Abstract
Phanerochaete velutina and the closely related species P. alnea, P. alnea subsp. lubrica, and P. rhodella are identified as distinct species based on morphological criteria and molecular analyses. Besides differences in macroscopic and microscopic basidiocarp features, the taxa differ in distribution and ITS sequence. Phanerochaete alnea is widely distributed in Eurasia and North America, whereas P. alnea subsp. lubrica is limited to the Pacific Coast of North America. Phanerochaete velutina is known from Eurasia and Alaska, and P. rhodella is found in North America only. Nomenclatural problems with the name Phanerochaete P. Karst. are discussed, and type specimens for Thelephora alnea Fr., the generic type of Phanerochaete, and Peniophora karstenii Massee are selected. Phanerochaete alnea, P. alnea subsp. lubrica, and P. velutina are described and illustrated. In addition, the synonymy of Phanerochaete robusta and P. aurantiobadia is confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phanerochaete P. Karst. (1889) is a large genus of so-called corticioid fungi, encompassing over 100 species (Index Fungorum 2016). Its current concept was developed by Eriksson (1958, as Membranicium, nom. prov.), Donk (1962), Parmasto (1968), and Burdsall (1985). Phanerochaete includes resupinate, crust-like fungi with a monomitic hyphal structure of predominantly clampless hyphae, and thin-walled, variably shaped, inamyloid, smooth basidiospores. Many species possess cystidia, and their presence, as well as shape, size and encrustation, is important for species recognition (Eriksson et al. 1978b; Burdsall 1985). Recent phylogenetic studies show that Phanerochaete s.l. is polyphyletic, and several new genera have been introduced, some of them placed into different families, or even orders (Greslebin et al. 2004; Wu et al. 2010; Binder et al. 2013; Floudas and Hibbett 2015). Most Phanerochaete species are still retained in a monophyletic lineage (Phanerochaetaceae sensu Larsson 2007) within Polyporales, along with genera such as Hyphodermella, Phlebiopsis, and Rhizochaete (Larsson 2007; Wu et al. 2010; Floudas and Hibbett 2015; Miettinen et al. 2016).
Nomenclatural problems with Phanerochaete revolve around different opinions about the generic type (Donk 1957; Burdsall 1977; Eriksson et al. 1978a, b). Cooke (1953) designated Thelephora alnea Fr. as the lectotype for Phanerochaete. Recent authors, however, consider Phanerochaete velutina (DC.) P. Karst. as the generic type (Eriksson et al. 1978b; Burdsall 1985; Chamuris 1988; Bernicchia and Gorjón 2010). Phanerochaete velutina has a number of synonyms, including Corticium decolorans P. Karst., Corticium rhodella Peck, and Peniophora karstenii Massee (Burdsall 1985). Recently, Floudas and Hibbett (2015) resolved a clade called the “Phanerochaete velutina complex” that included P. velutina, Phanerochaete conifericola Floudas & Hibbett, and P. rhodella (Peck) Floudas & Hibbett. In this paper we explore the species of this clade in more detail using morphological and DNA data, and suggest a solution to the nomenclatural issues surrounding the name Phanerochaete by selecting a neotype for Thelephora alnea and reinstate it as the generic type species.
Material and methods
Type material and specimens from H, GB, S, CFMR, G, LY, OULU, LE, and K, as well as from the private herbarium of Elia Martini (Switzerland), were studied. Herbarium acronyms follow Thiers (2016). Microscopic methods are described in Spirin et al. (2016).
DNA extraction, amplification of the ITS1-5.8S-ITS2 region of the rDNA, and sequencing are described in Volobuev et al. (2015). The partial coding region of tef1-α was amplified and sequenced using the primer pair 983 F and 1567R (Rehner and Buckley 2005). In total, 15 ITS and 10 tef1 sequences were prepared for this study and registered in GenBank or UNITE (Table 1).
Phylogenetic analyses
Three datasets were constructed and analyzed. The ITS dataset includes all available ITS sequences from the Phanerochaete velutina group (46 sequences). Sequences were selected by performing a BLAST search against DDBJ/EMBL-EBI/GenBank databases. The tef1 dataset includes 10 partial tef1 sequences. The tef1 and corresponding ITS sequences were combined into a third dataset.
We performed Bayesian inference with MrBayes 3.2 (Ronquist et al. 2012). Substitution models were determined with the aid of JModelTest (Darriba et al. 2012) and by comparing harmonic means of marginal probabilities of runs conducted with different settings. Models used were HKY + G for the ITS dataset (nst = 2, rates = gamma) and K80 (nst = 2, rates = equal, statfreqpr = fixed (equal)) for the tef1 and partitioned combined datasets. In the analyses, three parallel runs with eight chains each, temp = 0.1, were run for 2 million generations. All chains converged to <0.01 average standard deviation of split frequencies. Burn-in of 25% was used in final analyses.
Specimens examined (sequenced specimens are marked by asterisk)
Phanerochaete alnea. Canada. Alberta: Woodlands Co., McLeod Lake, Picea glauca and Populus sp., 30.VI.1969 Eriksson 12390, 12392 (GB); Yellowhead Co., William A. Switzer Provincial Park, Picea mariana, 24.VII.2015 Spirin 8829a* (H). British Columbia: Columbia-Shuswap, Bugaboo Provincial Park, coniferous log, 19.VIII.1982 Hallenberg 6672 (GB); Comox Valley, Forbidden Plateau, Abies amabilis, 6.IX.1967 Eriksson 8679 (GB). Ontario: Leeds Co., Lawrence Nat. Park, Pinus sp., 2.X.1975 Ginns (H ex DAOM 152157); Nipissing Dist., Algonquin Provincial Park, Thuja occidentalis, 19.IX.1982 Hallenberg 7396 (GB); Renfrew Co., Petawawa, Pinus banksiana, 8.X.1967 Eriksson 8840 (GB). Finland. Etelä-Häme: Tammela, “ad lignum corticemque Alni, Betulae et Populi tremulae”, Karsten 828 (H), 829 (Rabenhorst-Winter, Fungi Europaei #3231) (H, lectotype of Peniophora karstenii), Alnus sp., 18.IX.1880 Karsten (H), Betula sp., 29.IX.1882 Karsten (H), Juniperus communis, 6.III.1882 Karsten 807 (H), Populus tremula, 20.IX.1883 Karsten 1588 (H); Vilppula, Picea abies, 1.X.2003 Miettinen 7749.7* (H, holotype of Phanerochaete conifericola); Somerniemi, P. abies, 17.X.2003 Miettinen 8083 (H), 19.X.2003 Miettinen 8110 (H). Pohjois-Karjala: Lieksa, Pinus sylvestris, 23.IX.2003 Penttilä 14627* (H). Satakunta: Eura, P. abies, 6.X.2012 Uimonen (H); Loimaa, P. abies, 13.IV.2009 Poso* (OULU F080062). Kainuu: Kajaani, deciduous tree, 21.IX.2006 Helo 1534* (OULU F079522); Suomussalmi, P. abies, 1.VII.2014 Miettinen 18345 (H); Perä-Pohjanmaa: Rovaniemi, Betula sp., 17.VIII.1957 Kujala (H), P. abies, 31.VII.1979 Niemelä 1562 (H), 7.VIII.1987 Kotiranta 6278 (H). France. Pyrénées Atlantiques: Sansanet, Fagus sylvatica, 20.VII.1986 Gilles 580 (LY 11985, holotype of Phanerochaete binucleospordida). Germany. Nordrhein-Westfalen: Bielefeld, deciduous tree, 25.XI.2015 Miettinen 19852 (H). Schleiswig-Holstein: Ahrensburg, bark of Alnus and Betula, 14.V.1908 Jaap (Fungi Selecti #899) (H). Italy. Trentino: Paneveggio, Sorbus aucuparia, VIII.1893 Bresadola (S F15720, lectotype of Corticium cremeum). Netherlands. Noord-Brabant: Breda, Zundert, Salix sp., 1.VII.1958 Bas 1471 (H ex L). Norway. Telemark: Nome, Mörkvasslia Nat. Res., Betula sp., 23.IX.2003 K.H. Larsson 12054* (GB). Poland. Mazovia: Siedlce, Quercus robur, 1900 Eichler (S F15722, lectotype of Corticium eichlerianum). Russia. Karelia: Medvezhiegorsk Dist., Vitška, P. abies, 1.VII.1942 Laurila 3262 (H). Leningrad Reg.: Boksitogorsk Dist., Shidrozero, P. abies, 13.VII.2014 Spirin 6932 (H); Podporozhie Dist., Nemzha, P. abies, 1.X.2010 Spirin 3367 (H), Oksozero, P. abies, 3.X.2013 Spirin 6820* (H). Nizhny Novgorod Reg.: Lukoyanov Dist., Sanki, Q. robur, 20.VIII.2015 Spirin 9695 (H); Pavlovo Dist., Vorsma, Q. robur, 16.VII.2016 Spirin 10288 (H). Oryol Reg.: Znamenskoe Dist., Elenka, Q. robur, 13.VII.2011 Volobuev* (LE 298547). Perm Reg.: Krasnovishersk Dist., Kvarkush, Abies sibirica, 6.VIII.2005 Kotiranta 20848 (H), Betula sp., 6.VIII.2005 Kotiranta 20835 (H), Molebnyi Kamen, Abies sibirica, 12.VIII.2005 Kotiranta 20937* (H). Sweden. Östergötland, Alnus sp., 1891 Starbäck (herb. P. A. Karsten #675, H). Småland: Femsjö, Alnus sp., 27.VIII.1929 Nannfeldt 3408 (H, neotype of Thelephora alnea), Ribes uva-crispa, 25.VIII.1929 Nannfeldt 3382 (H); Ulfhult, Prunus padus (?), 5.VIII.1929 Nannfeldt 2918 (H). Upland: Lena, P. sylvestris, V.1927 Lundell 331 (H). Hälsingland: Delsbo, P. abies, 25.VII.1986 Kotiranta 6028 (H). Switzerland. Fribourg: Corminboeuf, deciduous tree, 3.X.1998 Martini 6715 (EM, dupl. H). Ticino: Alpe Püschett, Alnus viridis, 24.VI.1995 Martini 4128, 4129, 4191, 4192 (EM, dupl. H); Besso, Quercus sp., 3.X.1992 Martini 3417 (EM, dupl. H); Broglio, coniferous tree, 4.VIII.1996 Martini 6055 (EM, dupl. H); Faedo, Sarothamnus scoparius, 13.X.1985 Martini 290 (EM, H); Mogno, deciduous tree, 4.VIII.1985 Martini 227 (EM, dupl. H); Mondada, deciduous tree, 5.IX.1987 Martini 2237 (EM, H). USA. Arizona: Pima Co., Coronado Nat. Forest, Pinus sp., 26.VIII.1968 Burdsall 1399 (CFMR). Wisconsin: Dane Co., Madison, Pinus resinosa, 21.VI.1978 Burdsall 10228 (GB).
Phanerochaete alnea subsp. lubrica. Canada. British Columbia: Cowichan, fallen branches, 8.IX.1967 Eriksson 7748, 7750 (H), Alnus rubra, 9.IX.1967 Eriksson 7809 (GB), 12–14.IX.1967 Eriksson 7957, 8014, 8118 (GB), 17.IX.1967 Eriksson 8211 (GB), 5.IX.1982 Hallenberg 7050 (GB), Pseudotsuga menziesii, 9.IX.1967 Eriksson 7826 (GB), 10.IX.1967 Eriksson 7891 (GB), 12–13.IX.1967 Eriksson 7791, 8032 (GB),17–18.IX.1967 Eriksson 8208, 8264 (GB), 31.VIII.1982 Hallenberg 6917 (GB), drift wood, 27.IX.1967 Eriksson 8597 (GB); Squamish-Lillooet, Garibaldi Provincial Park, fallen log, 29.VIII.1982 Hallenberg 6877 (GB); Vancouver, fallen log of deciduous tree, 16.IX.1982 Hallenberg 7303 (GB). USA. Alaska: Chichagof Island, Pavlov Harbor, Picea sitchensis, 25.VII.1991 Burdsall 13753* (CFMR). Washington: Jefferson Co., Hoh River, P. sitchensis, 7.X.2014 Spirin 8229* (H), Spirin 8333* (H, holotype), Tsuga heterophylla, 20.X.2014 Spirin 8779b* (H), Acer macrophyllum, 20.X.2014 Spirin 8788 (H).
Phanerochaete martelliana. Iran. Golestan, Gorgan, dead hardwood twigs, 20.V.2008 Ghobad-Nejhad 1346 (H). Italy. Tuscany: Florence, Quercus, herb. Bresadola (H). Portugal. Trás-os-Montes e Alto Douro: Valpaços, Cytisus sp., 29.I.1991 Tellería 11358 (H ex LISU).
Phanerochaete rhodella. Canada. Alberta: Woodlands Co., McLeod Lake, Populus sp., 30.VI.1969 Eriksson 12346 (GB); Yellowhead Co., William A. Switzer Provincial Park, Populus tremuloides, 24.VII.2015 Spirin 8830 (H). Quebec: Cantley, deciduous branches, 10.VIII.1982 Hallenberg 6506 (GB); Outaouais, Gatineau National Park, Fagus grandifolia, 29.IX.1982 Hallenberg 7601 (GB). USA. Massachusetts: Worcester Co., Worcester, Quercus sp., 6.X.2013 Miettinen 17278* (H), Paxton, Quercus sp., 12.X.2013 Miettinen 17306 (H).
Phanerochaete robusta. Russia. Primorie: Khasan Dist., Kedrovaya Pad Nat. Res., angiosperm (fallen branch), IX.1961 Parmasto (LE 35895*, K ex TAAM 14125, isotypes).
Phanerochaete velutina. Finland. Varsinaissuomi: Tammisaari, Q. robur, 11.X.2006 Kotiranta 21402* (H). Uusimaa: Helsinki, Alnus glutinosa, 5.IX.2011 Miettinen 14694.3* (H), Betula sp., 19.VIII.2013 Rättel* (H), Salix sp., 30.X.2011 Miettinen 15027.1* (H). Etelä-Häme: Tammela, Salix sp., X.1879 Karsten 1405 (H, lectotype of Corticium decolorans). Pohjois-Karjala: Lieksa, Alnus incana, 25.VIII.1956 Kujala (H), P. tremula, 30.IX.2003 Penttilä 14759 (H). Russia. Bashkortostan: Burzyanskii Dist., Shulgan-Tash Nat. Res., Ulmus glabra, 8.VIII.2012 Kotiranta 25567* (H). Khabarovsk Reg.: Khabarovsk Dist., Bolshoi Khekhtsir Nat. Res., Corylus mandshurica, 5.IX.2013 Spirin 6671 (H), Malyi Niran, Picea ajanensis, 6.VIII.2012 Spirin 4986 (H), C. mandshurica, 8.VIII.2012 Spirin 5059* (H), Malyi Kukachan, P. ajanensis, 17.VIII.2012 Spirin 5344, 5353, 5357 (H), Povorotnaya, Rhododendron dauricum, 27.VIII.2012 Spirin 5740 (H), Ulun, P. ajanensis, 28.VIII.2012 Spirin 5765 (H); Solnechnyi Dist., Igdomi, P. tremula, 6.VIII.2011 Spirin 3854 (H), Abies nephrolepis, 7.VIII.2011 Spirin 3949* (H); Verkhnebureinskii Dist., Kyvyty, P. ajanensis, 17.VIII.2014 Spirin 7421* (H), Dublikan Nat. Res., P. ajanensis, 20.VIII.2014 Spirin 7666 (H). Krasnoyarsk Reg.: Turukhansk Dist., Mirnoe, Alnus sibirica, 19.VIII.2013 Kotiranta 26326 (H). Leningrad Reg.: Volkhov Dist., Zagubie, P. tremula, 3.X.2010 Spirin 3638 (H). Nizhny Novgorod Reg.: Lukoyanov Dist., Razino, Q. robur, 16.VIII.2015 Spirin 9343* (H), Tilia cordata, 16.VIII.2015 Spirin 9365 (H); Sharanga Dist., Kilemary Nat. Res., Ulmus laevis, 26.IX.1999 Spirin (LE 210942), T. cordata, 24.VIII.2000 Spirin (LE 210954). Switzerland. Jura, Quercus sp., 1813 Chaillet (G 00273767, lectotype of Thelephora velutina). Ukraine. Zakarpats’ka Reg.: Dilove, Fagus sylvatica, VIII.1938 Pilát (H ex PRM).
Results and discussion
Typification of Thelephora alnea Fr. and Phanerochaete P. Karst.
The genus Phanerochaete was introduced by Karsten (1889) with two species—P. alnea (Fr.) P. Karst. (= Thelephora alnea Fr.) and P. odorata (Fr.) P. Karst. (= T. odorata Fr.). For many years, Phanerochaete was considered a synonym of Peniophora when that genus included all corticioid species with cystidia. Cooke (1953) selected P. alnea as the generic type. Parmasto (1968), following Karsten (1898), Bresadola (1903), and Höhnel and Litschauer (1906), considered P. alnea a synonym of P. velutina, and therefore listed the latter species as the type for Phanerochaete. However, the absence of type material for Thelephora alnea that could support the synonymy was not addressed.
Burdsall (1977), recognizing that the identity of Thelephora alnea was unclear, chose Thelephora odorata Fr. as a lectotype of the genus because its identity was supported by an authentic material at UPS (see Hallenberg 1985). With this interpretation, Phanerochaete became an older name for Scytinostroma, and therefore Burdsall (1977) resurrected Grandiniella P. Karst. to replace Phanerochaete sensu Parmasto. A year later Eriksson et al. (1978a) made a counter proposal to preserve Phanerochaete. In their opinion, T. alnea could not be avoided as the generic type. In the protologue of Phanerochaete, Karsten (1889) included Corticium decolorans P. Karst. as a synonym of Thelephora alnea. Corticium decolorans was described 7 years earlier (Karsten 1882), and by listing it as a synonym of T. alnea, Karsten explained his interpretation of the latter name. By accepting C. decolorans as the lectotype of Phanerochaete, the modern concept of the genus could be preserved. Continuing in this vein, Eriksson et al. (1978b: 1038) selected a type specimen for C. decolorans from Karsten’s herbarium that was conspecific with T. velutina Fr., in keeping with Karsten’s 1893 intention for the genus. This specimen then also served as the lectotype for Phanerochaete (Eriksson et al. 1978b: 987). At this point, both Thelephora alnea and T. velutina were left non-typified, although the latter species was typified later by Burdsall (1985).
Our approach to typification of P. alnea, as well as the genus Phanerochaete in general, is based on the following arguments:
-
1.
Thelephora alnea Fr. is considered the type species (lectotype) of the genus Phanerochaete P. Karst., in accordance with Art. 10.5 of the Code (2011), because the lectotypification of the genus by Cooke (1953) precedes those proposed by Burdsall (1977) and Eriksson et al. (1978a, b). Cooke’s citation of the type species as “Phanerochaete alnea Karst.”, without formal reference to Fries’s T. alnea, is interpreted as an accidental omission and should not influence the validity of the lectotype selection.
-
2.
The lectotype of C. decolorans designated by Eriksson et al. (1978b) does not preclude independent typification of T. alnea and T. velutina DC., according to Art. 9.16 of the Code (2011).
-
3.
Only Karsten’s original description of the genus (Karsten 1889) and his previous work (Karsten 1882) can be used to interpret his concept of T. alnea. A description of T. alnea first appeared in 1882 (Karsten 1882: 137) alongside a protologue of C. decolorans (ibid.: 144), suggesting that he considered these taxa to be different species. In a later description of T. alnea, Karsten (1889) used nearly identical language to describe a species with reddish-yellowish basidiocarps, tubular cystidia, and ellipsoid basidiospores that occurs on wood of pine and alder in southern and western parts of Finland. These diagnostic features fit well with specimens distributed by Karsten as Corticium alneum (Fr.) P. Karst. in Rabenhorst-Winter’s Fungi Europaei exsiccates (#3231), which, as Donk (1957) already noted, belong to a species closely related to P. velutina. Burdsall (1977), however, argued that these exsiccati cannot be used for neotypification of T. alnea Fr. because Fries (1821) never studied them nor collected in Finland. Furthermore, the original description gives no clear indication whether Fries’ species is similar to Karsten’s species concept. This problem is best resolved by selecting a neotype for T. alnea from Fries’s home area in Sweden that also conforms to Karsten’s (1882, 1889) descriptions. In this way, the current generic concept of Phanerochaete is preserved. In his later works, Karsten (1893, 1898) placed T. alnea and C. decolorans in synonymy under P. velutina. Although Eriksson et al. (1978b) considered these later works as an emendation to the protologue of Phanerochaete, we consider them not relevant for clarifying the identity of Thelephora alnea. Neither should we take into account Karsten’s later decision to move Phanerochaete velutina to Peniophora (Karsten 1899) and to retain Phanerochaete for Stereum karstenii Bres. (≡ Dacryobolus karstenii (Bres.) Oberw.).
Molecular phylogenetic analyses
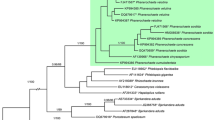
DNA analyses confirm that P. alnea as defined herein is closely related to P. rhodella and P. velutina. The ITS dataset resolves four clades in the Phanerochaete velutina group: P. velutina, P. alnea, P. rhodella and P. aff. velutina from California (GenBank KP814202). In addition, P. alnea is composed of two subclades based on two nucleotide differences in the ITS sequence (Fig. 1). The tef1 dataset analysis separated P. alnea from P. velutina (P. rhodella was not included) and grouped P. alnea samples slightly differently compared to the ITS tree (Fig. 2). The combined ITS-tef1 dataset tree was similar to the ITS tree (Fig. 3).
Phylogenetic tree based on the combined ITS+tef1 dataset – 1327 nucleotide positions of 10 taxa. Bayesian inference: MrBayes 3.2, HKY+G substitution model for the ITS partition, K80 substitution model for the TEF1 partition. Three runs with eight chains each, two million generations. 25% of trees discarded as burn-in
Phanerochaete aurantiobadia was recently introduced as a species belonging to the core Phanerochaete clade (Ghobad-Nejhad et al. 2015). Our analysis of ITS sequences confirms that this species belongs to Phanerochaete (as outlined by Floudas and Hibbett 2015) but also that it is distantly related to P. alnea and its allies (data not shown). Phanerochaete robusta, described from the same geographical region (Parmasto 1968), seems morphologically indistinguishable from P. aurantiobadia. ITS sequences from the types of P. robusta and P. aurantiobadia are identical.
Species descriptions
Phanerochaete alnea (Fr.) P. Karst., Bidr. Känned. Finlands Nat. Folk 48: 427, 1889. Figs. 4 and 7a, b
≡ Thelephora alnea Fr., Systema Mycologicum 1: 446, 1821.
Neotype. Sweden. Småland: Femsjö, Alnus sp., 27.VIII.1929 Nannfeldt 3408 (H, selected here).
= Peniophora karstenii Massee, Bot. J. Linnean Soc. 25: 153, 1889.
Lectotype. Finland. Etelä-Häme: Mustiala, “ad lignum corticemque Alni, Betulae et Populi tremulae”, Karsten 829 (Rabenhorst-Winter, Fungi Europaei #3231) (H, selected here).
= Corticium cremeum Bres., Fungi Tridentini 2: 63, 1898.
Lectotype. Italy. Trentino: Paneveggio, Sorbus aucuparia, VIII.1893 Bresadola (S F15720, studied) (selected by Eriksson et al. 1978b).
= Corticium eichlerianum Bres., Ann. Mycol. 1: 95, 1903.
Lectotype. Poland. Mazovia: Siedlce, Quercus robur, 1900 Eichler (S F15722, studied) (selected by Eriksson et al. 1978b).
= Phanerochaete velutina (DC.) P. Karst. var. cremicolor Parmasto (pr. p.), Consp. Syst. Cort.: 222, 1968.
= Phanerochaete binucleospordida Boidin, Lanq. & Gilles, Cryptogamie Mycologie 14: 195, 1993.
Holotype. France. Pyrénées Atlantiques: Sansanet, Fagus sylvatica, 20.VII.1986 Gilles 580 (LY 11985, studied).
= Phanerochaete conifericola Floudas & Hibbett, Fungal Biology 119: 705, 2015.
Holotype. Finland. Etelä-Häme: Vilppula, Picea abies, 1.X.2003 Miettinen 7749.7 (H, studied).
Basidiocarps resupinate, membranous, up to 20 cm in widest dimension, 0.2–0.5 mm thick. Margin first distinct, fibrillose, white, sometimes fimbriate with well-developed short hyphal strands, later more or less compact, thinning out, often paler than the hymenial surface, up to 1 mm wide. Hymenium ceraceous, pale pink to pale ochraceous, in vigorously growing specimens with reddish-vinaceous hues, in dry basidiocarps often darker, turning dark red in KOH, herbarium specimens stored for more than 20 years usually assume a yellowish-orange to bright orange colour.
Hyphal structure monomitic; hyphae mostly clampless.
Hyphae in strands parallel, with distinct (up to 2 μm thick) walls, clampless or with occasional clamps, 4–10 μm in diam., smooth or rarely covered by sparse angular crystals. Subicular hyphae parallel to interwoven, not adhering together, thin- to slightly thick-walled, mostly clampless but occasionally with large solitary or double clamps, branched at acute angles, (4.9–) 5.1–10.8 (−11.2) μm in diam. (n = 80/4), often easily collapsing, smooth or occasionally bearing small, irregularly arranged resinous grains or angular crystals. Subhymenial hyphae interwoven or more or less vertically arranged, clampless, mostly thin-walled, (3.1–) 3.2–5.1 (−5.2) μm in diam. (n = 80/4). Cystidia tubular to subfusiform, usually widened close to the base and more or less tapering towards the blunt or very rarely acute apex, (51.8–) 52.3–144.7 (−149) × (6–) 6.2–11 (−11.4) μm (n = 140/7), projecting up to 80 μm beyond the hymenial layer, easily collapsing, walls first gradually thinning towards apex, later more or less equally thickened throughout, up to 1.5 μm thick; encrustation irregularly present, often apical or located at the middle part of cystidia, crystals discrete, angular or very rarely aggregated to a solid sheath. Basidia long clavate, (20.8–) 21.4–49.4 (−50.1) × (4.7–) 4.8–6.8 (−6.9) μm (n = 92/6). Basidiospores narrowly ellipsoid to broadly cylindrical, (4.8–) 4.9–7.5 (−8.1) × (2.8–) 2.9–4 (−4.1) μm (n = 270/9), L = 6.06, W = 3.20, Q = 1.68–2.14, ventral side flat or slightly convex, occasionally slightly concave; oil droplets often present in cytoplasm. Resinous substance present as variably shaped particles, abundant in older basidiocarps and herbarium specimens, golden yellow, covering subhymenial hyphae and hymenial elements.
Notes. In 1821, Fries described a pale-reddish corticioid fungus collected on wood of alder and indicated he saw a living specimen (“v.v.”), implying that it occurs in Femsjö, Fries’ primary collecting place in the southern part of Sweden. Since no authentic specimens of T. alnea exist, a neotype from Femsjö is designated here. The same species was distributed by Karsten in Rabenhorst-Winter’s Fungi Europaei as Corticium alneum (Fr.) P. Karst. This exsiccati collection is the basis for Peniophora karstenii described by Massee (1890) and the lectotype of P. karstenii is designated above. We examined the type specimens of Corticium cremeum and Corticium eichlerianum, traditionally listed among the synonyms of P. sordida (P. Karst.) J. Erikss. & Ryvarden (Eriksson et al. 1978b; Volobuev et al. 2015). Both are conspecific with P. alnea. The more recent introductions of Phanerochaete binucleospordida (Boidin et al. 1993) and P. conifericola (Floudas and Hibbett 2015) have extended the list of synonyms further. Both species are morphologically identical to P. alnea. and in our phylogeny the type specimen sequence of P. conifericola clusters with P. alnea (Fig. 1).
Morphological identification of Phanerochaete alnea can be difficult. Herbarium specimens are often mislabelled as P. sordida or P. velutina, depending on the maturity of the basidiocarps. Young, thin basidiocarps of T. alnea lacking hyphal strands at the margin (e.g. types of C. cremeum, C. eichlerianum and P. binucleospordida) are strongly reminiscent of P. sordida, and tubular, sparsely encrusted cystidia with walls thinning towards the apex also points to the latter species. Cystidia of P. sordida are on average narrower than in P. alnea, 4–8 μm in diam. versus 6–11 μm in P. alnea. Additionally, subicular hyphae of P. sordida are distinctly thick-walled, variably interwoven, 5–7.5 μm in diam., with exceptionally rare clamps (Volobuev et al. 2015). Mature and senescent basidiocarps of P. alnea (for example, types of P. karstenii and P. conifericola) can be mistaken for P. velutina. Microscopically, cystidia in P. velutina are wider and more heavily encrusted with fused, indistinct hyaline crystals. In addition, cystidia of P. alnea are typically narrower and basidiospores larger than in P. velutina, although these differences are not absolute (Tables 2 and 3). Moreover, subicular hyphae of P. alnea lack the dense crystalline encrustation so characteristic of P. velutina.
The most easily observed difference between these species is the ability of P. alnea to produce a dark red coloration in KOH (see Floudas and Hibbett 2015, as P. conifericola), whereas there is no color change in P. velutina or P. sordida. Another striking feature of P. alnea is the distinct, yellowish-orange or bright orange coloration of basidiocarps kept in herbarium for decades, which appears to be the result of accumulating yellow, resinous substances. All herbarium specimens of P. velutina studied had retained their original colour or developed greyish hues (Fig. 7c, d). Aged basidiocarps of P. martelliana (Bres.) J. Erikss. & Ryvarden also turn orange as in P. alnea. However, P. martelliana has much shorter, subulate cystidia and larger basidiospores compared to P. alnea (Eriksson et al. 1978b).
Phanerochaete alnea is widely distributed in the Northern Hemisphere and is the only holarctic species in the P. velutina group. Its distribution is in general more boreal than that of P. velutina. Phanerochaete alnea seems to be the most common species of Phanerochaete in the middle and northern taiga subzones. It inhabits mostly coniferous hosts but regularly occurs on wood of deciduous trees. Specimens of P. alnea from the Pacific coast of North America belong to a new subspecies, subsp. lubrica, due to small (2 bp) differences in ITS sequences and slightly deviating morphology as described below.
Phanerochaete alnea subsp. lubrica Spirin & Volobuev, subsp. nova – Fig. 5
Holotype. USA. Washington: Jefferson Co., Hoh River, Picea sitchensis, 7.X.2014 Spirin 8333 (H).
Etymology. “Lubricus” (Lat., adj.) – ticklish, critical; referring to the small morphological differences separating it from closely related species.
MB 818235
Basidiocarps resupinate, membranous, up to 5 cm large, 0.1–0.3 mm thick. Margin first distinct, whitish, arachnoid or rarely with hyphal strands, later more or less compact, thinning out, concolorous to or paler than hymenial surface, up to 0.5 mm wide. Hymenium ceraceous to gelatinous, pale grey, in dried basidiocarps pale ochraceous, turning faintly or distinctly dark red in KOH, in specimens kept in herbarium for decades bright orange.
Hyphal structure monomitic; hyphae mostly clampless.
Hyphae in hyphal strands parallel, with distinct (up to 1 μm thick) walls, clampless or with occasional clamps, 4–14 μm in diam., often encrusted with densely arranged, angular crystals. Subicular hyphae parallel to interwoven, densely agglutinated, thin- to slightly thick-walled, mostly clampless but occasionally with large solitary or double clamps, branched at acute angles, (5.1–) 5.2–12.3 (−14.2) μm in diam. (n = 60/3), sometimes encrusted. Subhymenial hyphae interwoven or more or less vertically arranged, clampless, mostly thin-walled, (2.8–) 2.9–4.7 (−4.8) μm in diam. (n = 40/2). Cystidia tubular, usually basally widened, tapering to a blunt or very rarely acute apex, (56.4–) 59.6–104.2 (−117.9) × (6.3–) 6.5–10.8 (−11.3) μm (n = 60/3), projecting up to 70 μm beyond the hymenial layer, walls first gradually thinning out, later more or less equally thickened throughout, up to 1.5 μm thick; encrustation irregularly present, apical or scattered at the middle part of cystidia, rarely covering the whole cystidium, consisting of discrete angular crystals. Basidia clavate, (16.6–) 17.8–27.3 (−29.2) × (4.3–) 4.4–5.2 (−5.3) μm (n = 17/2). Basidiospores broadly cylindrical to narrowly ovoid, (4.2–) 4.3–6.1 (−6.2) × (2.3–) 2.4–3.2 (−3.3) μm (n = 120/4), L = 5.00, W = 2.83, Q = 1.71–1.86, ventral side often flat or slightly convex, in some spores indistinctly concave; oil droplets often present. Resinous droplets rare or absent. Basidiospores in some older collections occasionally reaching 8 μm long; they are excluded from measurements given above.
Notes. The best features for recognizing P. alnea subsp. lubrica are more or less gelatinized hymenium and strongly agglutinated basal hyphae. Its basidiospores are narrower than in P. alnea s. str. (Table 2). P. alnea subsp. lubrica is widely distributed along the Pacific Northwest coast, inhabiting fallen branches and logs of both conifer and hardwood trees.
Phanerochaete robusta Parmasto, Consp. Syst. Cort.: 220, 1968.
Holotype. Russia. Primorie: Khasan Dist., Kedrovaya Pad’ Nat. Res., angiosperm (fallen branch), IX.1961 Parmasto (LE, K ex TAAM 14125 – isotypes, studied).
= Phanerochaete aurantiobadia Ghobad-Nejhad, S.L. Liu & E. Langer, Mycological Progress 14 (68): 4, 2015.
Holotype. China. Jilin: Antu Co., Changbai Nat. Res., angiosperm (stump), 7.IX.2011 Ghobad-Nejhad 2288 (BJFC) (not studied, see description in Ghobad-Nejhad et al. 2015).
Phanerochaete robusta is an East Asian species with reddish-orange basidiocarps that turn bright red in KOH. Microscopically, it is recognizable by enclosed cystidia and by cylindrical to narrowly ovoid basidiospores, (4.9–) 5.0–7.1 (−8.1) × (2.1–) 2.2–3.0 (−3.1) μm (n = 30/1), L = 5.98 , W = 2.59 , Q = 2.32 (measured from the isotype in K). It was described from Primorie, Russian Far East (Parmasto 1968). Phanerochaete aurantiobadia, recently described from the adjacent part in China (Ghobad-Nejhad et al. 2015), shows no morphological differences when compared to P. robusta and is therefore considered its synonym. ITS sequences from the type specimens of P. robusta and P. aurantiobadia are identical.
Phanerochaete velutina (DC.) P. Karst., Kritisk Öfvers. Finlands Basidsvampar 3: 33, 1898. – Figs. 6 and 7c–d.
≡ Thelephora velutina DC., Flora Française 6: 33, 1815.
Lectotype. Switzerland. Jura, Quercus sp., 1813 Chaillet (G 00273767, studied) (selected by Burdsall 1985: 136, indicated as “holotype”).
= Corticium decolorans P. Karst., Bidr. Känned. Finlands Nat. Folk 37: 144, 1882.
Lectotype. Finland. Etelä-Häme: Tammela, Salix sp., X.1879 Karsten 1405 (H, studied) (selected by Eriksson et al. 1978b).
Basidiocarps resupinate, membranous, up to 20 cm large, 0.2–0.5 mm thick. Margin first distinct, fibrillose, white, sometimes fimbriate with well-developed short hyphal strands, later more or less compact, thinning-out, often paler than hymenial surface, up to 1 mm wide. Hymenium ceraceous, pale to bright pink, in vigorously growing specimens with scattered dark red dots, in dry basidiocarps pink to pinkish ochraceous, sometimes with greyish hues, almost unchanged in aged herbarium specimens, no color change in KOH.
Hyphal structure monomitic; hyphae mostly clampless.
Hyphae in hyphal strands parallel, with distinct (up to 2 μm thick) walls, clampless or with occasional clamps, 5–17 μm in diam., often densely encrusted with angular crystals. Subicular hyphae parallel to interwoven, densely packed but not agglutinated, with thickened walls, mostly clampless but occasionally with large solitary or double clamps, branched at acute angles, (4.6–) 5.3–11 (−12.2) μm in diam. (n = 120/6), often encrusted by tightly arranged angular crystals or bearing solid crystalline encrustation. Subhymenial hyphae interwoven or more or less vertically arranged, clampless, thin- to slightly thick-walled, (2.7–) 3–5.2 (−5.3) μm in diam. (n = 100/5). Cystidia tubular to obclavate, in most cases basally widened, tapering towards an obtuse apex, (45.5–) 55.1–142.3 (−159.6) × (6.3–) 6.6–15.8 (−17.7) μm (n = 200/10), projecting up to 100 μm beyond hymenium, walls more or less evenly thickened throughout, up to 3 μm thick; encrustation always present at apex, often covering the entire projecting part, first consisting of discrete angular crystals, later aggregated into a solid sheath. Basidia clavate, (19.4–) 21.2–40.8 (−41.0) × (4–) 4.4–6 (−6.2) μm (n = 62/5). Basidiospores ellipsoid, rarely broadly cylindrical (longest spores only), (4.7–) 4.8–7.2 (−7.3) × (2.6–) 2.7–3.6 (−3.8) μm (n = 340/11), L =5.55, W = 3.05, Q = 1.67–1.98, ventral side often slightly convex, rarely flat or somewhat concave; oil droplets often present. Resinous droplets usually abundant in mature specimens, brownish, found mostly on tramal and subhymenial hyphae, as well as on cystidia.
Notes. P. velutina was introduced by De Candolle (1815) as Thelephora velutina DC. The original specimen collected by Chaillet in Jura (G) was called holotype by Burdsall (1985) but is properly a lectotype. It is in perfect condition and agrees well with the species concept of P. velutina presented here. The protologue of C. decolorans P. Karst. was based on at least two collections so labelled by Karsten at H. One specimen is P. alnea and the other is P. velutina in the current sense. Eriksson et al. (1978) selected the latter specimen (Karsten 1405) as a lectotype of C. decolorans; therefore, C. decolorans is a later synonym of P. velutina.
Differences of P. velutina from P. alnea are discussed above. The North American P. rhodella, recently recognized as a distinct species (Floudas and Hibbett 2015), is similar to P. velutina macroscopically but has on average smaller cystidia and basidiospores (Tables 2 and 3).
The geographic distribution of P. velutina is uncertain because the name has been applied to several species. Most Canadian and US records of P. velutina on angiosperm hosts refer to P. rhodella; however, P. velutina occurs in Alaska based on three sequenced specimens (Floudas and Hibbett 2015). European and North American specimens of P. velutina collected from conifers probably represent P. alnea. Verified records of P. velutina from coniferous hosts are known so far from the Russian Far East. These collections possess wider cystidia than specimens of P. velutina from Europe; however, there is no DNA evidence to support their recognition as a separate taxon.
References
Bernicchia A, Gorjón SP (2010) Corticiaceae s.l. Fungi Europaei 12:1–1008
Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldes F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev I, Hibbett DS (2013) Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105:1350–1373
Boidin J, Lanquetin P, Gilles G (1993) Contribution a la conaissance des Phanerochaetoideae de France (Basidiomycotina). Cryptogam Mycol 14:195–206
Bresadola G (1903) Fungi Polonici. Ann Mycol 1:97–131
Burdsall HH (1977) A consideration of the names Phanerochaete, Membranicium, and Grandiniella. Taxon 26:327–330
Burdsall HH (1985) A contribution to the taxonomy of the genus Phanerochaete. Mycol Mem 10:1–165
Candolle, AP de (1815). Flore Française. Edition 3 [French Flora] 5-6: i-x, 1-662. Paris: Desray
Chamuris GF (1988) The non-stipitate stereoid fungi in the northeastern United States and adjacent areas. Mycol Mem 14:1–124
Cooke WB (1953) The genera of Homobasidiomycetes (exclusive of the Gastromycetes). USDA Div Mycol Dis Surv Spec Publ 3:1–100
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9: 772–772. http://www.nature.com/nmeth/journal/v9/n8/abs/nmeth.2109.html#supplementary-information
Donk MA (1957) The generic names proposed for Hymenomycetes 7. “Thelephoraceae”. Taxon 6:68–123
Donk MA (1962) Notes on resupinate Hymenomycetes 7. Persoonia 2:217–238
Eriksson J (1958) Studies in the Heterobasidiomycetes and Homobasidiomycetes – Aphyllophorales of Muddus National Park in North Sweden. Symbolae Bot Upsaliensis 16:1–172
Eriksson J, Weresub L, Burdsall HH (1978a) Phanerochaete vs Grandiniella. Taxon 27:51–52
Eriksson J, Hjortstam K, Ryvarden L (1978b) The Corticiaceae of North Europe. 5:887–1047
Floudas D, Hibbett DS (2015) Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol 119:679–719. doi:10.1016/j.funbio.2015.04.003
Fries EM (1821) Syst Mycol 1:1–520
Frӧhlich-Nowoisky J, Pickersgill DA, Després VR, Pӧschl U (2009) High diversity of fungi in air particulate matter. Proc Natl Acad Sci U S A 106:12814–12819. doi:10.1073/pnas.0811003106
Ghobad-Nejhad M, Liu SL, Langer EJ, Dai YC (2015) Molecular and morphological evidence reveal a new non-cystidiate species belonging to the core Phanerochaete (Polyporales). Mycol Prog 14(68):1–7
Greslebin A, Nakasone KK, Rajchenberg M (2004) Rhizochaete, a new genus of phanerochaetoid fungi. Mycologia 96:260–271
Hallenberg N (1985) The Lachnocladiaceae and Coniophoraceae of North Europe. Fungiflora, Oslo
Höhnel F, Litschauer V (1906) Beitrӓge zur Kenntnis der Corticeen. Sitzungsber Kaiserl Akad Wiss Math-Naturwiss Kl Abt I 115:1549–1620
Index Fungorum. http://indexfungorum.org. Accessed 25 Apr 2016
International Code of nomenclature for algae, fungi and plants (Melbourne Code, 2011). http://iapt-taxon.org/nomen. Accessed 25 Apr 2016
Karsten PA (1882) Rysslands, Finlands och den Skandinaviska halfӧns Hattsvampar. Bidrag Kӓnned Finl Nat Folk 37:1–257
Karsten PA (1889) Kritisk ӧfversigt af Finlands Basidsvampar (Basidiomycetes; Gastero- & Hymenomycetes). 1. Bidrag Kӓnned Finl Nat Folk 48:1–470
Karsten PA (1893) Kritisk ӧfversigt af Finlands Basidsvampar (Basidiomycetes; Gastero- & Hymenomycetes). 2: 1–32
Karsten PA (1898) Kritisk ӧfversigt af Finlands Basidsvampar (Basidiomycetes; Gastero- & Hymenomycetes). 3: 1–36
Karsten PA (1899) Finlands Basidsvampar. 11:1–186
Larsson KH (2007) Re-thinking the classification of corticioid fungi. Mycol Res 111:1040–1063. doi:10.1016/j.mycres.2007.08.001
Massee GE (1890) A monograph of Thelephoracerae. 1. Bot J Linn Soc 25:107–155
Miettinen O, Spirin V, Vlasák J, Rivoire B, Stenroos S, Hibbett DS (2016) Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys 17:1–46. doi:10.3897/mycokeys.17.10153
Parmasto E (1968) Cospectus systematis corticiacearum. Institutum Zool Bot Estoniae, Tartu
Pitkӓranta M, Meklin T, Hyvӓrinen A, Paulin L, Auvinen P, Nevalainen A, Rintala H (2008) Analysis of fungal flora in indoor dust by ribosomal DNA sequence analysis, quantitative PCR, and culture. Appl Environ Microbiol 74:233–244
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi:10.1093/sysbio/sys029
Spirin V, Vlasák J, Rivoire B, Kotiranta H, Miettinen O (2016) Hidden diversity in the Antrodia malicola group (Poylporales, Basidiomycota). Mycol Prog 15(51):1–12. doi:10.1007/s11557-016-1193-9
Thiers B (2016) Index Herbariorum: a global directory of public herbaria and associated stuff. New York botanical garden’s virtual herbarium. http://sweetgum.nybg.org/ih. Accessed 25 Apr 2016
Volobuev S, Okun M, Ordynets A, Spirin V (2015) The Phanerochaete sordida group (Polyporales, Basidiomycota) in temperate Eurasia, with a note on Phanerochaete pallida. Mycol Prog 14(80):1–13. doi:10.1007/s11557-015-1097-0
Wu SH, Nilsson HR, Chen CT, Yu SY, Hallenberg N (2010) The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phlebioid clade of the Polyporales (Basidiomycota). Fungal Divers 42:107–118. doi:10.1007/s13225-010-0031-7
Acknowledgements
Curators of herbaria GB, S, CFMR, G, LY, OULU, K arranged for specimen loans. Elia Martini (Switzerland) kindly provided us with valuable specimens. Aleksandr Sennikov (University of Helsinki) helped us with nomenclatural advice. Sequencing work was carried out at the Core Facility Center “Cell and Molecular Technologies in Plant Science”, Komarov Botanical Institute RAS (St. Petersburg, Russia). Research work of the author SV was supported by a grant from the President of Russian Federation (MK-6345.2015.4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Yu-Cheng Dai
Rights and permissions
About this article
Cite this article
Spirin, V., Volobuev, S., Okun, M. et al. What is the type species of Phanerochaete (Polyporales, Basidiomycota)?. Mycol Progress 16, 171–183 (2017). https://doi.org/10.1007/s11557-016-1267-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1267-8