Abstract
Poria eichelbaumii, a long-forgotten name, is transferred to Haploporus based on studies of the type specimen, comparison with new collections, and evidences from both morphology and phylogeny. The species is redescribed and illustrated. Haploporus grandisporus is described as new, on the basis of concordant morphological and phylogenetic species concepts. These two species form two very closely related clades within the Haploporus lineage in [28S-ITS]-based phylogenetic inferences. Haploporus eichelbaumii and H. grandisporus are distinguished by the size of their basidiospores (11.5–14.5 × 5.0–6.0 μm, average = 12.7 × 5.8 μm, vs 14–17.5 × 6.0–7.3 μm, average 15.4 × 6.6 μm), the size of their pores (2.5–3.5 / mm, vs 1.5–2.5 / mm), and, likely, divergent autecologies. Although both species occur in montane ecosystems of the eastern African rift, the data so far available suggest they occupy different habitats. Haploporus eichelbaumii has wider distribution, spanning over both branches of the eastern rift, at elevation ~ 1500–2500 masl, in various vegetation types, mostly on small-sized dead branches or twigs, and dead bamboo culms. It is known so far from Kenya, Tanzania, and Malawi to the East, and western Burundi, western Uganda, and Eastern Congo (DRC) in the Albertine mountain ranges. Haploporus grandisporus is known, hitherto, only from the Eastern slopes of Mount Elgon in Kenya, at the timberline, 2900–3200 masl, mostly on dead heather branches (Erica arborea, Ericaceae) in heather thickets. Haploporus nanosporus is currently the third known Haploporus species from tropical Africa, known from the western edge of the Guineo-Congolian rain forest in Gabon and Cameroon. This species is also redescribed and illustrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haploporus Bondartsev & Singer (core polyporoid clade, Basidiomycota, Justo et al. 2017), typified by H. odorus (Singer 1944), was characterized by the combination of a pileate basidiome, a trimitic hyphal system, and, most notably, thick-walled basidiospores ornamented with discontinuous, longitudinally oriented tubercles.
Keller (1974, 1986) studied and described the ontogenesis and resulting ultrastructure of these basidiospores. He (Keller 1986) also showed that this ontogenesis and ultrastructure were not unique in the polypores but had an equivalent in Pachykytospora tuberculosa (Fr.) Kotl. & Pouzar (Keller 1977), the type species of Pachykytospora Kotl. & Pouzar (Kotlaba and Pouzar 1963); the fine wall architecture in both species was found to be identical, which was interpreted as indicating affinities (Keller 1974, 1986). Haploporus odorus and P. tuberculosa also share the same hyphal system, and physiologically, both species cause a white rot of wood, with a marked preference, respectively, for Salix caprea (Salicaceae) and Quercus sp. (Fagaceae) (Ryvarden and Melo 2014). They differ, however, in their basidiome habit, resupinate vs pileate (Ryvarden and Melo 2014).
Haploporus remained monotypic for about 50 years [with the exception of Haploporus ljubarskyi (Pilát) Bondartsev & Singer ex Bondartsev (Bondartsev 1971), which is currently accepted in Trametes]; it was not until 1993 that Zeng and Bai (1993) added a second species, H. amarus X.L. Zeng & Y.P. Bai, nowadays a superfluous synonym of H. odorus (Zhou et al. 2019). Later on, emphasizing the microscopic characteristics and, in particular, the basidiospores morphology, Dai et al. (2002) argued to merge Haploporus and Pachykytospora; hence, they relegated the basidiome habit (pileate vs resupinate) as a subordinate element. Dai et al. (2002) then proposed the new combinations H. tuberculosus, H. alabamae [Pachykytospora alabamae (Berk. & Cooke) Ryvarden], and H. papyraceus [Pachykytospora papyracea (Schwein.) Ryvarden]. Subsequently, Piątek (2003, 2005) and Dai (in Yu et al. 2005) also proposed, respectively, the combinations H. nanosporus (Pachykytospora nanospora A. David & Rajchenb.), H. nepalensis [Pachykytospora nepalensis T. Hatt.], and H. thindii [Pachykytospora thindii Natarajan & Koland.]. Li et al. (2007), Shen et al. (2016), and Zhou et al. (2019) added several species from Eastern Asia (H. angustisporus, H. crassus, H. latisporus, H. cylindrosporus, H. microsporus, H. septatus, and H. subpapyraceus), Australasia (H. pirongia), and North America (H. gilbertsonii), whereas Lira et al. (2018) added two Neotropical taxa, Haploporus brasiliensis Nogueira-Melo & Ryvarden and Haploporus pileatus Ryvarden. Shen et al. (2016) and Zhou et al. (2019) provided the most comprehensive, multi-locus phylogenetic studies of the genus.
Regarding the genus in sub-Saharan Africa, still little is known. Two species are currently reported, H. papyraceus [as Poria papyracea (Schwein.) Cooke, fide Lowe (1966); as Pachykytospora papyracea, fide Ryvarden (2012)] and H. nanosporus (Ryvarden and Johansen 1980, David and Rajchenberg 1992, Piątek 2005).
Haploporus papyraceus was originally described from North America where it grows on angiosperms (Gilbertson and Ryvarden 1987, Lowe 1966). The reports of this species in sub-Saharan Africa dated from Lowe (1966) and are (partly) based on two long-forgotten names, Poria eichelbaumii P. Henn. and Poria pseudosinuosa P. Henn. (Hennings 1905, 1908). Types of both species were from Eastern Africa, respectively, continental (Tanzania) and insular (Madagascar), and both names were currently considered as synonyms of H. papyraceus (Lowe 1966, Ryvarden 2012).
Haploporus nanosporus was originally described from the western edge of Guineo-Congolian rain forest in Central Gabon (David and Rajchenberg 1992). In Africa, it was, hitherto, only known in literature from the type locality and a neighbouring area in Southwestern Cameroon (Piątek 2005).
As part of an ongoing survey of polypores (Basidiomycota) in sub-Saharan Africa (Decock 2001, 2007, 2011, Decock and Mossebo 2001, 2002, Decock and Ryvarden 2002, 2015, Decock and Masuka 2003, Decock et al. 2011, Decock and Bitew 2012), the types of P. eichelbaumii and P. pseudosinuosa have been re-examined, as well as a set of specimens originating from Eastern and Central Africa, and whose morphological characteristics, in particular the basidiospores, pointed to Haploporus. Their affinities also were inferred using phylogenetic inferences based on DNA sequence data from the nuclear ribosomal ITS (ITS1–5.8S–ITS2) and 5′ end of the 28S (region including the domains D1–D3).
As a result of these studies, the new combination Haploporus eichelbaumii and the new species Haploporus grandisporus are proposed. These species are described or redescribed and illustrated, and their ecologies and distribution ranges are discussed. Haploporus nanosporus is also redescribed and illustrated based on several specimens from the type locality or neighbouring areas, and its phylogenetic affinities are discussed. A fourth Haploporus species may also occur in Eastern Africa, but due to paucity of material, the species is left undescribed. The type specimen of Poria pseudosinuosa is sterile and could not be identified.
Materials and methods
Material and collection localities
Type and original specimens studied are preserved at BPI, MUCL, O, and S (herbarium acronyms according to Thiers, n.d., continuously updated (http://sciweb.nybg.org/science2/IndexHerbariorum.asp). African specimens collected by the author, C. Decock, originate from the Mount Elgon range, Kenya, the Kahuzi Biega mountain range, eastern Democratic Republic of Congo, and several spots of dense rain forest of the Guineo-Congolian phytogeographic region in Gabon. The nomenclature of the successive vegetation zones at Mt Elgon follows Niemelä and Pellikka (2014) and Kindt et al. (2011).
Description
Colours are described according to Kornerup and Wanscher (1981). Sections were carefully dissected under a stereomicroscope in hot (40 °C) NaOH 3% solution and later examined in NaOH 3% solution at room temperature (Decock et al. 2010). Sections were also examined in Melzer’s reagent and lactic acid cotton blue to show staining reaction. All the microscopic measurements were done in Melzer’s reagent. In presenting the size range of several microscopic elements, 5% of the measurements at each end of the range are given in parentheses when relevant. In the text, the following abbreviations are used: av., arithmetic mean; R, the ratio of length/width of basidiospores; and avR = arithmetic mean of the ratio R.
Molecular study and phylogenetic analysis
DNA extraction, amplification, and sequencing of the 5′ end of the nDNA 28S gene (region including the domains D1–D3) and the ITS regions were as described in Decock et al. (2007). Primers LR0R and LR6 (Vilgalys & Hester 1990) and ITS 4 and ITS 5 were used to amplify and to sequence the portion of the 28S gene and the ITS regions, respectively. Materials and sequences used in this study are listed in Table 1. Two DNA data sets were compiled.
The first global data set comprised 28S and ITS sequences of all Haploporus species described to date and several unidentified collections from MUCL. The second data set comprised 28S and ITS sequences of specimens of Haploporus originating from montane areas of the African eastern rift and H. gilbertsonii, as outgroup. Both data sets are deposited at TreeBASE (study accession numbers http://purl.org/phylo/treebase/phylows/study/TB2:S26676 and S26688).
The methodologies and parameters for running phylogenetic analyses [maximum parsimony as implemented in PAUP* ver. 4.0b10 (Swofford 2002), Bayesian inference as implemented in MrBayes ver. 3.1.2 (Huelsenbeck & Ronquist 2001), and maximum likelihood as implemented in RAxML 7.0.4 (Stamatakis 2006)] are described in Yombiyeni et al. (2015). Bayesian analyses were implemented with two independent runs, each with four simultaneous independent chains for 1,000,000 generations, starting from random trees, and keeping one tree every 1000th generations. All trees sampled after convergence (average standard deviation of split frequencies 0.01, confirmed with Tracer 1.4 Rambaut and Drummond 2007), were used to reconstruct a 50% majority rule consensus tree (BC) and to estimate posterior probabilities. The posterior probability (BPP) of nodes was estimated based on the frequency at which the node was resolved among the sampled trees with the consensus option of 50% majority rule (Simmons et al. 2004). Clades with BPP above 0.95 were considered strongly supported by the data. Maximum likelihood (ML) searches conducted with RAxML involved 1000 replicates under the GTRGAMMAI model, with all model parameters estimated by the program. In addition, 1000 bootstrap (ML BS) replicates were run with the same model. Clades with maximum likelihood bootstrap values of 85% or greater were considered to be significantly supported.
Results
Phylogenetic analysis
The Haploporus [28S + ITS] data set comprised 59 sequences (55 28S; 58 ITS) and 1971 positions. However, 125 positions in ITS1/2, whose alignment was judged to be ambiguous, were excluded, resulting in a final data set of 1846 positions.
The alignment of several 28S sequences was problematic, however, involving sequence KU941885, first cited as Haploporus sp. 4 (Shen et al. 2016) then as H. microsporus (Zhou et al. 2019), and sequences KU941883 and KU941884 cited as H. nanosporus (Shen et al. 2016, Zhou et al. 2019). A blast search at GenBank and UNITE databases showed that these three sequences were identical. More importantly, the blast search revealed that they do not correspond to a species of Haploporus but belong to species of Hymenochaetaceae, mostly likely Inonotus species; these three sequences (KU941885, KU941883, KU941884) were 97% similar to sequences KX832920 of Inonotus casuarinae L.S. Bian and KX832918 of Inonotus henanensis Juan Li & Y.C. Dai. By comparison, the similarity between KU941885, registered as H. microsporus and the 28S sequences of its presumed closest relative, H. nanosporus (Zhou et al. 2019), based on the sequence from MUCL 47447, was only 87%. These three 28S sequences were removed from the analyses.
The ITS sequence KU941859, H. nanosporus (voucher LYAD2044a, Gabon, Shen et al. 2016, Zhou et al. 2019), was also problematic. This sequence differed in three base pairs only from sequence KY264039, identified as H. alabamae (voucher specimen JV-0610-K16-Kout, Belize, Meso-America). By comparison, the ITS sequences of H. nanosporus KU941859 and of H. microsporus KU961841 (China), two presumably closely related species (Zhou et al. 2019), differed in 84 positions (including 26 gaps). Our own ITS sequences of H. nanosporus (e.g., MUCL 47447, MUCL 51966), obtained from pure cultures, and whose voucher specimens originated from the species type locality, differed from H. nanosporus KU941859 in 75 positions (including 24 gaps). Hence, sequence KU941859 does not represent H. nanosporus; instead, it clustered within the H. alabamae lineage.
In Table 1 of Zhou et al. (2019), we noted two related errors in matching specimens and sequences. The ITS and 28S sequences of H. crassus, voucher Dai 13580 and of H. subpapyraceus voucher Dai 9324, are linked to GenBank accession numbers FJ627252/KU941886 and KU941841/KU 941865, respectively. A blast search at GenBank showed that FJ627252 and KU941886 corresponded to Dai 9324, whereas sequences KU941841 and KU 941865 corresponded to Dai 13580. Furthermore, sequence KU941886 corresponded to H. angustisporus Dai 10951 (1 bp difference), and then with H. alabamae JV1704/75 (16 bp differences), and not at all to H. subpapyraceus Cui 2651, the species type (52 bp differences).
The MP analysis (1846 characters, 1372 constant, 408 parsimony informative, 66 parsimony uninformative) produced 750 most parsimonious trees (914 steps, consistency index = 0.631, retention index = 0.907). The variation between the 750 trees occurred exclusively within terminal clades.
The general time reversible model (GTR + I + G), using proportion of invariant sites and distribution of rates at variable sites modelled on a discrete gamma distribution with four rate classes, was estimated as the best-fit likelihood model of evolution for the [28S + ITS] full data set, for BI and ML, using the Akaike information criterion (AIC) as implemented in Modeltest 3.7 (Posada and Crandall 1998). The two Bayesian runs (2.000.000 generations) converged to stable likelihood values after 350.000 generations; the first 35% of the tree was discarded as burn-in. The remaining stationary trees from each analysis were used to compute a 50% majority rule consensus tree (BC) and to calculate posterior probabilities. In the ML searches, the alignment had 641 distinct patterns with a proportion of gaps and undetermined characters of 16.63%.
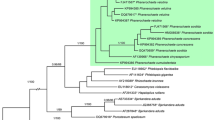
The consensus of the most parsimonious trees, the consensus tree of the BI and the maximum likelihood tree were nearly congruent. The ML (− lnL = − 7511.402) is presented in Fig. 1.
Bayesian Inference consensus tree based on [28S + ITS] sequence data for species of Haploporus. Values above nodes indicate posterior probabilities obtained through Bayesian inference and bootstrap values (1000 replicates) obtained from Maximum Likelihood analysis. Au = Australia, Ch = China, CR = Costa Rica, Cu = Cuba, FG = French Guiana, Fi = Finland, Ga = Gabon, Ke = Kenya, Ma = Malawi, Mar = Martinique, Th = Thailand, USA = United States of America, SK = South Korea, Sw = Sweden
The topologies of the trees regarding the recovery and the relative positions of the different species of Haploporus were, overall, similar in all the phylogenetic inferences, and in accordance with previous published results (Shen et al. 2016, Zhou et al. 2019) except for the relationships of H. nanosporus sensu Shen et al. 2016. This discrepancy resulted from the exclusion of the 28S sequences of H. nanosporus KU941883 and KU941884 (cf. above).
The phylogenetic analyses recovered our specimens from the eastern African mountain range as a single lineage with three slightly divergent clades (cf. Fig. 1). This lineage is distant from all other species clades shown to date (Fig. 1, Shen et al. 2016, Zhou et al. 2019). Its closest relative is H. gilbertsonii (Fig. 1).
The second data set comprised 24 [28S + ITS] sequences of the East African Haploporus specimens. The final alignment resulted in 1697 positions.
The two Bayesian runs converged to stable likelihood values after 225.000 generations. The remaining stationary trees from each analysis were used to compute a 50% majority rule consensus tree (BC) and to calculate posterior probabilities. In the ML searches, the alignment had 97 distinct patterns with a proportion of gaps and undetermined characters of 5.05%.
The consensus tree of the BI and the maximum likelihood tree (− lnL = −2852.313) were nearly congruent. The Bayesian consensus tree is presented in Fig. 2. The Haploporus specimens from East Africa are distributed into three closely related clades (Fig. 2) that could be equated to three phylogenetic species.
Bayesian Inference consensus tree based on [28S + ITS] sequence data for species of Haploporus from the Afromontane range, with H. gilbertsonii as outgroup. Values above nodes indicate posterior probabilities obtained through Bayesian inference and bootstrap values (1000 replicates) obtained from Maximum Likelihood analysis. Ke = Kenya, Ma = Malawi, USA = United States of America
Haploporus nanosporus belongs to a different sublineage, and is related to several specimens from the Neotropics, viz., H. cf. nanosporus from French Guiana, and an unidentified Haploporus, also from French Guiana (Fig. 1, Table 1).
Taxonomic conclusions
The three East African phylogenetic species can be differentiated morphologically mainly by their pore and basidiospore sizes (Table 2). They can also be differentiated as ecological species by their autecological parameters, including host and habitat. From these results, we conclude that the East African Haploporus corresponds to three very closely related species.
The re-examination of the type specimen of Poria eichelbaumii showed that it was, morphologically, identical to one of the three East African species (Table 2); hence, the species name is reinstated and transferred to Haploporus. The type of Poria pseudosinuosa is sterile and the hyphal system is dimitic with typical skeletal hyphae; its affinities are uncertain.
Of the other two East African species, one represents an undescribed species, proposed below as H. grandisporus. The third species is represented by a single specimen, originating from Malawi. It differs from the two other species in having much smaller pores. However, because of the paucity of material available so far, we refrain from describing it for the time being and is left as Haploporus sp. 1.
Taxonomy
Haploporus eichelbaumii (P. Henn.) Decock, comb. nov. [Mycobank: MB835536] Figs. 3, 5a–b, 6a.
Basionym: Poria eichelbaumii P. Henn., Engl. Bot. Jahrb. 39: 109, 1905 [MycoBank: MB233318].
Type: Tanzania, Tanga Region, East Usambara Mountain range, Amani [approx. 04.048° N, 052.677° W] [on dead branch, unidentified angiosperm, ~ 1 cm diam.], Sep 1903, Eichelbaum (isotypes at S! and BPI!).
Basidiome seasonal, resupinate, adnate, effused and thin, occasionally slightly cushion-shaped, confluent, individual patches first circular, down to 3 × 3 mm diam., then ellipsoid to elongated, up to 95 × 15 mm, or up to 150 × 15 mm when several patches merged together, up to 1 mm at the thickest, overall with a soft corky consistency when fresh, corky to brittle when dry; pores surface plane to slightly convex, pale greyish orange (5A3, orange white) to mostly greyish orange (5B[3–5], greyish orange); margin 0.5–2 mm wide, whitish to pale cream (4A[1–2], white to yellowish white); pores variable, round to angular, diamond-shape on oblique substrate, then elongated, becoming lacerate, sublamellar, sinuous, especially in marginal areas, (1.5)2.5–3.5(4)/mm, mostly (125)200–470(625) μm wide (av. = 275 μm); dissepiments entire, the very edge variably lanose, or agglutinated on weathering, thin, 25–55 μm thick (av. = 37 μm); tubes layer single, up to 1 mm deep, mostly whitish to very pale greyish orange, with a corky consistency when fresh, drying fragile, brittle; context reduced to absent, < 0.1-mm thick, whitish.
Hyphal system dimitic, both in the subiculum and the hymenophoral trama; generative hyphae hyaline, clamped, sparingly branched, 1.5–2.0 μm wide; vegetative hyphae as skeleto-binding hyphae, of the arboriform type, the branching pattern loose in the subiculum, progressively denser toward the hymenophoral trama and dissepiments, hyaline, dextrinoid, cyanophilous; in the subiculum, skeleto-binding hyphae with a poorly developed arboriform branching pattern, 1.3–2.5 μm diam. All over; in the hymenophoral trama, skeleto-binding hyphae with a basal stalk and apical branching; stalk arising from a generative hypha, clamped at the basal septum, 40–95 μm long (av. = 67 μm, n = 30), straight to geniculated then often with small, lateral, aborted processes, progressively thick-walled from the basal septum, non-septate, progressively widening from 1.5–2.0 μm diam. at the basal septum (av = 1.8 μm) to 2–3 μm diam. at the apex (av. = 1.6 μm), with 2–4 subapical or apical branches; the apical branched part intermingled, measured 120–250 μm long (av. = 185 μm), mostly straight, occasionally geniculated, occasionally with aborted processes, regularly thick-walled, 1.3–1.7 μm diam. at the branching point to 1.0–1.3 μm diam. at the thin-walled, whip-like end; in areas close to the hymenium, skeleto-binding hyphae with the same construction but shorter, with shorter stalk and branches; dendrohyphidia lining the very margin of dissepiments, variably abundant, hyaline, thin-walled, variably apically branched.
Hymenium: basidia and basidioles club-shaped to slightly pyriform, clamped at the basal septum; mature basidia 20 × 10 μm, with 4 sterigmata; basidiospores ellipsoid to oblong, the abaxial side plane to slightly incurved, the apex rounded to slightly truncate, with a small basal apiculus, thick-walled, the wall hyaline, without reaction in Melzer’s reagent, cyanophilous, slightly swelling in Melzer’s reagent, roughened with longitudinal warts or ridges, discontinuous to continuous to variable extents, ridges simple to occasionally furcate, with a single internal ellipsoid gutta, (10)11.0–14.0(15) × (5.0)5.3–6.5(6.8) μm, R = (1.8)1.9–2.6(2.8) (av. = 12.7 × 5.8 μm, avR = 2.2, n = 240/8); chlamydospores absent.
Physiology (type of rot): white rot;
Ecology (substrate, host, habitat): dead fallen branches of various angiosperms, including Chassalia subochreata (Rubiaceae), and dead bamboo canes, Sinarundinaria alpina (Poaceae), which could be, in some areas, a preferential substrate; medium elevation forest or bamboo thicket, known from 1500 to 2500 (− 2900) masl.
Phylogenetic affinities: the closest known relative of Haploporus eichelbaumii is H. grandisporus and Haploporus sp. 1. (Table 1). Outside Africa, the closest relative of H. eichelbaumii is H. gilbertsonii.
Additional specimens examined: Burundi, Kibira National Park, Mt Teza, 09 Jul 1974, J. Rammeloo 3799, O (F-915707) (a duplicate should be housed in BR); République Démocratique du Congo, Sud Kivu Prov., Kahuzi Biega National Park, road Bukavu-Walikale [as Wakisake on the label], on a dead twig, unidentified angiosperm, 23 Dec 1971, P. Van der Veken w/o #, O (F-915701) (a duplicate should be housed in BR); ibid., Kahuzi Mountains Range, elev. ~ 2300 masl, on dead hanging twigs (0.5–1 cm diam.) on the ground, with mosses, Chassalia subochreata (Rubiaceae), 31 Oct 2018, A. Balezi and C. Decock, CO-18-101 (MUCL); ibid., CO-18-102 (MUCL); ibid., CO-18-103 (MUCL); Kenya, Central Prov., Nyeri district, Mt Kenya, Southern slope, Regati Forest Station, approx. S 0°20′–E 37°15′ [approx. 2200 masl], 02 Feb 1973, L. Ryvarden #9794, O (F-915703); ibid. L. Ryvarden #9851, O (F-915705); Trans-Nzoia County, Mt Elgon National Park, N 01°02′20.5″–E 034°44′12.2″, elev. approx. 2580 masl, bamboo belt, on dead cane of bamboo (Sinarundinaria alpina K. Schum), on the ground, in bamboo thicket, 3 Apr 2018, C. Decock & B. Masai, KE-18-292 (MUCL); ibid., KE-18-293 (MUCL); ibid., KE-18-295 (MUCL 57031); ibid., KE-18-296 (MUCL 57032); ibid., N 01°01′41.8″ – E 034°45′19.3″, elev. approx. 2370 masl, bamboo belt, on dead cane of bamboo (S. alpina), on the ground, in bamboo thicket, 6 Apr 2018, C. Decock & B. Masai, KE-18-330 (MUCL); ibid., KE-18-333 (MUCL); ibid., KE-18-339 (MUCL); ibid., KE-18-341 (MUCL57055); Malawi, Southern Prov., Mulanje district, Mulanje Mountain, Lichenya Plateau, approx. 15°58’ S – 35°30′ E, approx. [1800 masl], 09 Mar 1973, L. Ryvarden, #11353, O (F-915699); Tanzania, Mount Kilimanjaro, Southern slope, above Mweka, ~ S 3°14′ – E 37°20′, elev. 1800–2300 masl, 12 Feb 1973, L. Ryvarden 10,346, O (915700); Uganda: Kabale District, Bwindi Impenetrable Forest National Park (~ S 01°01′ – E 29°41′), Ruhija, bamboo belt, on dead bamboo cane (S. alpina), 23 Nov 2002, P. Ipulet, F1268, O (F-918796); Kabarole District, Kibale Forest National Park, Kanyawara, Parinari forest (K30 Red), approx. 1500 masl, on dead hanging branch, unidentified angiosperm, 26 Nov 2001, P. Ipulet, F160, O (F-918577).
Comments—Haploporus eichelbaumii was considered as a synonym of H. papyraceus (as Poria papyraceae, Lowe 1962, 1966, or as Pachykytospora papyracea, Ryvarden 2012). Haploporus papyraceus differs in having larger basidiospores, 14–17 × 6–8 μm (fide Gilbertson and Ryvarden 1987) (mostly 11.0–14.0 × 5.3–6.5 in H. eichelbaumii), and a distribution restricted to the new world. Earlier, Bresadola (1916) proposed synonymy with Trametes serpens Fr. which is currently accepted as Cerioporus mollis (Sommerf.) Zmitr. & Kovalenko (Zmitrovich and Kovalenko 2016).
Haploporus grandisporus Decock, sp. nov. Figs. 4, 5c–e, 6a–c, 7
[Mycobank: MB835536].
Similar to H. eichelbaumii, differing by the combination of larger pores, 1.5–2.5 / mm, 225–550 μm wide (av. = 389 μm), larger basidiospores, mostly 14–17.5(19) × 6.0–7.3 μm, R = 1.9–2.7 (av. = 15.4 × 6.6 μm, avR = 2.3), and growing from Ericaceae.
HOLOTYPE. Kenya, Trans-Nzoia County, Mount Elgon National Park, ~ N 01.04′29.9″–E 034°39′34.6″, elev. ~ 3150 masl, Ericaceous belt, heather thicket, on dead hanging branch, approx. 1.5 cm diam., Erica arborea L. (Ericaeae), 28 Feb. 2017, C. Decock and B. Masai, KE-17-228 (MUCL 56368). ITS/LSU reference sequences GenBank MT758244. MycoBank: MB835537.
Basidiome seasonal, resupinate, adnate, effused to slightly cushion-shaped, in confluent patches, merging at their margin, individual patches circular at first, down to 3 × 3 mm, becoming ellipsoid to elongated, up to 65 × 8 mm, up to 1.8 mm thick, overall with a corky consistency, drying brittle; pore surface plane to slightly convex, whitish, pale cream (4A2, yellowish white) to pale corky, greyish orange (5B[3–5], greyish orange), sometimes with a fleshy tinge (6B[3–4]; margin 0.5–2 mm wide, whitish to pale cream (4A2, yellowish white), well delimited, the very margin with distant hyphae; pores round to mostly angular, or elongated, diamond-shape on oblique substrate, becoming lacerate, sublamellar, sinuous especially in marginal areas, 1.5–2.5(3)/mm, mostly (175)225–550(625) μm wide (av. = 389 μm), occasionally up to 800 × 500 μm; dissepiments thin to thick, (50)75–125(150) μm thick (av. = 103 μm), the very edge variably lanose, agglutinated on weathering; tube layer single, 1–1.8 mm deep, mostly whitish to very pale greyish orange, with a corky consistency when dry; context reduced to absent, < 0.1 mm thick, whitish.
Hyphal system dimitic, both in the subiculum and the hymenophoral trama; generative hyphae hyaline, clamped, sparingly branched, 1.5–2.0 μm wide; vegetative hyphae as skeleto-binding type, of the arboriform type, the branching pattern loose in the subiculum, progressively denser toward the hymenophoral trama and dissepiments, hyaline, dextrinoid, cyanophilous; in the subiculum, skeleto-binding hyphae with a poorly developed arboriform branching pattern, 1.3–1.8 μm diam. All over; in the hymenophoral trama arboriform skeleto-binding hyphae with a basal stalk and an apical branching; basal stalk arising from a generative hypha, clamped at the basal septum, 30–85 μm long (av. = 61 μm, n = 30), straight to geniculated then often with small, lateral, aborted processes, progressively thick-walled from the basal septum, non-septate, progressively widening from 1.3–2.0 μm diam. at the basal septum (av. = 1.6 μm) to 1.5–3.0 μm diam. at the apex (av. = 2.2 μm), with 2–4 subapical or apical branches; the apical branched part intermingled (occasionally enrolled), measuring 90–260 μm long (av. = 174 μm), mostly straight, occasionally geniculated, regularly thick-walled, 1.2–1.5 μm diam. at the branching point to 1.0–1.3 μm wide. at the thin-walled, whip-like end; in areas close to the hymenium skeleto-binding with the same construction but shorter, with shorter stalk and branches; dendrohyphidia lining the very margin of dissepiments, variably abundant, hyaline, thin-walled, variably apically branched.
Hymenium: basidia and basidioles club-shaped to slightly pyriform, clamped at the basal septum; mature basidia, 18–20 × 8–13 μm, with 4 sterigmata; basidiospores ellipsoid to oblong, the apex rounded to slightly truncate, with a small basal apiculus, with a single (mostly central) gutta, thick-walled, the wall hyaline, without reaction in Melzer’s reagent, cyanophilous, with longitudinal rows of warts or discontinuous to continuous ridges, simple to occasionally furcate, straight to irregularly sinuous, or geniculated, swelling in Melzer’s reagent, (12)14–17.5(19) × (5.7–)6.0–7.3(7.7) μm, R = (1.8)1.9–2.7(2.8), (av. = 15.4 × 6.6 μm, avR = 2.3, n = 120); chlamydospores absent;
Physiology (type of rot): white rot;
Ecology (substrate, host, habitat): dead, hanging or fallen branches, approx. 1–5 cm diam., heather (Erica arborea, Ericaceae), which seems to be, at least locally, the preferential substrate, also known from dead fallen branches of Agauria salicifolia (Ericaceae) and Hagenia abyssinica (Rosaceae), often among lichens; in Erica thicket or mixed Erica thicket with scattered H. abyssinica (Rosaceae) (2900–3200 masl);
Phylogenetic affinities: The closest known relative to Haploporus grandisporus are H. eichelbaumii and Haploporus sp. 1. Outside Africa, the closest relative of H. grandisporus is H. gilbertsonii.
Distribution—Known so far only from elevation ~ 2900–3200 masl, at Mt Elgon, Kenya.
Additional specimens examined: Kenya, Trans-Nzoia County, Mount Elgon National Park, ~ N 01.04′29.9″–E 034°39′34.6″, elev. ~ 3150 m a.s.l., Ericaceous belt, heather thicket mixed with Hagenia abyssinica (Rosaceae), on dead fallen branch, approx. 1.5 cm diam., Erica arborea (Ericaeae), 01 Apr 2016, C. Decock and B. Masai, KE/16–130 (MUCL 56079); ibid., on dead fallen branch, Hagenia abyssinica, 28 Feb. 2017, C. Decock and B. Masai, KE-17-229 (MUCL 56369); ibid., N 01°04′29.7–E 034°39′36″, elev. ~ 3160 masl, on a dead fallen branch, 30 Oct 2017, C. Decock and B. Masai, KE-17-239 (MUCL 56735); ibid., N 01°04′29.7, E 034°39′358.5″, elev. ~ 3160 masl, on a dead hanging branch, 1–1.5 cm diam., E. arborea, 30 Oct 2017, C. Decock and B. Masai, KE-17-240 (MUCL 56821); ibid., on a dead twig, 1 cm diam., E. arborea, 30 Oct 2017, C. Decock and B. Masai, KE-17-242 (MUCL 56736); ibid., N 01°04′18.2″ – E 034°40′06.13, elev. ~ 3065 masl, on a dead hanging branch at 1.5 m high, 2–3 cm diam., 02 Nov 2017, C. Decock and B. Masai, KE-17-282 (MUCL 56826); ibid., dead hanging branch at 1.5 m above soil, 1–1.5 cm diam., E. arborea, 02 Nov 2017, C. Decock and B. Masai, KE-17-284 (MUCL); ibid., elev. ~ 3000 masl, dead fallen branch, unidentified, 07 Apr 2018, C. Decock and B. Masai, KE-18-352 (MUCL).
Comments—The combination of a resupinate, thin, effused to slightly cushion-shaped basidiome, a cream to cork-coloured pore surface (Figs. 4 and 5), large pores, variably abundant dendrohyphidia along the dissepiment edges (Fig. 6), vegetative hyphae of the skeleto-binding type (Fig. 7), and elliptical to oblong, longitudinally ornamented basidiospores (Figs. 5 and 6) characterize both H. eichelbaumii and H. grandisporus. Their basidiospore ornamentation, as seen a light microscope, consists of rows of dot-like or discontinuous to continuous crest-like protuberances having a predominantly longitudinal orientation (Fig. 5). Their wall is also covered by a hyaline “layer” of uncertain nature, slightly swelling in Melzer’s reagent and alkali, a feature unreported hitherto in Haploporus. Dendrohyphidia lining the dissepiments are also present in other species of Haploporus, such as H. papyraceus (Gerber and Loguercio-Leite 1997) and H. microsporus (Zhou et al. 2019).
Haploporus eichelbaumii and H. grandisporus differ from each other mainly by their basidiospores size, respectively, 11.5–14.5 × 5.0–6.0 μm (av. = 12.7 × 5.8 μm) vs 14–17.5 × 6.0–7.3 μm (av. 15.4 × 6.6 μm), and more marginally by their pores size, 2.5–3.5(4)/mm, mostly 190–485 μm wide (av. = 278 μm) vs (1)1.5–2.5(3)/mm, mostly 225–550 μm wide (av. = 389 μm) (Table 2).
Based on the data available so far, the autecologies of H. eichelbaumii and H. grandisporus, including host relationships, habitats, and distribution, could also be important discriminating features. Both species occur on the Eastern slopes of Mount Elgon, where their autecologies could be best compared. Locally, H. eichelbaumii was found mainly on dead bamboo culms (Sinarundinaria alpina, Poaceae), fallen or standing, in bamboo thickets, a vegetation that extends locally at an elevation range of ~ 2500–2900 masl (Kindt et al. 2011). Haploporus grandisporus was found mostly on dead heather branches, standing or on the ground, in heather thickets at the Ericaceous zone, which extends locally at an elevation range of ~ 2900–3200 masl, at the timberline. The woody vegetation in the Ericaceous zone is mostly shrubby, dominated by heather in pure stands or with scattered Hagenia abyssinica. Haploporus grandisporus was found also growing on a small, dead branch of another Ericaceae, Agauria salicifolia, and on a dead, fallen branch of Hagenia abyssinica (Rosaceae) in a transition vegetation at the bamboo upper range toward the heather thickets lower range at ~ 2900 masl. In this transition vegetation, H. grandisporus and H. eichelbaumii were observed sympatrically, the H. eichelbaumii basidiome emerging from a dead, small (2 cm diam.) branch of an unidentified angiosperm.
Haploporus grandisporus is only known to date from the Kenyan slopes of Mt. Elgon. Nonetheless, it could follow heather wherever it occurs in the mountains of the African rift. In eastern Africa, H. eichelbaumii is widely distributed; it is known from dead sticks of unidentified angiosperms in Tanzania and Malawi (cf. list of specimens). It was found also on various substrates in montane forests of the Albertine rift (cf. specimens examined), including bamboo (S. alpina) at Bwindi Impenetrable Forest National Park, Southwestern Uganda, at about 1500 masl; dead fallen sticks of Chassalia subochreata (Rubiaceae) at the Kahuzi mountain range (Kahuzi Biega National Park), at ~ 2300 masl, eastern Democratic Republic of Congo (cf. specimens examined); dead sticks of unidentified angiosperms at Kibale National Park (western Uganda), at about 1500 masl; and dead sticks of unidentified angiosperms at Kibira National Park (western Burundi).
In tropical Africa, H. eichelbaumii and H. grandisporus should be compared to Haploporus sp. 1 and H. nanosporus. Haploporus sp. 1 differs from both H. eichelbaumii and H. grandisporus in having smaller pores (4–5/mm) and from H. grandisporus in having also smaller basidiospores. Haploporus nanosporus differs in having comparatively much smaller pores and basidiospores (8–9/mm, 80–110 μm diam.; average 5.3 × 3.4 μm, Table 2), and by inhabiting a very different ecosystem, the lowland rainforest at the western edge of the Guineo-Congolian Phytochorion (cf. below).
Outside Africa, H. eichelbaumii and H. grandisporus should be compared to H. gilbertsonii, so far their closest phylogenetic relative: these three species form a well-supported lineage (Fig. 1). Haploporus gilbertsonii shares with both African species the pale buff pore surface, large pores (2–3/mm), and basidiospores of a similar shape and size range, averaging 14 × 6.9 μm (fide Zhou et al. 2019). Haploporus gilbertsonii also is characterized by a much reduced or absent sterile margin, absence of dendrohyphidia, and basidiospores ornamented by discontinuously tubercles (Zhou et al. 2019), features that differ from both African species. Furthermore, H. gilbertsonii is known so far only from Quercus sp. in Southern USA (Zhou et al. 2019); it resembles the European H. tuberculosus, in basidiome habit, basidiospores, substrate, and host.
Haploporus eichelbaumii and H. grandisporus also could be compared to the East Asian H. latisporus Juan Li & Y.C. Dai. Haploporus latisporus shares with these two species the patchy, effused basidiomata, a whitish cream pore surface, large pores (~ 2–3/mm), and basidiospores of a comparable size (13–16.5 × 8–10 μm, av. = 14.3 × 8.7 μm). However, H. latisporus has no or a much reduced sterile margin, lacks dendrohyphidia at the edges of the dissepiment, and has wider basidiospores (8–10 μm wide) with discontinuous and thick “warts” (Li et al. 2007), features that differ from both H. eichelbaumii and H. grandisporus. Haploporus latisporus also differs in its autecological parameters and distribution range; it is known so far on gymnosperms (Pinus sp., Pinaceae) in North-Central China (Li et al. 2007). Haploporus latisporus also is, phylogenetically, distantly related to both African species.
Haploporus nanosporus (A. David & Rajchenb.) Piątek, Ann. Bot. Fennici 42: 24, 2005 [MycoBank: MB510489]. Fig. 8.
Basionym: Pachykytospora nanospora A. David & Rajchenb., Mycotaxon 45:197, 1992 [MycoBank: 358930].
Basidiomata seasonal to pluri-seasonal, resupinate, adnate (the marginal areas occasionally peeling off on drying), effused, following the substrate, extending to neighbouring plant debris, forming small, circular, 5–10 mm diam. Patches to extended sheets up 200 mm long × 150 mm wide, 1–4 mm thick, overall with a soft corky consistency when fresh, drying corky; pore surface whitish to pale cream ([4–5]A2, yellowish white to orange white), on ageing slightly darker, discolouring to corky on bruising when fresh, occasionally (especially when the basidiomes develop on an oblique surface) with sterile patches, pale cinnamon to pinkish brown (8D3, greyish red); margin 0.5–2 mm wide, well delimited, concolorous with the pore surface ([4–5]A2, yellowish white to orange white) with age, in multi-layered specimens discolouring to cork-coloured to light brown (6D[7–8], 6E7, cinnamon, autumn leaf); pores round (drying more angular), regular (7)8–9(10)/mm, (60)80–110(125 μ) m wide (av. = 80 μm, n = 60), sometimes elongated, ellipsoid, up to 180 × 100 μm; dissepiments entire, thick, (20)25–65(75) μm thick (av. = 40 μm, n = 60); tube layer single or multiple, then observed up to 3, up to 4 mm thick in total, each individual layer 1–2 mm thick, separated by a thin layer of flesh (~ 100 μm thick) lying on a thin dark line, whitish to pale creamy in the upper part near the pore surface, discolouring to pale greyish orange, cork-coloured deeper, with a corky consistency when dry; context almost absent or reduced to a very thin layer, from < 0.1 to 0.25-mm thick, fibrous, whitish to pale creamy.
Hyphal system dimitic, both in the subiculum and the hymenophoral trama; generative hyphae hyaline, clamped, sparingly branched, 1.5–2.0 μm wide; vegetative hyphae skeleto-binding of the arboriform type, little branched however, hyaline; in the subiculum, skeleto-binding hyphae with a poorly developed arboriform branching pattern, 1.3–1.8 μm diam. All over; in the hymenophoral trama skeleto-binding hyphae arboriform but with a reduced branching pattern, with a short to medium basal stalk and long apical branches; basal stalk arising from a generative hypha, clamped at the basal septum, 20–55 μm long, progressively thick-walled from the basal septum, non-branched, straight to occasionally geniculated then sometimes with small lateral aborted processes, non-septate, progressively slightly widening from 1.5–1.8 μm diam. at the basal septum (av. = 1.6 μm) to 1.5–2.0 μm diam. at the apex (av. = 1.7 μm); with 2–4 subapical or apical filiform branches; the apical branched part tightly intermingled and difficult to tease apart, measured up to 160 μm long, mostly unbranched and straight, thick-walled, gradually tapering from 1.2–1.5 μm diam. at the branching point to 1.0–1.3 μm wide at the thin-walled end.
Hymenium: cystidioles few, fusiform; basidia and basidioles pyriform to subglobose, clamped at the basal septum; basidia with 4 sterigmata; basidiospores ellipsoid to broadly ellipsoid, the apex rounded, with a small basal apiculus, thick-walled, hyaline, (non-) to dextrinoid, cyanophilous, with numerous isolated warts in longitudinal rows, (4.5)5.0–5.8(6.0) × 3.0–3.5(4.0) μm, R = 1.4–1.8, (av. = 5.3 × 3.4 μm, avR = 1.5, n = 90); chlamydospores absent;
Physiology (type of rot): white rot;
Ecology (substrate, host, habitat): mostly on dead fallen trunks or branches (3–30 cm diam), or dead stump, known from Dacryodes buettneri H.J. Lam (Burseraceae) and other unidentified angiosperms, Guineo-Congolian rainforest.
Phylogenetic affinities: the species has a basal position in the present phylogeny and has no close relatives so far except for H. microsporus.
Distribution—Known from the lower Guinean sub-region, Guineo-Congolian rain forest, in Gabon and Cameroon (cf. Piątek 2005).
Specimens examined–Gabon, Ogooué Ivindo Prov., Ipassa Makokou Biosphere Reserve, ~ N 0°30′87″06–E 12°48′11″, on a dead hanging branch, ~ 10 cm diam., unidentified angiosperm, 06 Apr 2006, C. Decock, GA-06-39 & 43, MUCL 47559 & MUCL 47444 (culture ex. MUCL 47444); ibid., side of dead fallen trunk, unidentified angiosperm, GA-06-44, MUCL 47445 (culture ex. MUCL 47445); ibid., dead standing tree, unidentified angiosperm, GA-06-51, MUCL 47447 (culture ex. MUCL 47447); ibid., side of dead fallen trunk, unidentified angiosperm, 07 Apr. 2006, C. Decock, GA-06-76, MUCL 47515 (culture ex. MUCL 47515); ibid., side of dead fallen trunk, unidentified angiosperm, GA-06-77, MUCL 47516 (culture ex. MUCL 47516); ibid., side of dead fallen trunk, unidentified angiosperm, 08–09 Apr 2006, C. Decock, GA-06-131, MUCL 47522 (culture ex. MUCL 47522); ibid., dead stump, unidentified angiosperm, C. Decock, GA-06-139, MUCL 47524 (culture ex. MUCL 47524); ibid., side of a dead fallen trunk, 15–20 cm diam., unidentified angiosperm, 10 Apr 2006, C. Decock, GA-06-154, MUCL 47470 (culture ex. MUCL 47470); ibid., dead standing trunk, unidentified angiosperm, 13 Apr 2006, C. Decock, GA-06-210, MUCL 47485 (culture ex. MUCL 47485); ibid., on a dead fallen trunk 50–60 cm diam., unidentified angiosperm, 27 Mar 2008, C. Decock and P. Yombiyeni, GA-08-310, MUCL 51166 (culture ex. MUCL 51166); ibid., bark of living tree, ~ 80 cm diam., Dacryodes buettneri (Burseraceae), 01 Apr 2008, C. Decock and P. Yombiyeni, GA-08-353, MUCL 51182 (culture ex. MUCL 51182); ibid., dead fallen trunk, ~ 30 cm diam., unidentified angiosperm, GA-08-380, MUCL 51195 (culture ex. MUCL 51195); Estuaire Prov., Forêt Classée de la Mondah, ~ N 0°36.7′–E 9°23.3′, on a dead fallen trunk, 20–30 cm diam., unidentified angiosperm, 22 Mar 2008, C. Decock and P. Yombiyeni, GA-08-280, MUCL 51154 (culture ex. MUCL 51154); ibid., Akanda National Park, N 0°37′–E 9°33′, on a dead fallen trunk, ~ 30 cm diam., deeply rotten, 05 Apr 2009, C. Decock and P. Yombiyeni, GA-09-505, MUCL 51959 (culture ex. MUCL 51959); ibid., Monts de Cristal National Park, ~ 00°27.23′N–010°16.7′E, elev. ~ 80 masl, on a dead branch on the ground, ~ 5 cm diam., 07 Apr 2009, C. Decock and P. Yombiyeni, GA-09-514, MUCL 51962 (culture ex. MUCL 51962); Ngounié Prov., Waka National Park, ~ S 1°07′–E 011°09, elev. ~ 450 masl, on a dead fallen trunk, 40–50 cm diam., 14 Apr 2010, C. Decock and P. Yombiyeni, GA-10-697, MUCL 52818 (culture ex. MUCL 52818); ibid., dead branch fallen, ~ 20 cm diam., GA-10-705, MUCL 52820 (culture ex. MUCL 52820); Ogooue Maritime Prov., Rabi, Smithsonian Forest Monitoring Plot, ~ S 01°55′34″–E 009°52′49″, elev. ~ 60 masl, on a dead hanging branch, ~ 3 cm diam., 07 Apr 2012, C. Decock and P. Yombiyeni, GA-12-790 (MUCL 54306; culture ex. MUCL 54306); ibid., on a dead fallen branch, 20–25 cm diam., 09 Apr 2012, GA-12-841, MUCL 54317 (culture ex. MUCL 54317).
Discussion—Haploporus nanosporus is characterized by a pale cream pore surface, small pores, mostly 8–9/mm, little branched, dextrinoid skeleto-binding hyphae (Fig. 8), and slightly ovoid to broadly ellipsoid basidiospores, 4.8–6 × 3–3.5 μm (Fig. 8). The species grows mostly on dead fallen trunks or branches of various diameters. It is known from the western edge of the Guineo-Congolian rain forest, in Cameroon and Gabon.
It is in Gabon that the species is best known. Locally, H. nanosporus was collected, from west (coastal areas) eastward, at La Mondah forest (forêt classée de la Mondah, ~ N 0°36.7′–E 9°23.3′), rabi forest monitoring plots (~ S 1°55″, E 9°52′), the Mont de Cristal National Park (~ N 0°42′–1 E 0°17′), Waka National Park (~ S 1°16′, 1 E 1°02′), and the Ipassa Makokou Biosphere Reserve (~ N 0°30′–E 12°48′), so far its easternmost locality in Central Africa. Although statistical evidence is lacking, based on our field observations in Gabon, the abundance of this species might decline from east (Guineo-Congolian forest) westward (lower Guinean forest) which also corresponds to a moisture gradient; the easternmost locality at Ipassa Makokou is, overall, comparatively dryer than, for instance, the hyper humid La Mondah or the Mont de Cristal forest areas.
Piątek (2005) reported a collection from the Akok low land forest reserve in southwestern Cameroon, bordering equatorial Guinea, so far its northernmost locality. This species has not been observed in the Dja Biosphere Reserve (~ 3°00′N–13°00′E), in southeastern Cameroon during two 2-week long field trips (C. Decock, pers. obs.).
From both morphological (Table 2) and ecological perspectives, H. nanosporus is readily distinguishable from H. grandisporus and H. eichelbaumii (cf. discussion under H. grandisporus). It is also phylogenetically distantly related to these species (Fig. 1).
Outside Africa, H. nanosporus should be compared to two specimens of Haploporus originating from French Guiana (C. Decock, MUCL 55303 and MUCL 55313); both had been tentatively identified as H. nanosporus, following Ryvarden and Iturriaga (2004) who had reported the species from Venezuela. These two specimens form a clade closely related to the H. nanosporus s.s. clade (Fig. 1); they could be considered as representing a distinct species. Lira et al. (2018) described H. brasiliensis, from Brazil. It shares with MUCL 55303 and MUCL 55313, the resupinate habit and the basidiospores shape (oblong ellipsoid) and size (6–8 × 5–4 μm), but differs in having much larger pores, 1–3/mm (Lira et al. 2018). There is no DNA sequence available for H. brasiliensis.
A third specimen from French Guiana (MUCL 55027), morphologically reminiscent of H. papyraceus, is also related to H. nanosporus (Fig. 1) and could be considered as representing a distinct species.
Haploporus nanosporus is also much reminiscent of H. microsporus, described from Hainan, southern, insular China (Zhou et al. 2019). Both species share most of their morphological characters and are hardly distinguishable on morphological grounds. They are also phylogenetically related, although H. microsporus, represented by a single ITS sequence, stands on a long branch. Piątek (2005) reported previously H. nanosporus from Papua New Guinea, Southeast Asia. That specimen was not available for comparison, but it should be compared with H. microsporus.
Poria pseudosinuosa Henn., Reise in Ostafrika in den Jahren 1903-1905, 3: 20, 1908 [MycoBank: MB180609].
Ryvarden (2012) noted that the type of P. pseudosinuosa was not found at S (cf. also Hein 1988). Nonetheless, Lowe (1966) mentioned an isotype was available at BPI; re-examination revealed that it is sterile. The hyphal system is dimitic with skeletal hyphae. The affinities of this specimen remain uncertain.
Key to the species of Haploporus found in sub-Saharan, continental and insular Africa .
1. Pores 8–9/mm; basidiospores ≤6 μm…......H. nanosporus
Known from the western edge of the Guineo-Congolian phytochorion, in Gabon and Cameroon, inhabiting rain forest.
1* Pores 1–5 mm; basidiospores >10 μm long .................2
2. Pores 4–5 mm…………….…………Haploporus sp. 1
Known so far from a single specimen (LR11231) from Malawi.
2* Pores 1–3(4)/mm)……………………..………….… 3
3. Basidiospores on average ~ 15 μm long, and up to 19 μm long; pores ~ 1.5–2.5/mm, 225–550 μm wide, averaging 390 μm ……........................ H. grandisporus
Known so far only at high elevation (~2900–3200 masl), eastern mountain ranges of the African rift, on Ericaceae, mostly Erica, in heather thicket, Kenya.
3* Basidiospores on average ≤ 13 μm long, and up to 15 μm long; pores ~ 2.5–3.5/mm, 200–470 μm wide, averaging 275 μm ………………....... H. eichelbaumii. Known at medium elevation (~ 1500–2500 masl) in both branches of the African rift (Eastern and Albertine mountain ranges), montane forests and bamboo thickets, known from Burundi, Democratic Republic of Congo, Kenya, Malawi, Tanzania, and Uganda.
Discussion
Haploporus eichelbaumii, H. grandisporus, and Haploporus sp.are closely related from the perspectives of phylogeny, morphology, or ecology. They form three closely related terminal clades and a well-supported “East African” sublineage within the Haploporus lineage. These species inhabit montane forests of the East African mountain ranges. However, and pending confirmation, they inhabit different ecosystems at different elevation ranges. Haploporus nanosporus is the fourth tropical African species. It is very distinct from the three other species and phylogenetically isolated. It is known so far only from the humid, low land rain forest of the Western edge of the Guineo-Congolian phytochorion.
Zhou et al. (2019) described H. angustisporus based on specimens from Southern China (Guangdong Prov.). This species is here reported from a southern locality, in northern Thailand (Fig. 1, C. Decock, MUCL 44667; Table 1).
The hyphal system in Haploporus has been variably described as dimitic with skeletal hyphae (e.g., H. angustisporus, H. crassus, H. gilbertsonii, H. latisporus, H. nepalensis, H. papyraceus, H. thindii, Yu et al. 2005, Shen et al. 2016, Zhou et al. 2019), trimitic with skeletal and binding hyphae (e.g., H. alabamae, H. odorus, or H. tuberculosus, Piątek, 2003, Ryvarden and Melo 2014, Shen et al. 2016, Zhou et al. 2019), or the uncertain di- trimitic with skeletal hyphae (e.g., H. latisporus, Li et al. 2007, H. subtrameteus, Shen et al. 2016, Zhou et al. 2019). These skeletal hyphae in the dimitic or di-trimitic hyphal systems sensu Yu et al. 2005, Li et al. (2007), Shen et al. (2016), or Zhou et al. (2019) were further characterized as “frequently branched”; they are of uncertain interpretation, and raise two issues. To characterize skeletal hyphae as “frequently branched” is intrinsically contradictory; skeletal hyphae are by definition unbranched, except for the sparingly branched mediate hyphae (Corner 1932). On the other hand, “frequently branched” does not adequately describes their branching patterns.
The hyphal system in H. eichelbaumii, H. grandisporus, and to a lesser degree in H. nanosporus is better characterized as dimitic with vegetative hyphae of the skeleto-binding type, with a branching pattern defined as loosely arboriform. The vegetative hyphae have a short basal stem and an apical branching system, with a slight range of variations in the length and number of branches. Haploporus eichelbaumii and H. grandisporus have a similar branching pattern. Clémençon (2004) reported a branching pattern in H. tuberculosus that resembles the one in H. eichelbaumii. The branching system is extremely loose in the case of H. nanosporus, with few, long, sinuous branches. In this sense, it is the “less” branched of the African Haploporus species. This branch pattern is also shared by specimens MUCL 55313 & MUCL 55303, both from French Guiana, which are closely related to H. nanosporus (Fig. 1).
Haploporus nanosporus, MUCL 55313 and MUCL 55303, is phylogenetically distant from Haploporus eichelbaumii, and does not belong in the core (type) Haploporus lineage (Fig. 1). Haploporus alabamae and H. angustisporus form a sister clade to the H. nanosporus clade. Dollinger and Vlasák (2018) questioned the monophyly of Haploporus sensu Zhou et al. (2019); their ITS-based phylogenetic inference, based on a much reduced species sampling, showed that H. alabamae was on an isolated branch, distant from H. tuberculosus. Their results could suggest that H. alabamae and H. angustisporus would form an independent genus. In that scenario, H nanosporus and related taxa (Fig. 1) would also represent a distinct genus. This should be assessed using additional data, especially DNA sequences from protein coding genes.
Data availability
Scientific data concerning DNA sequences and DNA data sets are made available through the platform NCBI and Treebase (cf. accession numbers in the text).
References
Bondartsev AS (1971) Polyporaceae of the European part of the USSR and the Caucasus. Keter Press, Jerusalem
Bresadola J (1916) Synonymia et adnotanda mycologica. Annale Mycologici 14:221–242
Corner EJH (1932) A Fomes with two systems of hyphae. Trans Br Mycol Soc 17:51–81
Clémençon H (2004) Cytology and plectology of the Hymenomycetes. Bibl Mycol 199:1–488
Dai YC, Niemelä T, Kinnunen J (2002) The polypore genera Abundisporus and Perenniporia (Basidiomycota) in China, with notes on Haploporus. Ann Bot Fenn 39:169–182
David A, Rajchenberg M (1992) West African polypores. New species and combinations. Mycotaxon 45:131–148
Decock C (2001) Studies in Perenniporia (Basidiomycetes, Aphyllophorales): African taxa. I. Perenniporia dendrohyphidia and Perenniporia subdendrohyphidia, sp. nov. Systematic and Geograph Plants (Belgium National Botanical Garden) 71:45–51
Decock C (2007) On Microporellus with two new species and one recombination (M. papuensis sp. nov., M. adextrinoideus sp. nov., M. terrestris comb. nov.). Czech Mycol 59:153–170
Decock C (2011) Studies in Perenniporia s.l. (Polyporaceae): African taxa VII. Truncospora oboensis sp. nov., an undescribed species from cloud forest in São tome. Cryptogam Mycol 32:383–390
Decock C, Bitew A (2012) Studies in Perenniporia. African taxa VI. A new species and a new record of Perenniporia from Afromontane forests of Ethiopia. Plant Ecol Evol 145(2):272–278
Decock C, Masuka AJ (2003) Studies in Perenniporia (Basidiomycetes, Aphyllophorales): African taxa IV. Perenniporia mundula and its presumed taxonomic synonym, Vanderbylia ungulata. Syst Geogr Plants 73:161–170
Decock C, Mossebo DC (2001) Studies in Perenniporia (Basidiomycetes, Aphyllophorales): African taxa. II. Perenniporia centrali-africana, sp. nov. from Cameroon. In E. Robbrecht, J. Degreef & I. Friis (Eds), plant systematics and phytogeography for the understanding of African biodiversity. Proceedings of the XVIth AETFAT congress. Systematic and Geograph Plants (Belgium National Botanical Garden) 71(2):607–612
Decock C, Mossebo D (2002) Studies in Perenniporia (Basidiomycetes, Polyporaceae): African taxa. III. The new species Perenniporia djaensis and some records of Perenniporia for the Dja biosphere reserve, Cameroon. Syst Geogr Plants 72:55–62
Decock C, Ryvarden L (2002) Two undescribed Microporellus species and notes on M. clemensiae, M. setigerus, and M. subincarnatus. Czech Mycol 54:19–30
Decock C, Ryvarden L (2015) Studies in Perenniporia s.l. African taxa IX. Perenniporia vanhullii sp. nov. from open woodlands. Synop Fung 33:43–48
Decock C, Herrera Figueroa S, Robledo G, Castillo G (2007) Fomitiporia punctata (Basidiomycota, Hymenochaetales) and its presumed taxonomic synonyms in America: taxonomy and phylogeny of some species from tropical / subtropical area. Mycologia 99:733–752
Decock C, Valenzuela R, Castillo G (2010) Studies in Perenniporia s.l.: Perenniporiella tepeitensis comb. nov., an addition to Perenniporiella. Cryptogam Mycol 31:419–429
Decock C, Mossebo D, Yombiyeni P (2011) The genus Perenniporia s.l. (Polyporaceae) in Africa V. Perenniporia alboferruginea sp. nov. from Cameroon. Plant Ecol Evol 144:226–232
Dollinger J, Vlasák J (2018) New polypore records from Florida. Synop Fung 38:44–55
Gerber AL, Loguercio-Leite C (1997) New records of polypores (Aphyllophorales) from southern Brazil. Mycotaxon 62:305–318
Gilbertson RL, Ryvarden L (1987) North American polypores 2. Fungiflora, Oslo
Hein B (1988) Liste uber Arten und infraspecifischen taxa von P. Hennings Englera 10:1–374
Hennings P (1905) Fungi Africae orientalis IV. Bot Jb 38:102–118
Hennings P (1908) Fungi von Madagaskar, den Comoren und Ostafrika. Reise in Ostafrika in den Jahren 1903–1905(3):15–33
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Jang Y, Jang S, Lee J, Lee H, Lim YW, Kim C, Kim JJ (2016) Diversity of wood-inhabiting polyporoid and corticioid fungi in Odaesan National Park, Korea. Mycobiology 44:217–236. https://doi.org/10.5941/MYCO.2016.44.4.217
Justo A, Miettinen O, Floudas D, Ortiz-Santana B, Sjökvist E, Lindner D, Nakasone K, Niemelä T, Larsson K-H, Ryvarden L, Hibbett DS (2017) A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol 121:798–824
Keller J (1974) Contribution à la connaissance de l’infrastructure de la paroi sporique des Aphyllophorales. Thèse Univ, Neuchatel
Keller J (1977) Ultrastructure des parois sporiques des Aphyllophorales IV. Ontogenèse des parois sporiques de Pachykytospora tuberculosa et Ganoderma lucidum. Bull Soc Bot Suisse 87:34–51
Keller J (1986) Ultrastructure des parois sporiques de quelques aphyllophorales. Mycol Helvetica 2:1–34
Kindt R, Van Breugel P, Lillesø J-PB, Gachathi F, Omondi W, Jamnadass R, and Graudal L (2011) Potential natural vegetation of eastern Africa. Volume 8. Atlas and tree species composition for Kenya. Forest & landscape working paper-2013
Kornerup A, Wanscher JH (1981) Methuen handbook of colour, Ed. 3, London, Methuen
Kotlaba F, Pouzar Z (1963) A new genus of the polypores – Pachykytospora gen. Česká Mykol 17:27–34
Li J, Dai YC, Yuan HS (2007) A new species of Haploporus (Basidiomycotina) from China. Mycotaxon 99:181–188
Lira C, Nogueira-Melo GS, Ryvarden L, Gibertoni T (2018) Two new species of Haploporus from Brazil. Synop Fung 38:62–65
Lowe JL (1962) Studies in the genus Poria. VI. Papers. Mich Acad Sci, Arts Lett 47:81–187
Lowe J (1966) Polyporaceae of North America. The genus Poria. State University College of Forestry, Syracuse University. Techn Public 90:1–183
Niemelä T, Pellikka P (2014) Zonation and characteristics of the vegetation of Mt. Kenya. In: Pellikka P, Ylhäisi J, Clark B (eds) Taita Hills and Kenya, 2004 – expedition reports of the Department of Geography, Univ Helsinki. University of Helsinki, Helsinki
Piątek M (2003) Haploporus tuberculosus, a new polypore genus and species in Belarus, with a new combination in Haploporus. Polish Bot J 48:81–83
Piątek M (2005) Taxonomic position and world distribution of Pachykytospora nanospora (Polyporaceae). Ann Bot Fenn 42:23–25
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818. https://doi.org/10.1093/bioinformatics/14.9.817
Rambaut A, Drummond A (2007) Tracer v1.4 (internet). http://tree.bio.ed.ac.uk/software/tracer/. Accessed May 2020
Ryvarden L (2012) Type studies in Polyporaceae 27. Species described by P. Ch. Hennings. Czech Mycol 64:13–21
Ryvarden L, Iturriaga T (2004) Studies in Neotropical polypores 21. New and interesting species from Venezuela. Synop Fung 18:68–75
Ryvarden L, Johansen I (1980) A preliminary flora of East Africa. Fungiflora, Oslo
Ryvarden L, Melo I (2014) Poroid fungi of Europe. Synopsis Fungorum 31. Fungiflora, Oslo
Shen LL, Chen JJ, Wang M, Cui BK (2016) Taxonomy and multi-gene phylogeny of Haploporus (Polyporales, Basidiomycota). Mycol Prog 15:731–742. https://doi.org/10.1007/s11557-016-1203-y
Simmons MP, Pickett KM, Miya M (2004) How meaningful are Bayesian support values? Mol Biol Evol 21:188–199. https://doi.org/10.1093/molbev/msh014
Singer R (1944) Notes on the taxonomy and nomenclature of Polypores. Mycologia 36:65–69. https://doi.org/10.2307/3754880
Stamatakis A (2006) Raxml-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Massachusetts
Thiers B [continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium http://sweetgum.nybg.org/ih/
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
Yombiyeni P, Balezi A, Amalfi M, Decock C (2015) Hymenochaetaceae from the Guineo-Congolian rainforest: three new species of Phylloporia based on morphological, DNA sequences, and ecological data. Mycologia 107:996–1011. https://doi.org/10.3852/14-298
Yu CJ, Zuo L, Dai YC (2005) Three polypores from Xizang new to China. Fungal Sci 20:61–68
Zeng XL, Bai YP (1993) The genus Haploporus in China. Acta Mycol Sin 12:12–15
Zhou M, Wang L, May TW, Vlasák J, Chen J-J, Dai Y-C (2019) Phylogeny and diversity of Haploporus (Polyporaceae, Basidiomycota). MycoKeys 54:77–88. https://doi.org/10.3897/mycokeys.54.34362
Zmitrovich IV, Kovalenko AE (2016) Lentinoid and polyporoid fungi, two generic conglomerates containing important medicinal mushrooms in molecular perspective. Int J Med Mushrooms 18(1):23–38. https://doi.org/10.1615/IntJMedMushrooms.v18.i1.40
Acknowledgements
Cony Decock also acknowledges the Research Institute in Tropical Ecology (IRET) of the National Centre of the Scientific Research (CENAREST) of Gabon for facilitating field works in Gabon; the staff of the CNRS Nouragues Research Station in French Guiana for facilitating field work in French Guiana; Prof. R. Courtecuisse, Université de Lille, for his invitation to participate to the survey of polypores in Martinique. The authors also extend their gratitude to the Kenyan Wildlife Service for granting permission to collect at Mount Elgon National Park; the staff at Mount Elgon National Park, particularly Mr. Bonface Masai, for his invaluable help during field work. The authors also warmly thank Mrs. Stéphanie Huret for her help with the sequencing program. Sequences of several specimens from Martinique were kindly provided by Prof. Anne Favel, from the CIRM-CF Fungal collection, INRA, Marseille, to whom we are thankful.
Funding
Funding was provided to Cony Decock by Belgian State–Belgian Federal Science Policy (BELSPO), through the BCCM program; to Cony Decock and Isabel Wagara by the FNRS through an ERAFRICA (project ASAFEM); funding was provided to Cony Decock and Prudence Yombiyeni by the FNRS through a FRFC project (FRFC # 2.4515.06); Funding was provided to Cony Decock and Alphonse Balezi by ARES through the PRD project “Mycologie et développement dans la Région des Grands Lacs: approche raisonnée et filières de production ex-situ de champignons comestibles”.
Author information
Authors and Affiliations
Contributions
Isabelle Wagara and Cony Decock contributed to the study conception and design. Material collections were performed by all the authors, including Cony Decock, Alphonse Balezi, Prudence Yombiyeni and Isabel Wagara. Data collection and analysis were performed by Cony Decock. The first draft of the manuscript was written by Cony Decock, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Yu-Cheng Dai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on diversity and phylogeny of wood-decaying Basidiomycota
Rights and permissions
About this article
Cite this article
Decock, C.A., Wagara, I., Balezi, A. et al. Haploporus (Basidiomycota, Polyporales) in sub-Saharan Africa: Poria eichelbaumii, a long-forgotten name, is reinstated in Haploporus and H. grandisporus sp. nov. is proposed. Mycol Progress 20, 149–168 (2021). https://doi.org/10.1007/s11557-020-01660-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-020-01660-x