Abstract
The genus Muscodor comprises fungal endophytes which produce mixtures of volatile compounds (VOCs) with antimicrobial activities. In the present study, four novel species, Muscodor musae, M. oryzae, M. suthepensis and M. equiseti were isolated from Musa acuminata, Oryza rufipogon, Cinnamomum bejolghota and Equisetum debile, respectively; these are medicinal plants of northern Thailand. The new Muscodor species are distinguished based on morphological and physiological characteristics and on molecular analysis of ITS-rDNA. Volatile compound analysis showed that 2-methylpropanoic acid was the main VOCs produced by M. musae, M. suthepensis and M. equiseti. The mixed volatiles from each fungus showed in vitro antimicrobial activity. Muscodor suthepensis had the highest antifungal activity.
Similar content being viewed by others
Introduction
Endophytes colonize healthy inter- and intracellular living tissue of host plants, typically without causing any visible symptoms of disease (Azevedo et al. 2000; Saikkonen et al. 2004; Hyde and Soytong 2008). Endophytes also protect their hosts from infectious agents and adverse conditions by secreting bioactive secondary metabolites (Azevedo et al. 2000; Gao et al. 2010). The highest plant biodiversity biomes are found in tropical and temperate rainforest regions, and plants in these areas also possess a high endophyte diversity (Strobel 2003). Muscodor species are volatile, producing endophytes known from certain tropical tree and vine species in Australia, Central and South America, and Central and Southeast Asia (Worapong et al. 2001, 2002; Daisy et al. 2002; Sopalun et al. 2003; Ezra et al. 2004; Atmosukarto et al. 2005; Strobel et al. 2007; Mitchell et al. 2008; González et al. 2009; Suwannarach et al. 2010; Zhang et al. 2010). The volatile organic compounds (VOCs) produced by Muscodor species are active against many plant pathogenic fungi and Gram-positive and Gram-negative bacteria, and the genus is thus potentially important for biocontrol (Strobel et al. 2001; Strobel and Daisy 2003; Macías-Rubalcava et al. 2010).
Eight species of Muscodor have been described on the basis of morphological, physiological, biological and genetic features (www.MycoBank.org; Kudalkar et al. 2012). These fungi are classified in the family Xylariaceae and have a unique molecular identity as compared to other genera in the family (Ezra et al. 2004; Zhang et al. 2010). Gas chromatography and mass spectrometry (GC/MS) can also be used to identify Muscodor species; this is based on the differences in the VOCs that they produce (Strobel et al. 2001).
We are investigating the endophytes of various plants, including medicinal plants in northern Thailand. The purpose of the present paper is to introduce four new species of Muscodor isolated from medicinal plants based on their morphology, antimicrobial activities, volatile chemical composition and phylogeny.
Materials and methods
Study sites
Study sites were Chiang Mai University (18°47′74″N, 98°57′41″E, altitude 328 m), Queen Sirikit Botanic Garden (18°53′93″N, 98°51′11″E, altitude 640 m) and Medicinal Plant Garden, Doi Suthep-Pui National Park (18°48′22″N, 98°54′51″E, altitude 1,076 m), located in Chiang Mai Province, northern Thailand. Specimens were collected during November 2010 to May 2011.
Fungal isolation
The endophytic fungi were isolated from four plant species, Cinnamomum bejolghota and Musa acuminata (Medicinal Plant Garden site), Equisetum debile (Queen Sirikit Botanic Garden site) and Oryza rufipogon (Chiang Mai University site) by previously described methods (Mitchell et al. 2008). The plant samples (leaf and stem) were collected, placed in sterile plastic bags, stored in an icebox and transported to the laboratory within 48 h of sampling. A total of 40 samples were cut into segments (leaf, 5 mm × 5 mm; stem, 5 mm long). All segments were surface-sterilized in 75 % ethanol for 30 s, 2 % sodium hypochlorite for 3 min and 95 % ethanol for 30 s under a laminar flow hood. Potato dextrose agar (PDA) was poured into a half-plate of two-compartment plastic plates (90 mm × 15 mm). The other side of each plate contained ½ strength PDA, 0.05 % streptomycin sulfate and 0.03 % rose bengal. An agar plug (6 mm diam) of actively growing culture of Muscodor cinnamomi was placed into the PDA. The plant tissues then were placed onto ½PDA on the other side. The Petri dishes were sealed with Parafilm M and incubated at room temperature for 2 weeks. Fungi growing out from the samples were aseptically transferred to PDA. Pure culture isolates were maintained in PDA slants and tested three times by exposure to M. cinnamomi in order to exclude false positive growth in the initial isolation. The fungi were then grown on PDA for 12 days before small squares of colony were cut and placed into vials containing sterile 20 % glycerol, distilled water or mineral oil. The former vials were stored at −20 °C, while the latter two vials were stored at 4 °C, and pure fungi were deposited to the Research Laboratory for Excellence in Sustainable Development of Biological Resources (SDBR), Faculty of Science, Chiang Mai University, Thailand and Japan Collection of Microorganisms (JCM), Japan.
Fungal DNA extraction
Genomic DNA was extracted by a SDS-CTAB method (Suwannarach et al. 2010). Muscodor species were subcultured onto PDA and incubated for 10 days. Mycelium was harvested, freeze-dried, and ground into a fine powder with a pestle and mortar. About 15 mg of powdered mycelium was suspended in 1 mL of ice-cold lysis buffer (150 mM NaCl, 50 mM EDTA, 10 mM Tris–HCl, pH 7.4, 20 mg/mL proteinase K), transferred into a 1.5-mL Eppendorf tube and kept at 4 °C to prevent endonuclease activity during rehydration of the sample. SDS was added to a final concentration of 2 %, vortexed and incubated for 30 min at 65 °C. After centrifugation for 15 min at 14,000 rpm, the supernatant was transferred to a new sterile 1.5-mL Eppendorf tube. The volume of supernatant was measured and the NaCl concentration was adjusted to 1.4 M, and one-tenth volume of 10 % CTAB buffer (10 % CTAB, 500 mM Tris–HCl, 100 μM EDTA, pH 8.0) was added. The solution was thoroughly mixed and incubated for 10 min at 65 °C. After cooling for 2 min at 15 °C, an equal volume of chloroform:isoamyl alcohol (24:1, v/v) was added, thoroughly mixed, and the tube was centrifuged for 15 min at 14,000 rpm. The extraction was repeated until the interface was clear. The supernatant was moved to a new Eppendorf tube, containing 2 volumes of cold 100 % ethanol. After DNA precipitation, the pellet was centrifuged for 15 min at 14,000 rpm and 4 °C. The pellet was washed with 70 % ethanol and dried at room temperature. It was resuspended in 100 mL of 0.002 % RNase (5 mg/mL) in TE buffer and incubated for 1 h at 37 °C. The suspension was stored at −20 °C pending use for PCR amplification.
ITS sequencing and phylogenetic analysis
The internal transcribed spacer (ITS) regions 1 and 2, including 5.8S rDNA, were separately amplified in a 25-μL reaction on a GeneAmp 9700 thermal cycler (Applied Biosystems) under these reaction conditions: 1 μL of template DNA extraction, 0.2 μM dNTP, 0.2 μL of FastTaq (Applied Biosystems), 0.2 μM each of primers, 2.5 μL of the supplied PCR buffer with MgCl2, and sterile water to bring the volume to 25 μL. The ITS regions were amplified using ITS4 and ITS5 primers. Amplification of ITS regions was initialed by denaturation at 95 °C for 2 min, followed by 30 cycles of 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. PCR products were analyzed by electrophoresis in 1 % agarose gels in TAE buffer (20 mM Tris-Acetate, 1 mM EDTA, pH 8.0) and viewed by staining with ethidium bromide. PCR products were purified using PCR clean up Gel Extraction NucleoSpin® Extract II Purification Kit (Macherey-Nagel, Germany) following the manufacturer’s protocol. The purified PCR products were directly sequenced.
Sequencing reactions were performed and sequences determined automatically in a genetic analyzer (1st Base, Malaysia) using the PCR primers mentioned above. Sequences obtained in this study were compared to those from GenBank database using the BLAST software on the NCBI website: (http://www.ncbi.nlm.nih.gov/BLAST/). A multiple sequence alignment was carried out using the alignment subroutines in Clustal X (Thompson et al. 1997) and deposited at TreeBASE under study number 12911. The data was analyzed to determine the phylogenetic relationship based on distance and parsimony criteria phylogenetic tree was inferred with PAUP beta 10 software program, v.4.0 (Swofford 2002).
Bioassay tests
A dual culture volatile assay was used to investigate antimicrobial activity against 5 bacteria, 2 yeasts and 14 fungi test microorganisms. One side of a two-compartment plastic plate was loaded with PDA while the other side was loaded with nutrient agar (NA), PDA or yeast extract peptone dextrose agar (YPDA) for bacteria, fungi, and yeast tests, respectively. A 12-day-old Muscodor isolate was inoculated on the PDA side of the plate and grown for 3 days at room temperature (25 ± 2 °C). Twenty-one strains including 14 plant pathogenic fungi, 2 yeasts, 3 Gram-positive bacteria, and 2 Gram-negative bacteria were used in this experiment (Table 3). An agar plug taken from the margin of 7-day-old fungal pathogen mycelium was placed into the other compartment containing PDA. Bacteria and yeasts were streaked (1.5-cm-long streak) onto either NA or YPDA. All incubated plates were sealed with Parafilm and incubated at room temperature for 6 days. Percentage inhibition of growth of each tested microorganism was then recorded and the viability of the tested microorganisms were investigated by subculture of each microorganism in the tested plate onto fresh medium. The experiment was repeated two times with three replicates.
Qualitative analysis of fungal volatiles
Analyses of volatiles produced by Muscodor spp. grown for 1 week at 25 ± 2 °C on PDA were carried out according to the protocols described earlier (Strobel et al. 2001). A baked solid phase micro-extraction (SPME) fiber consisting of 50/30 divinylbenzene/carboxen on polydimethylsiloxane on a stable flex fiber was exposed for 45 min to the vapor phase of the headspace of the grown culture. The fiber was inserted into the splitless injection port of a gas chromatograph GC 2010 (Shimadzu, Japan) equipped with a 30 m × 0.25 mm I.D. DB-Wax capillary column with a film thickness of 0.25 μm. The column was subjected to a thermal program as follows: 40 °C for 2 min increased to 200 °C at 5 °C/min. Ultra-high purity helium was used as a carrier gas with an initial column head pressure of 60 kPa. The fiber was conditioned at 250 °C for 57 min under a flow of helium gas prior to trapping the volatiles. A 30-s injection time was used to introduce the adsorbed volatiles into the GC. The gas chromatograph was interfaced to a mass spectrometer MS-QP2010 Plus (Shimadzu) mass selective detector mass spectrometer operating at unit resolution. Data acquisition and data processing were performed on the software system. The compounds produced by Muscodor spp. were tentatively identified through a library comparison with the NIST database, hence all chemical compounds described in this report use the NIST database chemical terminology and are compared to the type strains (Daisy et al. 2002; González et al. 2009; Kudalkar et al. 2012; Mitchell et al. 2008; Suwannarach et al. 2010; Worapong et al. 2001, 2002; Zhang et al. 2010).
Results
Fungal isolation
Four volatile-producing endophytic fungal isolates, Muscodor equiseti, M. musae, M. oryzae and M. suthepensis were isolated from medicinal plants, Equisetum debile, Musa acuminata, Oryza rufipogon and Cinnamomum bejolghota, respectively. Two isolates produced white mycelium on PDA, while isolate M. suthepensis developed a pale pink-colored mycelium in natural light, and isolate M. oryzae produced pale orange mycelium (Table 1). All isolates produced rope-like and coiled hyphae. Only swollen hyphae cells were found in isolate M. equiseti. The morphology characteristics of all isolates were different from other Muscodor species reported previously (Table 1) and therefore are introduced as new species. In addition, the morphological taxonomy was confirmed by molecular and physiological analysis. The characteristics of the Muscodor species are shown in Figs. 1, 2, 3 and 4.
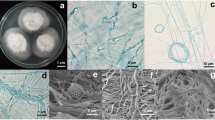
Muscodor suthepensis (CMU-Cib462) a culture on PDA, bar 1 cm; b light microscope micrographs of coiling formation of fungal hyphae, bar 10 μm; c–e scanning electron micrographs, bars 10 μm: c coiling formation of fungal hyphae; d, e hyphal cells from the colony edge showing fused, rope-like hyphal cells
Muscodor equiseti (CMU-M2) a culture on PDA, bar 1 cm; b, c light microscope micrographs of coiling formation of fungal hyphae, bars 10 μm; d–h scanning electron micrographs: d cottony-like mycelium, bar 50 μm; e triangular branching pattern; f, g swollen cells, bars 10 μm; h hyphal cells from the colony edge showing fused, rope-like hyphal cells, bar 10 μm
Molecular phylogeny analysis
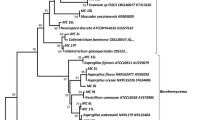
The ITS sequences data of the four isolates of Muscodor were obtained and compared with species downloaded from GenBank. The aligned dataset of 32 sequences consisted of 745 characters, of which 324 characters were constant, 184 variable characters were parsimony uninformative, and 237 characters were parsimony informative. Heuristic searches resulted in six equally parsimonious trees with a length of 904 steps, CI = 0.683, RI = 0.752, RC = 0.531 and HI = 0.318. One of the maximum parsimony trees is shown in Fig. 5. Muscodor species from GenBank formed a monophyletic clade with a high bootstrap support (83 %) and are closely related to other members within Xylariaceae. A phylogenetic dendrogram strongly supported the node separating M. musae and M. suthepensis from other Muscodor species with 96 and 100 % bootstrap support, respectively (Fig. 5). In addition, M. equiseti formed a sister group with 100 % bootstrap support to M. vitigenus and M. sutura. The remaining novel species, M. oryzae, was distant from the other novel species.
Maximum parsimonious trees inferred from a heuristic search of the internal transcribed spacer 1, 5.8S ribosomal RNA gene and internal transcribed spacer 2 sequence alignment of 32 sequences. Peziza badia and Taphrina sadebeckii were used to root the tree. Branches with bootstrap values ≥50 % are shown at each branch and the bar represents 10 substitutions per nucleotide position. New species are in bold
Antimicrobial activity
Twenty-one strains including 14 plant pathogenic fungi, 2 yeasts, 3 Gram-positive bacteria and 2 Gram-negative bacteria were tested. The growth and viability of the tested microorganisms were recorded after 7 days of exposure to volatile compounds from each novel Muscodor species. All new species have inhibitory activity on all tested microorganisms (Table 2). The volatile compounds produced by all Muscodor species inhibited the growth (100 %) and caused the death of the tested yeasts and bacteria. Percentage inhibition of tested plant pathogenic fungi ranged from 62.3 ± 2.1 to 100 % (Table 2). Most pathogens were killed by most Muscodor species. Muscodor suthepensis showed 100 % inhibition and killed all tested plant pathogenic fungi.
Volatile compound analysis
SPME-GC/MS analysis of 1-week-old cultures of the four Muscodor species grown on PDA revealed strikingly different patterns of volatile compounds production (Table 3). The four novel Muscodor species produced VOCs consisting mainly of esters, alcohols and small molecular weight acids. Muscodor suthepensis produced 27 VOCs which was the highest number, followed by M. musae (18 VOCs), M. equiseti (16 VOCs) and M. oryzae (15 VOCs). 2-methylpropanoic acid was the main VOC produced by M. musae, M. suthepensis and M. equiseti, whereas M. oryzae produced 3-methylbutan-1-ol as the major VOCs. Most taxa produced azulene derivatives, except for M. equiseti. Only naphthalene derivative compounds were detected in M. suthepensis cultures.
Taxonomy
Muscodor musae N. Suwannarach & S. Lumyong, sp. nov. Fig. 1
MycoBank No.: MB800811
GenBank No.: JX098323
Diagnosis: Colonies cotton white, form a hairy mycelium pattern on PDA. Hyphae 0.9–3.4 μm thick, coils 24–28.9 × 26–27.4 μm diam and produced a musty odor.
Etymology: musae, refers to the name of the host plant, Musa acuminata.
Holotype: THAILAND, Chiang Mai Province, Doi Suthep-Pui, Medicinal Plant Garden, from a leaf of Musa acuminata (Musaceae), May 2011, Nakarin Suwannarach, dried culture (SDBR CMU-MU3), ex-type living culture JCM 18230.
Teleomorph: Unknown
Description: In nature, the fungus is associated with Musa acuminata and is an ascomycete with sterile mycelium. Fungal colonies are cotton white and form a hairy mycelium pattern on PDA when grown in darkness and natural light (Fig. 1a, b). Hyphae (0.9–3.4 μm thick) with coils (24–28.9 × 26–27.4 μm diam.; Fig. 1c, d), commonly appearing as fused rope-like hyphal strands and branching (5.3–16.1 μm thick; Fig. 1e, f). Mycelium on PDA has a growth rate of 3.50 ± 0.35 mm day−1, producing a musty odor. Spores and other fruiting bodies did not develop under any conditions tested.
Muscodor oryzae N. Suwannarach & S. Lumyong, sp. nov. Fig. 2
MycoBank No.: MB800812
GenBank No.: JX098321
Diagnosis: Colonies pale orange on PDA. Hyphae 1.1–3.9 μm thick, coils 19.6–39.4 × 18.1–36.7 μm diam and produced a fruity odor.
Etymology: oryzae, refers to the name of the host plant, Oryza rufipogon.
Holotype: THAILAND, Chiang Mai Province, Chiang Mai, Chiang Mai University, from a leaf of Oryza rufipogon (Poaceae), May 2011, Nakarin Suwannarach and Jaturong Kumla, dried culture (SDBR CMU-WR2), ex-type living culture JCM 18231.
Teleomorph: Unknown
Description: In nature, the fungus is associated with Oryza rufipogon and is an ascomycete with sterile mycelium. Fungal colonies pale orange on PDA when grown in darkness and natural light (Fig. 2a). Hyphae (1.1–3.9 μm thick) with coils (19.6–39.4 × 18.1–36.7 μm diam.; Fig. 2b, c); commonly appearing as fused rope-like hyphal strands and branching (6.3–20.9 μm thick; Fig. 2d, e). Mycelium on PDA has a growth rate of 3.65 ± 0.30 mm day−1, producing a fruity odor. Spores and other fruiting bodies did not develop under any conditions tested.
Muscodor suthepensis N. Suwannarach & S. Lumyong, sp. nov. Fig. 3
MycoBank No.: MB800813
GenBank No.: JN558830
Diagnosis: Colonies pale pink in natural light and white in dark on PDA. Hyphae 1–4.6 μm thick, coils 16.1–31.8 × 13.4–29.5 μm diam and produced a fruity odor.
Etymology: suthepensis, refers to the collection site, Doi Suthep-Pui National Park.
Holotype: THAILAND, Chiang Mai Province, Doi Suthep-Pui, Medicinal Plant Garden, from a stem of Cinnamomum bejolghota (Lauraceae), November 2010, Nakarin Suwannarach, dried culture (SDBR CMU-Cib462), ex-type living culture JCM 18232.
Teleomorph: Unknown
Description: In nature, the fungus is associated with Cinnamomum bejolghota and is an ascomycete with sterile mycelium. Fungal colonies pale pink on PDA when grown in natural light (Fig. 3a), and white mycelium when grown in dark condition. Hyphae (1–4.6 μm thick) have coils (16.1–31.8 × 13.4–29.5 μm diam.; Fig. 3b, c); commonly appearing as fused rope-like strands and branching (8.2–35.6 μm thick; Fig. 3d, e). Mycelium on PDA has a growth rate of 3.00 ± 0.61 mm day−1, producing a fruity odor. Spores and other fruiting bodies did not develop under any conditions tested.
Muscodor equiseti N. Suwannarach & S. Lumyong, sp. nov. Fig. 4
MycoBank No.: MB800814
GenBank No.: JX098322
Diagnosis: Colonies cottony pattern, white on PDA. Hyphae 0.9–2.9 μm thick, coils 16.5–29.4 × 19.1–26.9 μm diam, tri-angle branching, subglobose swollen cells (1–4.2 × 1.8–6.7 μm and produced a fruity odor.
Etymology: equiseti, refers to the name of the host plant, Equisetum debile.
Holotype: THAILAND, Chiang Mai Province, Queen Sirikit Botanic Garden, from a stem of Equisetum debile (Equsetaceae), May 2011, Nakarin Suwannarach, dried culture (SDBR CMU-M2), ex-type living culture JCM 18233.
Teleomorph: Unknown
Description: In nature, the fungus is associated with Equisetum debile and is an ascomycete with sterile mycelium. Fungal colonies are cotton white on PDA when grown in darkness (Fig. 4a). Hyphae (0.9–2.9 μm thick) with coils (16.5–29.4 × 19.1–26.9 μm diam.; Fig. 4b, c) and a cottony pattern (Fig. 4d); tri-angle branching (Fig. 4e); subglobose swollen cells (1–4.2 × 1.8–6.7 μm; Fig. 4f, g) commonly appearing as fused rope-like strands and branching (5.3–17.1 μm thick; Fig. 4h). Mycelium on PDA has a growth rate of 2.82 ± 0.06 mm day−1, producing a fruity odor. Spores and other fruiting bodies did not develop under any conditions tested.
Discussion
Muscodor is essentially a genus of endophytic volatile-producing tropical fungi. Its diversity, host range and habitats are being expanded (Atmosukarto et al. 2005; Strobel et al. 2007). The morphological identification of Muscodor species is difficult because the genus does not produce any reproductive structrues on any substrate or medium tested. Colony and mycelial characters are the only available morphological traits (Strobel et al. 2001; Zhang et al. 2010). Consequently, the physiological characteristics such as VOC production, biological activities and molecular analysis are necessary tools for characterization. We have described four novel Muscodor species, based on differences in colony and hyphal morphology, VOC production and ITS sequence data. In general, Muscodor species produce white mycelium (Worapong et al. 2001; Daisy et al. 2002; Ezra et al. 2004; Mitchell et al. 2008; González et al. 2009; Suwannarach et al. 2010; Zhang et al. 2010; Kudorka et al. 2012), However, M. roseus produces a light rose-colored mycelium (Worapong et al. 2002). Muscodor oryzae produced a pale orange mycelial pigment similar to M. cinnamomi, but M. cinnamomi produces pigment only under natural light conditions (Suwannarach et al. 2010). Muscodor suthepensis produced pale pink pigment in natural light similar to M. crispans, but M. crispans produced wavy growing hypha and cauliflower-like bodies (Mitchell et al. 2008). In addition, hyphal growth of M. equiseti on agar had a cottony-like pattern, while M. musae produced hairy-like mycelium in contrast to other species of Muscodor (Table 1). The ITS sequence analysis of the rDNA of the four novel species showed that they are clearly separated from the other Muscodor species (Fig. 5). A phylogenetic dendrogram of the Muscodor group is in concordance with previous studies that show Muscodor as a member of the Xylariaceae, but in a district phylogenetic clade (González et al. 2009; Suwannarach et al. 2010; Zhang et al. 2010).
GC/MS of Muscodor species consistently found alcohols, esters and small molecular weight acid in the gas phase, when grown on PDA. Both major and minor VOCs produced by different Muscodor species are distinguishable (Table 1). The most abundant VOC produced by M. suthepensis, M. equiseti and M. musae was 2-methylpropanoic acid which is similar to M. albus, M. crispans, M. sutura and M. fengyangensis (Worapong et al. 2001; Ezra et al. 2004; Mitchell et al. 2008; Zhang et al. 2010; Kudorka et al. 2012). However, in the present study M. oryzae produced 3-methylbutan-1-ol as a major volatile compound. Muscodor suthepensis produced both azulene and naphthalene derivatives similar to M. albus, M. roseus, M. vitigenus and M. fengyangensis (Worapong et al. 2001, 2002; Daisy et al. 2002; Ezra et al. 2004; Zhang et al. 2010). In addition, M. musae and M. oryzae produced only azulene derivatives similar to M. cinnamomi (Suwannarach et al. 2010). Neither azulene nor naphthalene derivatives were found in M. equiseti culture which is similar to M. crispans and M. yucatanensis (Mitchell et al. 2008; González et al. 2009). The biological activity of mixed volatile compounds produced by the novel species showed antifungal and antibacterial activity (Tables 1 and 2). This result agrees with previous studies that mixed VOCs produced by Muscodor spp. were inhibitory to growth and killed tested bacteria and fungi (Worapong et al. 2001; Ezra et al. 2004; Mitchell et al. 2008; Suwannarach et al. 2010; Zhang et al. 2010). Muscodor roseus and M. sutura showed only antifungal activities (Kudalkar et al. 2012; Worapong et al. 2002). Only M. yucatanensis has been reported to have phytoinhibitory activity (González et al. 2009). Moreover, Worapong et al. (2002) reported that M. vitigenus has anti-insect activity. In addition, previous studies reported that 8 VOCs: aciphyllene, 3-methylbutanoyl acetate, 2-butanone, 2-methylbutan-1-ol, ethyl butyrate, 2-methyl furan, isobutyric acid, and tetrahydofuran from M. albus showed antibiotic activity (Atmosukarto et al. 2005; Ramin et al. 2005; Mercier et al. 2007). Each VOC produced by the four novel Muscodor species but not produced by M. albus will be further investigated using biological tests. Further studies are necessary to determine their potential for biological control, food storage, agricultural production, and industrial applications.
References
Atmosukarto I, Castillo U, Hess WM, Sears J, Strobel G (2005) Isolation and characterization of Muscodor albus I-41.3 s, a volatile antibiotic producing fungus. Plant Sci 169:854–861. doi:10.1016/j.plantsci.2005.06.002
Azevedo JL, Ereira JOP, Araujo WL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biotechnol 3:40–65
Daisy B, Strobel G, Ezra D, Castillo U, Bairn G, Hess WM (2002) Muscodor vitigenus anam. sp. nov., an endophyte from Paullinia paullinioides. Mycotaxon 81:463–475
Ezra D, Hess WM, Strobel GA (2004) New endophytic isolates of Muscodor albus, a volatile antibiotic producing fungus. Microbiology 150:4023–4031. doi:10.1099/mic.0.27334-0
Gao F, Dai C, Liu X (2010) Mechanisms of fungal endophytes in plant protection against pathogens. Afr J Microbiol Res 4:1346–1351
González MC, Anaya AL, Glenn AE, Macías-Rubalcava ML, Hernández-Bautista BE, Hanlin RT (2009) Muscodor yucatanensis, a new endophytic ascomycete from Mexican chakah, Bursera simaruba. Mycotaxon 110:363–372
Hyde KD, Soytong K (2008) The fungal endophyte dilemma. Fungal Divers 33:163–173
Kudalkar P, Strobel G, Hassan S, Geary B, Sears J (2012) Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience. doi:10.1007/s10267-011-0165-9
Macías-Rubalcava ML, Hernández-Bautista BE, Oropeza F, Duarte G, González MC, Glenn AE, Hanlin RT, Anaya AL (2010) Allelochemical effects of volatile compounds and organic extracts from Muscodor yucatanensis, a tropical endophytic fungus from Bursera simaruba. J Chem Ecol 36:1122–1131. doi:10.1007/s10886-010-9848-5
Mercier J, Jiménez-Santamaría I, Tamez-Guerra P (2007) Development of the volatile-producing fungus Muscodor albus Worapong, Strobel, and Hess as a novel antimicrobial biofumigant. Rev Mex Fitopatol 25:173–179
Mitchell AM, Strobel GA, Hess WM, Vargas PN, Ezra D (2008) Muscodor crispans, a novel endophyte from Ananas ananassoides in the Bolivian Amazon. Fungal Divers 31:37–44
Ramin AA, Braun PG, Prange RK, DeLong JM (2005) In vitro effects of Muscodor albus and three volatile components on growth of selected postharvest microorganisms. Hortscience 40:2109–2114
Saikkonen K, Wali P, Helander M, Faeth SH (2004) Evolution of endophyte–plant symbioses. Trends Plant Sci 9:275–280. doi:10.1016/j.tplants.2004.04.005
Sopalun K, Strobel GA, Hess WM, Worapong J (2003) A record of Muscodor albus, an endophyte from Myristica fragrans in Thailand. Mycotaxon 88:239–247
Strobel GA (2003) Endophytes as source of bioactive products. Microbes Infect 5:535–544. doi:10.1016/S1286-4579(03)00073-X
Strobel GA, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67:491–502. doi:10.1128/mmbr.67.4.491-502.2003
Strobel GA, Dirske E, Sears J, Markworth C (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943–2950. doi:10.1099/mic.0.27334-0
Strobel GA, Kluck K, Hess WM, Sears J, Ezra D, Vargas PN (2007) Muscodor albus E-6, an endophytic of Guazuma ulmifolia making volatile antibiotics: isolation, characterization and experimental establishment in the host plant. Microbiology 153:2613–2620. doi:10.1099/mic.0.2007/008912-0
Suwannarach N, Bussaban B, Hyde KD, Lumyong S (2010) Muscodor cinnamomi, a new endophytic species from Cinnamomum bejolghota. Mycotaxon 114:15–23
Swofford DL (2002) PAUP*: Phylogenetic analysis using parsimony (*and other methods), beta version 4.0b10. Sinauer, Sunderland
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higguns DG (1997) The CLUSTAL_X Windows Interface: Flexible stratgies for multiple sequene alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Worapong J, Strobel GA, Ford EJ, Li JY, Brird G, Hess WM (2001) Muscodor albus anam. nov., an endophyte from Cinnamomum zeylanicum. Mycotaxon 79:67–79
Worapong J, Strobel GA, Daisy B, Castillo UF, Baird G, Hess WM (2002) Muscodor roseus anam. sp. nov., an endophyte from Grevillea pteridifolia. Mycotaxon 81:463–475
Zhang C, Wang G, Mao L, Komon-Zelazowska M, Yuan Z, Lin F, Druzhinina IS, Kubicek CP (2010) Muscodor fengyangensis sp. nov. from southeast China: morphology, physiology and production of volatile compounds. Fungal Biol 114:797–808. doi:10.1016/j.funbio.2010.07.006
Acknowledgments
This work was supported by The Higher Education Commission, Thailand, under the National Research University (A1) program, Chiang Mai University, and TRF Research-Team promotion Grant RTA5580007. We are grateful to Keegan Kennedy for improving the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suwannarach, N., Kumla, J., Bussaban, B. et al. Molecular and morphological evidence support four new species in the genus Muscodor from northern Thailand. Ann Microbiol 63, 1341–1351 (2013). https://doi.org/10.1007/s13213-012-0593-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-012-0593-6