG 407 kb)
11557_2021_1743_MOESM4_ESM.docx
Supplementary file4 (DOCX 15 kb) Online Resource 4 Mycelial growth of Induratia isolates in different culture media and temperatures
Rights and permissions
About this article
Cite this article
da Silva Costa Guimarães, S., Tavares, D.G., Monteiro, M.C.P. et al. Polyphasic characterization and antimicrobial properties of Induratia species isolated from Coffea arabica in Brazil. Mycol Progress 20, 1457–1477 (2021). https://doi.org/10.1007/s11557-021-01743-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-021-01743-3