Abstract
The larch bark beetle (Ips subelongatus), which occurs in larch plantations over a vast area of eastern Asia, infects both dying and fallen trees. When its population reaches a high density, the beetle may also infect healthy trees, resulting in tree decline and, eventually, death. Leptographium spp., in both their sexual and asexual states, are mainly associated with conifer-infesting bark beetles; some species are important tree pathogens. The aims of this study were to identify the Leptographium spp. associated with I. subelongatus infestations of Larix spp. in northern China and to examine their pathogenicity towards the tree. Morphological studies and phylogenetic approaches based on multilocus DNA sequence data (ITS2- partial r28S, partial β-tubulin, and EF-1α gene regions) showed that three Leptographium species occur in association with I. subelongatus in the areas investigated: Leptographium taigense, which is recorded in China for the first time, and two new species, namely L. innermongolicum sp. nov. and L. zhangii sp. nov. Leptographium innermongolicum is closely related to L. taigense, whereas L. zhangii belongs to the Grosmannia piceaperda species complex. The pathogenicity of these Leptographium species towards mature Larix spp. was tested by stem inoculation in forests. All inoculations only resulted in small lesions on the inner bark; therefore, the three Leptographium species were not considered to be pathogenic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ips subelongatus Motschulsky (Scolytidae, Coleoptera) is an important pest that mainly infects larches (Larix spp., Pinacae) in eastern Asia. The bark beetle can infect dying or fallen trees and may also infect healthy trees at high population densities, with subsequent morbidity or death (Yin et al. 1984; Van der Westhuizen et al. 1995). Because of their morphological similarities, I. subelongatus and several other eight-spined larch bark beetles were considered synonyms of Ips cembrae Heer (Wood and Bright 1992; Pfeffer 1995). However, mitochondrial gene sequence analysis indicates that the European and Asian populations of I. cembrae are genetically diverse and encompass several haplotypes (Stauffer et al. 2001); at species level, the East Asian haplotypes correspond to I. subelongatus. Bark beetles cause serious damage; therefore, these species have been added to the European and Mediterranean Plant Protection Organization (EPPO) alert list A2 (http://www.eppo.int/QUARANTINE/listA2.htm).
In China, I. subelongatus, which mainly occurs in northern areas, infects several species of Larix: Larix gmelinii in the Da Hinggan and Xiao Hinggan mountain ranges in the Inner Mongolia autonomous region and the Heilongjiang Province, Larix olgensis in south-eastern Heilongjiang Province and the Chang Bai mountain range in Liaoning Province, and Larix principis-ruprechtii in middle Inner Mongolia, as well as some areas of Beijing, Hebei, and Shanxi Provinces, with allopatric distribution, consequently threatening local plantations (Yang et al. 2007). Ips subelongatus is commonly found in association with ophiostomatoid fungi, including species of Ophiostoma, Grosmannia/Leptographium, Ceratocystiopsis, Ceratocystis, and Graphilbum (Aoshima 1965; Yamaoka et al. 1997, 1998, 2009; Paciura et al. 2010; De Beer and Wingfield 2013). At present, six species of Grosmannia/Leptographium have been recorded in association with I. subelongatus in eastern Asia, viz. Grosmannia laricis Zipfel et al., Grosmannia olivacea Zipfel et al., Leptographium altius Paciura et al., Leptographium manifestum Paciura et al., and two unnamed species (Yamaoka et al. 1998, 2009; Paciura et al. 2010).
Grosmannia, which was discovered in 1936 (Goidánich 1936), has historically been considered a synonym of the genus Ophiostoma. It was reconsidered as an independent genus on the basis of data from multilocus DNA sequence analysis (Zipfel et al. 2006). Grosmannia species are characterized by ascomata with a globose base ending in a neck of variable length and ascospores embedded in a mucilaginous sheath (Jacobs and Wingfield 2001; Zipfel et al. 2006). Leptographium, the main asexual form of Grosmannia species, was first described in 1927 as a fungus that causes a blue stain on timber (Lagerberg et al. 1927; Zipfel et al. 2006). It is characterized by dematiaceous, erect conidiophores terminating in penicillated branches that give rise to conidiogenous cells. These cells produce single-celled conidia that accumulate in mucilaginous drops (Kendrick 1962; Jacobs and Wingfield 2001).
Species of Leptographium are mainly associated with conifer-infesting bark beetles (Grosmann 1931; Harrington and Cobb 1988; Wingfield et al. 1993; Jacobs and Wingfield 2001; Kirisits 2004; Paciura et al. 2010). Only a few species of this genus are known to be associated with non-coniferous hosts (Jacobs and Wingfield 2001; Jacobs et al. 2006; Paciura et al. 2010). Some Leptographium species, such as L. wageneri Zipfel et al., which causes the black-stain root disease (Wagener and Mielke 1961; Harrington and Cobb 1988), or Grosmannia serpens Goid, which is linked to pine decline in the USA (Eckhardt et al. 2007), are primary pathogens that cause significant economic losses (Lagerberg et al. 1927; Wingfield et al. 1988; Seifert et al. 1993).
In recent years, several species of Grosmannia and their Leptographium asexual forms have been recorded in association with various bark beetles in China: G. koreana Q Lu et al., L. procerum K Jacobs et al., and L. sinoprocerum Q Lu et al. associated with Dendroctonus valens LeConte (Lu et al. 2008, 2009a, b); G. yunnanense Tsiang associated with Tomicus yunnanensis Kirkendall and Faccoli (Zhou et al. 2000; Kirkendall et al. 2008; Yamaoka et al. 2008); and L. sinense Lour associated with Hylobitelus xiaoi Zhang (Yin et al. 2015). However, to date, few investigations on Grosmannia/Leptographium associated with I. subelongatus have been carried out: only two species, namely L. manifestum and L. altius (Paciura et al. 2010), have been reported in China.
In a recent survey of ophiostomatoid fungi associated with I. subelongatus and their galleries in northern larch forests in China, several strains of Leptographium were isolated. The primary aim of this study was to identify these strains using a combination of morphological observations and multilocus DNA sequence data. This study additionally evaluated the pathogenicity of the identified species via inoculation tests in the field.
Materials and methods
Collection of samples and isolation of fungi
Samples of I. subelongatus and their galleries were collected from L. gmelinii in the Heilongjiang Province and the Inner Mongolia autonomous region in China. Fungi were isolated from galleries as described by Seifert et al. (1993) and incubated at 25 °C. Fungi were also isolated from young adult beetles by crushing them onto the surface of 2 % malt extract agar (MEA) with 0.05 % cycloheximide. All strains were purified by hyphal tip isolation. Representative cultures were deposited in the BCCM/MUCL culture collection and the culture collection of the Chinese Academy of Forestry (CXY).
Cultural and morphological studies
The strains of Leptographium spp. were grown on 2 % MEA and oatmeal agar (OA) (Gams et al. 1998) at 25 °C for 20 days. All microscopic measurements were performed in 85 % lactic acid. Fifty measurements were performed for each morphological character.
The optimal growth temperature of the various strains was determined by placing a 5-mm (diameter) plug from an actively growing fungal colony at the center of MEA plates for three replicates. Plates were incubated in the dark at temperatures ranging from 5 to 35 °C, at 5 °C intervals. Colony diameters on each dish were measured along two perpendicular lines and the averages were calculated for each of the seven temperatures. Colony color was determined according to the method of Rayner (1970).
DNA extraction, PCR, and sequencing
The fungal strains were grown in liquid malt at 25 °C in the dark for 7 days. DNA was extracted using an Invisorb Spin Plant Mini Kit (Invitek, Berlin), following the manufacturer’s instructions. Three gene regions, viz. the internal transcribed spacer 2 (ITS2) and part of the 28S (containing domains D1 and D2), partial β-tubulin, and partial elongation factor 1-α (EF1-α) were amplified. The ITS2 and 28S regions were amplified using primers ITS3 and LR3 (White et al. 1990). The primers Bt2a and Bt2b (Glass and Donaldson 1995) were used to amplify part of the β-tubulin gene region. The transcription elongation factor-1α gene region was amplified with primers EF1F and EF2R (Jacobs et al. 2004).
Polymerase chain reaction (PCR) assays were performed in 25-μL volumes (2.5 mM MgCl2, 1 × PCR buffer, 0.2 mM dNTP, 0.2 mM of each primer, and 2.5 U Taq polymerase enzyme). The PCR conditions for the ITS2 and 28S gene regions were: an initial denaturation step at 95 °C for 2 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 54 °C, and 1 min at 72 °C, and a final chain elongation at 72 °C for 8 min. The partial β-tubulin and EF1-α genes were amplified using a denaturation step at 95 °C for 2 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 56 °C, and 1 min at 72 °C, and a final chain elongation at 72 °C for 8 min. PCR products were cleaned using an MSB Spin PCRapace Kit (250) (Invitek, Berlin), following the manufacturer’s instructions.
Sequencing reactions were performed with a CEQ DTCS Quick Start Kit (Beckman Coulter), following the manufacturer’s instructions, with the same PCR primers as above. Nucleotide sequences were determined with a CEQ 2000XL capillary automated sequencer (Beckman Coulter).
Phylogenetic analyses
BLAST searches were conducted for preliminary identification, after which datasets were compiled including published GenBank sequences. Datasets were aligned using MAFFT 6 (Katoh et al. 2002). Phylogenetic analyses were performed using maximum parsimony (MP) as implemented in PAUP* 4.0b10 (Swofford 2003), Bayesian inference (BI) as implemented in MrBayes v3.1.2 (Huelsenbeck and Ronquist 2001), and maximum likelihood (ML) using RAxML 7.0.4 (Stamatakis 2006).
For phylogenetic inferences based on the ITS2-28S dataset, O. piliferum Syd et al., O. karelicum Linnakoski et al., and O. novo-ulmi Brasier were used as the outgroup (Linnakoski et al. 2012). The phylogenetic inferences based on the β-tubulin and EF1-α datasets were performed without outgroups (unrooted trees).
ML analyses were performed using RAxMLv7.0.4 (Stamatakis et al. 2006) assuming the GTR + G Substitution model, and run on the CIPRES cluster at the San Diego Supercomputer Center. Supports for the nodes were estimated from 1000 bootstrap replicates. MP was performed using PAUP* version 4.0b10 (Swofford 2001), with gaps treated as fifth base. The most parsimonious trees were identified using heuristic searches with random addition sequence (1000), with MAXTREES set to 200, and further evaluated by bootstrap analysis, retaining clades compatible with the 50 % majority rule in the bootstrap consensus tree. The analysis conditions were tree bisection reconnection branch swapping (TBR); starting tree obtained via stepwise addition; steepest descent not in effect; MULTREES effective.
BI was carried out using MrBayes v3.1.2 (Huelsenbeck and Ronquist 2001). The most appropriate substitution models were selected using the Akaike information criterion (AIC) in MrModeltest v2.3. In order to calculate posterior probabilities, BI analyses were performed using the Markov chain Monte Carlo (MCMC) approach with 5,000,000 generations.
Pathogenicity tests
The pathogenicity of three Leptographium species was tested by artificial inoculation of mature larch trees in the field. The possible host specificity of the fungi was additionally assessed by cross-inoculation between two larch species. Two separated forest plots were selected for inoculation, viz. the Genhe forest farm (north-eastern Inner Mongolia, N: 50°54′18.9″, E: 121°29′59.9″) and the Huanggangliang forest farm (middle Inner Mongolia, N: 43°36′24.5″, E: 117°30′45.6″). The local larch species inoculated were L. gmelinii and L. principis-ruprechtii, respectively.
Three strains were grown on 2 % MA in 9-cm Petri dishes at 25 °C for 2 weeks before being used as inoculum. Twelve 25-year-old healthy trees were selected for inoculation, with three trees used for each of the three Leptographium strains. Sterile MEA was inoculated as the control for each experiment. Tree stems were inoculated a height of 150 cm above the ground, and 6-mm-diameter holes were drilled horizontally up to the sapwood. A plug of 5-mm-diameter mycelium disk, cut from the actively growing margin of the colony, was inserted into the bark hole using a sterilized toothpick, according to the method described by Yamaoka et al. (1998). Sterilized Eppendorf tube caps were used to cover the inoculation holes to prevent invasion by insects and air contamination.
Field inoculations were performed on 4 July 2014. After periodic inspection for the development of external symptoms over 10 weeks, the trees were cut in September. The lesions formed in the inner bark around the inoculation points were measured. The stems were then split at the center of each inoculated area in order to evaluate the extent of the stained area inside the wood. Tissues at the margin of the reaction zone were collected for reisolation of the fungi under aseptic conditions. The data for the lesions were then analyzed using one-way analysis of variance (ANOVA).
Results
Fungal strains and phylogenetic analyses
Six strains of Leptographium were obtained from bodies or galleries of I. subelongatus, at relatively low frequencies (≤5 % of the total isolates) (Table 1).
Amplification of the ITS2-28S region, partial β-tubulin gene, and partial EF1-α gene yielded fragments of 900 bp, 410 bp, and 820 bp, respectively. For each of the sequence datasets, the topologies of MP and Bayesian tree were similar to that of ML.
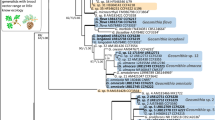
Phylogenetic inferences based on the ITS2-LSU dataset grouped the strains isolated from the larch trees into two clades (Fig. 1). Together with G. aenigmatica Zipfel et al., G. piceaperda Goid et al., and G. laricis, the strains CXY1552 (=MUCL 55162) and CXY1553 (=MUCL 55163) formed a well-supported clade (the G. piceaperda clade). The strains CXY1547 (=MUCL 55158), CXY1548 (=MUCL 55159), CXY1549, and CXY1554 (=MUCL 55160) grouped together with L. taigense Linnakoski et al. to form a second well-supported clade; the ITS2-LSU sequences of these four strains were found to be 100 % identical to those of L. taigense.
Phylogram obtained from ML analyses of the ITS2 and 28S regions; novel sequences obtained in this study are indicated in bold type. ML bootstrap support values (1000 replicates) (normal type) and MP jackknife values (10,000 replicates) (bold type) above 70 % are indicated at the nodes. Posterior probabilities (above 95 %) obtained from BI are indicated by bold lines at the relevant branching points. Values less than 70 % are indicated by an asterisk
Analysis of the β-tubulin dataset yielded trees (Fig. 2) with a topology similar to that obtained from the ITS2-28S dataset (Fig. 1). The present strains isolated from Larix also clustered into two clades, viz. the G. piceaperda and the L. taigense clades. The strains CXY1552 and CXY1553 were resolved as a distinct lineage within the G. piceaperda clade, with very high bootstrap support (Fig. 2), suggesting a possibly distinct phylogenetic species. The β-tubulin sequence of the strains CXY1547, CXY1548, CXY1549, and CXY1554 was identical to that of L. taigense.
Phylogram obtained from ML analyses of the β-tubulin gene regions; novel sequences obtained in this study are indicated in bold type. ML bootstrap support values (1000 replicates) (normal type) and MP jackknife values (10,000 replicates) (bold type) above 70 % are indicated at the nodes. Posterior probabilities (above 95 %) obtained from BI are indicated by bold lines at the relevant branching points. Values less than 70 % are indicated an asterisk
Phylogenetic relationships inferred from the EF-1α dataset yielded a tree topology (Fig. 3) similar to that obtained from the analysis of the β-tubulin dataset (Fig. 2). The strains CXY1552 and CXY1553 were also resolved as a distinct lineage within the G. piceaperda clade, with very high bootstrap support (Fig. 3). The strains CXY1549 and CXY1554 still grouped together with the type strain of L. taigense (Fig. 3). However, the strains CXY1547 and CXY1548 were resolved as a distinct clade with strong support (Fig. 3).
Phylogram obtained from ML analyses of the EF1-α gene region; novel sequences obtained in this study are indicated in bold lines. ML bootstrap support values (1000 replicates) (normal type) and MP jackknife values (10,000 replicates) (MP Jackknife values) above 70 % are indicated at the nodes. Posterior probabilities (above 95 %) obtained from BI are indicated by bold lines at the relevant branching points. Values less than 70 % are indicated by an asterisk
Culture morphology and characteristics
On the basis of cultural characteristics and micromorphology, the present strains from I. subelongatus and their galleries could be grouped into three distinct morphological groups or phenotypes. Group 1 comprised of the strains CXY1552 and CXY1553, group 2 the strains CXY1547 and CXY1548, and group 3 the strains CXY1549 and CXY1554.
The strains belonging to group 1 produced fertile perithecia on MEA within 8 days. However, the asexual form was not observed. The colony color of these strains was white, with abundant aerial mycelium. Perithecia were not formed by strains from groups 2 and 3 on MEA. However, strains of both groups 2 and 3 developed a dark brown synnematous asexual form, whose conidia differed between strains of the two groups. The colony color of strains from groups 2 and 3 was white, becoming respectively dark celadon and light brown with age on MEA.
The optimal temperature of growth for all three groups was 25 °C, with no growth observed at 5 °C and 35 °C. Strains of group 1 grew much faster than those of groups 2 and 3, reaching 76-mm diameters after 6 days at 25 °C. In comparison, colonies of strains from groups 2 and 3 reached 24 and 23 mm, respectively, after 6 days of growth at 25 °C (Fig 4).
Taxonomy
Leptographium zhangii X. W. Liu, Q. Lu & X. Y. Zhang, sp. nov. Fig. 5
MycoBank: MB 811205
Etymology: “zhangii” (L), named in honor of Prof. Xingyao Zhang, the senior author of the paper.
Colonies on 2 % MEA were fast growing, reaching 84 mm in diameter in 7 days at the optimal growth temperature of 25 °C; no growth at 5 °C and 35 °C; colonies on malt extract agar white, with abundant aerial mycelium, hyaline on OA.
Perithecia appearing over the colony surface, more abundant at its edge, after 8 days; perithecia with a globose base, (126–)141–215(−316) μm diameter, dark, ornamented with hyphae, ending in black, smooth, straight to slightly curved perithecial necks, (190–)200–430(−445) μm long, (37–)42–69(−78) μm wide at the base down to (24–)29–37(−43) μm at the apex; ostiolar hyphae absent; ascospores with hyaline gelatinous sheets, hyaline, aseptate, oblong, 5.5–10.0 × 2.7–3.8 μm (excluding the mucilaginous sheath). Asexual form unknown.
Type material: Holotype CXY1552 (dried culture), CHINA, Heilongjiang, Mohe, from I. subelongatus infecting L. gmelinii, 2012, collected by X. Liu, ex-holotype culture CXY1552 = MUCL55162.
Hosts/substrate: L. gmelinii
Known distribution: China
Leptographium innermongolicum X. W. Liu, Q. Lu & X. Y. Zhang, sp. nov. Fig. 6
Leptographium innermongolicum. A–B: Growing on 2 % MEA and OA, respectively. C–D: Rhizoids, conidiophores, conidiogenous apparatus (bar = 20 μm). E: Conidiogenous cells showing tapering apex with conidia (bar = 10 μm). F: Aggregated conidiophores of Leptographium-like anamorph (bar = 20 μm). G: Conidia (bar = 10 μm)
MycoBank: MB 811204
Etymology: “innermongolicum” (L), in reference to the type locality of this species, Inner Mongolia.
Colonies on 2 % MEA reaching 29 mm in diameter in 7 days at the optimal growth temperature of 25 °C; no growth observed at 5 °C and 35 °C; colony color on MEA white, becoming dark celadon with age; colony color on OA hyaline, becoming dark brown with age.
Sexual form unknown. Synnematous anamorph predominant in culture; single or in groups, dark brown, (80–)120–272(−385) μm high, (12–)20–39(−48) μm wide at the base, with rhizoid-like structures occasionally present; mononematous Leptographium-like synanamorph present, but sparse on MEA, soon aggregated to form synnematous structures; stipes hyaline to light brown, cylindrical, (15–)60–165(−250) μm long and (1.3–)2–2.0(−2.5) μm wide, apical cell not swollen, basal cell not swollen. Primary branches 2–3, cylindrical, 0–1-septate, (7–)9–22(−35) μm long and (1–)1.5–2(−2.5) μm wide. Secondary branches occasionally swollen, (4–)6–11(−14) μm long and (1–)1.5–2.5(−3.5) μm wide. Tertiary branches sometimes observed, typically swollen, (6–)7–12(−12.5) μm long and (2–)2.5–4(−4.5) μm wide. Conidiogenous cells discrete, 2–7 per branch, cylindrical, tapering slightly at the apex, (4–)10–15(−21) μm long and 1.0–2.0 μm wide. Conidia hyaline, aseptate, oblong, 2.2–3.7 × 0.9–2.2 μm.
Type material: Holotype CXY1547 (dried culture), CHINA, Inner Mongolia, Genhe wood reservation station from I. subelongatus infecting L. gmelinii, 2010, collected by Q. Lu, ex-holotype culture CXY1547 = MUCL55158.
Hosts/substrate: L. gmelinii
Known distribution: China
Pathogenicity tests
Two months after inoculation, none of the 12 inoculated trees in the two plots showed any visible disease symptoms in the crowns. Trees inoculated with the control exhibited a slight brown discoloration in inner bark, extending up to 1.4 mm long from the border of the hole; this was observed after removal of the outer bark. Lesions produced by the Leptographium strains for each species (CXY1552 for L. zhangii, CXY1547 for L. innermongolicum, and CXY1554 for L. taigense) were not significantly longer than those observed in control-inoculated trees (Table 2). Moreover, no significant differences were found between the lesion lengths produced by the Leptographium strains in Genhe and Huanggangliang. The inoculated fungi were readily reisolated from selected lesions, while the control holes did not yield Leptographium spp.
Discussion
The present study reveals that three species of Leptographium are associated with I. subelongatus infestations of larch forests in northern China, including L. taigense and two new species, L. zhangii and L. innermongolicum. The strains of these three species occurred at a very low frequency, not exceeding 5 % of the total isolates.
According to the Melbourne code 2011 (Hawksworth 2011; McNeill et al. 2012) and the “one fungus, one name” principle, we adopted Leptographium as the formal genus name based on priority and usage of the name (Jacobs and Wingfield 2013). Accordingly, the two new species were named under the genus Leptographium.
Leptographium taigense has been described on the basis of a few collections made from various bark beetles on pine and spruce in the Karelia forest of north-western Russia (Linnakoski et al. 2012). In three-locus phylogenetic studies, this species formed a standalone taxon distinct from the other known species complexes (Linnakoski et al. 2012).
To our knowledge, the present study is the first to report the occurrence of L. taigense on L. gmelinii in association with I. subelongatus in northern China; our data indicate a broader host range and geographic distribution for the fungal species. In addition, a genetically closely related taxon, L. innermongolicum, was found to co-occur in the same environment. According to our analysis, L. innermongolicum differs from L. taigense only in terms of its EF1-α DNA sequence data (Fig. 3). The partial sequences of ITS-28S and β-tubulin were found to be identical between these two species. Furthermore, these two species are morphologically similar, producing a similar macronematous, synnematous asexual form resulting from the aggregation of mononematous conidiophores, as described by Linnakoski et al. (2012). However, the synnemata of L. innermongolicum are much shorter than those of L. taigense: 120–272 μm and 287–566 μm, respectively (Linnakoski et al. 2012). Leptographium innermongolicum may additionally be distinguished from L. taigense based on colony characteristics. The colony color of L. innermongolicum is initially white, becoming dark celadon with age, whereas the colony color of L. taigense is hyaline, later becoming light brown.
In addition to L. taigense, L. innermongolicum may be compared with G. galeiformis Zipfel et al., which also produces a synnematous form in culture (Zhou et al. 2004). However, the conidia of L. innermongolicum are smaller to those of G. galeiformis. The conidiophores of L. innermongolicum are dark brown, whereas those of G. galeiformis are middle brown in color. The colony color of L. innermongolicum is white, becoming dark celadon with age. However, the colony color of G. galeiformis is light gray, becoming dark brown with age. The sexual forms of L. innermongolicum and L. taigense are unknown at present. Linnakoski et al. (2012) failed to obtain perithecia in vitro for L. taigense via mating studies.
Leptographium was originally characterized by the presence of mononematous conidiophores (Jacobs and Wingfield 2001). However, recent phylogenetic studies have shown that members of the Leptographium lineage may exhibit diverse asexual forms, including Hyalorhinocladiella, reduced Leptographium structures, loose and tight aggregates of Leptographium-like structure, or even Phialographium-like structure (Zipfel et al. 2006; Paciura et al. 2010; Linnakoski et al. 2012; Huang and Chen 2014).
Leptographium zhangii belongs to the G. piceaperda clade (Figs. 1 and 2), which differs from G. piceaperda and from G. aenigmatica in terms of the shape of the ascospores, including the mucilaginous sheath; L. zhangii has oblong ascospores which are cucullate in G. piceaperda and G. aenigmatica (Jacobs et al. 1998; Jacobs et al. 2000). No asexual form was found for L. zhangii, despite searching for conidiogenous cells in cultures.
Leptographium zhangii is also similar to G. laricis, which has been described in association with several bark beetles and is considered pathogenic to larch trees (Stauffer et al. 2001; Yamaoka et al. 1998, 2009). Leptographium zhangii differs in having much shorter perithecial necks, viz. 140–215 μm in length, compared with those of G. laricis, which are 400–1320 μm long (Jacobs and Wingfield 2001).
Little is known about the ecology of L. zhangii and L. innermongolicum. To date, these species have only been described in the context of L. gmelinii ecosystems in Inner Mongolia, northern China, in association with I. subelongatus. Both species were isolated from the insect itself as well as its breeding galleries, suggesting that I. subelongatus may act as a vector. Leptographium species are adapted to be carried by bark-infesting beetles or other insects that act as vectors (Harrington and Cobb 1988; Wingfield et al. 1993; Jacobs and Wingfield 2001).
These three species do not seem to be pathogenic to larch in the L. gmelinii ecosystems. After 2 months of incubation, stem inoculation with the three Leptographium species resulted in non-significant lesions that were visible at the inner bark, with no discernible symptoms in the crowns of mature larch trees.
As L. innermongolicum is closely related to L. taigense, it should not be surprising that the two species may exhibit the same pathogenicity. The pathogenicity of L. zhangii to L. gmelinii in northern China is weaker than that of other members of the G. piceaperda complex, of which G. piceaperda and G. laricis are pathogenic to trees (Yamaoka et al. 1998; Sallé et al. 2005).
Leptographium species typically occur in conifer forests, in association with bark beetles. Ten species of Leptographium have been isolated from larch worldwide (Bakshi 1950; Mielke 1979; Yamaoka et al. 1998, 2009; McBeath et al. 2004; Paciura et al. 2010). To date, there are few reports of the occurrence of Leptographium in association with I. subelongatus in China. The present study reports the isolation of L. taigense, L. zhangii, and L. innermongolicum from L. gmelinii. Future studies should contribute interesting insights into the ecology, biodiversity, and biogeography of the fungi.
References
Aoshima K (1965) Studies on wood-staining fungi of Japan. Doctoral thesis, University of Tokyo, Tokyo
Bakshi BK (1950) Fungi associated with ambrosia beetles in Great Britain. Trans Br Mycol Soc 33(1):111–IN11
De Beer ZW, Wingfield MJ (2013) Emerging lineages in the Ophiostomatales. In: Seifert KA, De Beer ZW, Wingfield MJ (eds) The ophiostomatoid fungi: expanding frontiers. CBS, Utrecht, The Netherlands, pp 21–46
Eckhardt LG, Weber AM, Menard RD, Jones JP, Hess NJ (2007) Insect–fungal complex associated with loblolly pine decline in central Alabama. For Sci 53:84–92
Gams W, Hoekstra ES, Aptroot A (1998) CBS course of mycology, 4th edn. Centraalbureau voor Schimmelcultures (CBS), Baarn, The Netherlands
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61(4):1323–1330
Goidánich G (1936) II genere di Ascomiceti ‘Grosmannia’ G. Goid. Boll Staz Pat veg Roma 16:26–60
Grosmann H (1931) Contributions to the knowledge concerning the life partnership between bark beetles and fungi. Z Parasitenkd 3:56–102
Harrington TC, Cobb FW (1988) Leptographium root diseases on conifers. American Phytopathological Society Press, St. Paul, MN
Hawksworth DL (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2:155–162. This article was first published in MycoKeys, 1:7–20 (2011)
Huang YT, Chen CY (2014) Leptographium globosum sp. nov., a new species with globose conidia. Mycol Progress 13:841–848
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jacobs K, Wingfield MJ (2001) Leptographium species: tree pathogens, insect associates, and agents of blue-stain. American Phytopathological Society Press, St. Paul, MN
Jacobs K, Wingfield MJ (2013) An overview of Leptographium and Grosmannia. In: Seifert KA, De Beer, Wingfield MJ (eds) The ophiostomatoid fungi: expanding frontiers. CBS, Utrecht, The Netherlands, pp 47–56
Jacobs K, Wingfield MJ, Wingfield BD, Yamaoka Y (1998) Comparison of Ophiostoma huntii and O. europhioides and description of O. aenigmaticum sp. nov. Mycol Res 102:289–294
Jacobs K, Wingfield MJ, Crous PW (2000) Ophiostoma europhioides and Ceratocystis pseudoeurophioides, synonyms of O. piceaperdum. Mycol Res 104(2):238–243
Jacobs K, Wingfield MJ, Wingfield BD (2001) Phylogenetic relationships in Leptographium based on morphological and molecular characters. Can J Bot 79(6):719–732
Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA, Bright DE, Wingfield BD (2004) Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol Res 108(4):411–418
Jacobs K, Solheim H, Wingfield BD, Wingfield MJ (2005) Taxonomic re-evaluation of Leptographium lundbergii based on DNA sequence comparisons and morphology. Mycol Res 109(10):1149–1161
Jacobs K, Eckhardt LG, Wingfield MJ (2006) Leptographium profanum sp. nov., a new species from hardwood roots in North America. Can J Bot 84:759–766
Jacobs K, Krokene P, Solheim H, Wingfield MJ (2010) Two new species of Leptographium from Dryocetes authographus and Hylastes cunicularius in Norway. Mycol Prog 9(1):69–78
Katoh K, Misawa K, Kuma KI, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acid Res 30:3059–3066
Kendrick WB (1962) The Leptographium complex Verticicladiella Hughes. Can J Bot 40:772–797
Kim JJ, Lim YW, Breuil C, Wingfield MJ, Zhou XD, Kim GH (2005) A new Leptographium species associated with Tomicus piniperda infesting pine logs in Korea. Mycol Res 109(03):275–284
Kirisits T (2004) Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer, Dordrecht, The Netherlands, pp 181–235
Kirkendall LR, Faccoli M, Ye H (2008) Description of the Yunnan shoot borer, Tomicus yunnanensis Kirkendall & Faccoli sp. n. (Curculionidae, Scolytinae), an unusually aggressive pine shoot beetle from southern China, with a key to the species of Tomicus. Zootaxa 1819:25–39
Lagerberg T, Lundberg G, Melin E (1927) Biological and practical researches into blueing in pine and spruce. Svenska Skogsvårdsfören Tidskr 25:145
Lim YW, Alamouti SM, Kim JJ, Lee S, Breuil C (2004) Multigene phylogenies of Ophiostoma clavigerum and closely related species from bark beetle-attacked Pinus in North America. FEMS Microbiol Lett 237(1):89–96
Linnakoski R, de Beer ZW, Duong TA, Niemelä P, Pappinen A, Wingfield MJ (2012) Grosmannia and Leptographium spp. associated with conifer-infesting bark beetles in Finland and Russia, including Leptographium taigense sp. nov. Antonie Van Leeuwenhoek 102:375–399
Lu Q, Decock C, Zhang XY, Maraite H (2008) Leptographium sinoprocerum sp. nov., an undescribed species associated with Pinus tabuliformis–Dendroctonus valens in northern China. Mycologia 100:275–290
Lu Q, Decock C, Zhang XY, Maraite H (2009a) Ophiostomatoid fungi (Ascomycota) associated with Pinus tabuliformis infested by Dendroctonus valens (Coleoptera) in northern China and an assessment of their pathogenicity on mature trees. Antonie Van Leeuwenhoek 96:275–293
Lu M, Zhou XD, De Beer ZW, Wingfield MJ, Sun JH (2009b) Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Divers 38:133–145
McBeath JH, Cheng M, Gay P, Ma M (2004) First report of Leptographium abietinum associated with blue stain on declining western Siberian larch in Alaska. Plant Health Progress (March):1–2
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme van Reine WF, Smith GF, Wiersema J, Turland NJ (eds) (2012) International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum vegetabile 154. Koeltz Scientific Books, Königstein
Mielke PW (1979) On asymptotic non-normality of null distributions of MRPP statistics. Commun Stat Theory Methods 8(15):1541–1550
Paciura D, De Beer ZW, Jacobs K, Zhou XD, Ye H, Wingfield MJ (2010) Eight new Leptographium species associated with tree-infesting bark beetles in China. Persoonia 25:94–108
Pfeffer A (1995) Zentral- und Westpaläarktische Borken- und Kernkäfer. Naturhistorishes Museum Basel, Basel, 310 pp
Rayner RW (1970) A mycological colour chart. Commonwealth Mycological Institute and the British Mycological Society, Kew, Surrey
Sallé A, Monclus R, Yart A, Garcia J, Romary P, Lieutier F (2005) Fungal flora associated with Ips typographus: frequency, virulence, and ability to stimulate the host defence reaction in relation to insect population levels. Can J For Res 35:365–373
Seifert KA, Webber JF, Wingfield MJ (1993) Methods for studying species of Ophiostoma and Ceratocystis. In: Wingfield MJ, Seifert KA, Webber JF (eds) Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. American Phytopathological Society Press, St. Paul, MN, pp 255–259
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stauffer C, Kirisits T, Nussbaumer C, Pavlin R, Wingfield MJ (2001) Phylogenetic relationships between the European and Asian eight spined larch bark beetle populations (Coleoptera, Scolytidae) inferred from DNA sequences and fungal associates. Eur J Entomol 98:99–105
Swofford DL (2001) PAUP*: Phylogenetic analysis using parsimony (and other methods) 4.0.b5
Swofford DL (2003) PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, MA
Van der Westhuizen K, Wingfield MJ, Yamaoka Y, Kemp GHJ, Crous PW (1995) A new species of Ophiostoma with a Leptographium anamorph from larch in Japan. Mycol Res 99(11):1334–1338
Wagener WW, Mielke JL (1961) A staining-fungus root disease of pondersoa, Jeffrey, and pinyon pines. Plant Dis Rep 45:831–835
White TJ, Bruns T, Lee SJWT, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18(1):315–322
Wingfield MJ, Capretti P, Mackenzie M (1988) Leptographium spp. as root pathogens on conifers. An international perspective. In: Harrington TC, Cobb FW Jr (eds) Leptographium root diseases on conifers. American Phytopathological Society Press, St. Paul, MN, pp 113–128
Wingfield MJ, Seifert KA, Webber JF (1993) Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. American Phytopathological Society Press, St. Paul, MN
Wood SL, Bright DE (1992) A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index Volume A. Great Basin Naturalist Memoirs, No. 13. Brigham Young University, Provo, UT, 833 pp
Yamaoka Y, Wingfield MJ, Takahashi I, Solheim H (1997) Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. japonicus in Japan. Mycol Res 101:1215–1227
Yamaoka Y, Wingfield MJ, Ohsawa M, Kuroda Y (1998) Ophiostomatoid fungi associated with Ips cembrae in Japan and their pathogenicity of Japanese larch. Mycoscience 39:367–378
Yamaoka Y, Masuya H, Chung WH, Goto H, To-Anun C, Tokumasu S, Zhou X, Wingfield MJ (2008) The teleomorph of Leptographium yunnanense, discovered in crosses among isolates from Thailand, China, and Japan. Mycoscience 49:233–240
Yamaoka Y, Chung WH, Masuya H, Hizai M (2009) Constant association of ophiostomatoid fungi with the bark beetle Ips subelongatus invading Japanese larch logs. Mycoscience 50:165–172
Yang JL, Lin Q, Chen GF (2007) Risk analysis of Ips subelongatus Motschulsky. J Northeast For Univ 35(3):60–63
Yin HF, Huang FS, Li ZL (1984) Economic insect fauna of China. Science and Technology Press, Beijing, pp 54–55
Yin M, Duong TA, Wingfield MJ, Zhou X, De Beer ZW (2015) Taxonomy and phylogeny of the Leptographium procerum complex, including Leptographium sinense sp. nov. and Leptographium longiconidiophorum sp. nov. Antonie Van Leeuwenhoek 107:547–563
Zhou XD, Jacobs K, Morelet M, Ye H, Lieutier F, Wingfield MJ (2000) A new Leptographium species associated with Tomicus piniperda in southwestern China. Mycoscience 41:573–578
Zhou XD, De Beer ZW, Harrington TC, McNew D, Kirisits T, Wingfield MJ (2004) Epitypification of Ophiostoma galeiformis and phylogeny of species in the O. galeiformis complex. Mycologia 96:1306–1315
Zhou XD, Jacobs K, Kirisits T, Chhetri DB, Wingfield MJ (2008) Leptographium bhutanense sp. nov., associated with the root collar weevil Hylobitelus chenkupdorjii on Pinus wallichiana in Bhutan. Persoonia Molecular Phylogeny Evol Fungi 21(1):1–8
Zipfel RD, De Beer ZW, Jacobs K, Wingfield BD, Wingfield MJ (2006) Multi-gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud Mycol 55:75–97
Acknowledgments
This study was supported by the Special Fund for Forest Scientific Research in the Public Welfare (201204501), the National Natural Science Foundation of China (project no.: 31070571), and the Special Fund for Public Welfare Institutes of the Central Government (CAFRIFEEP201102). This work was conducted under the auspices of the Chinese Academy of Forestry International Cooperation and Innovation Team. Quan Lu gratefully acknowledges a postdoctoral scholarship granted by the Wallonia-Brussels International Excellent Grants Program. Cony Decock gratefully acknowledges the financial support received from the Belgian State and Belgian Federal Science Policy through the BCCM™ research program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Gerhard Rambold
Rights and permissions
About this article
Cite this article
Liu, XW., Wang, HM., Lu, Q. et al. Taxonomy and pathogenicity of Leptographium species associated with Ips subelongatus infestations of Larix spp. in northern China, including two new species. Mycol Progress 16, 1–13 (2017). https://doi.org/10.1007/s11557-016-1245-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1245-1