Abstract
Species of Leptographium are generally characterized by mononematous conidiophores and are commonly associated with bark beetles and weevils. These species are responsible for sapstain and in some cases serious diseases on a range of primarily coniferous trees. In comparison with coniferous trees, the occurrence of Leptographium species on hardwood trees has been poorly studied in Europe. During a survey of ophiostomatoid fungi on various tree species in Norway and Poland, three unusual species, which fit the broader morphological description of Leptographium spp., were found in association with Scolytus ratzeburgi, Dryocoetes alni and Trypodendron domesticum on a variety of hardwoods, and from wounds on Tilia cordata. Phylogenetic analyses of sequence data for three gene regions (ITS2-LSU, β-tubulin, and TEF1-α) showed that these Leptographium species are phylogenetically closely related to each other and form a well-supported lineage that included Grosmannia grandifoliae and Leptographium pruni. The first species could be distinguished from the other Leptographium species based on conidiophores arising from spiral hyphae, chlamydospore-like structures and a hyalorhinocladiella-like synanamorph in culture. The second species differs from the previous one by having distinctly shorter conidiophores and smaller conidia. This species also produces a well-developed sporothrix-like synanamorph with denticulate conidiogenous cells. Based on these unusual morphological characteristics and distinct DNA sequences, these fungi were recognised as new taxa for which the names Leptographium trypodendri sp. nov. and L. betulae sp. nov. are provided. The third group of isolates belonged to Grosmannia grandifoliae, representing the first report of this species outside of the USA. The newly defined G. grandifoliae complex is the first species complex in Leptographium s.l. consisting of only hardwood-infecting species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Leptographium Lagerb. & Melin and Grosmannia Goid. (Ophiostomatales, Ascomycota) are well-known agents of sapstain and disease of respectively conifer wood and trees in North America, Europe and Asia (Harrington 1988; Jacobs and Wingfield 2001). These fungi are specifically adapted for dispersal by bark beetles and weevils (Coleoptera: Scolytinae) (Malloch and Blackwell 1993). The associations with these beetles vary from very specific to fairly unspecific (Kirisits 2004). Only a few of these fungi is considered primary pathogens, and the damage they cause are usually in association with their bark beetle vectors (Jacobs and Wingfield 2001).
Probably the best known of the conifer pathogens are the three host-specific varieties of Grosmannia wageneri (Goheen & F.W. Cobb) Zipfel et al., responsible for black stain root disease on native Pinus and Pseudotsugae spp. in disturbed stands in Western North America (Harrington and Cobb 1987; Cobb 1988). Another species, Grosmannia clavigera (R.C. Rob. and R.W. Davidson) Zipfel et al., is vectored by the mountain pine beetle (Dendroctonus ponderosae Hopkins) in an ongoing epidemic and range expansion of the beetle in Western Canada that is driven by climate change (Hicke et al. 2006; Alamouti et al. 2011). Grosmannia clavigera has been shown to be moderately virulent when inoculated in living pine trees (Solheim and Krokene 1998), but suggestions that the beetle depends on these fungi to kill trees have been challenged by Six and Wingfield (2011). In another scenario, Leptographium procerum (W.B. Kendr.) M.J. Wingf., a common associate of several bark beetle and weevil species, has been implicated as contributing to the death of millions of native pine trees in China in association with the red turpentine (Dendroctonus valens Le Conte) that was introduced into China from North America (Lu et al. 2010). The fact that L. procerum was originally described from Canada and the USA (Kendrick 1962), together with earlier population studies including only Chinese and North American isolates, led to assumptions that L. procerum was, like the beetle, introduced into China from the USA (Lu et al. 2009, 2011). However, several recent reports of L. procerum from various pine-infesting beetles in Europe (Jankowiak 2012; Jankowiak and Bilański 2013a, b, c), as well as a more comprehensive population study (Taerum et al. 2017), suggest that Europe is most likely the centre of origin of the fungus, implying that the L. procerum—D. valens is a most likely new host-vector association (Wingfield et al. 2016).
Of the more than 90 species currently treated in Leptographium sensu lato (De Beer et al. 2013), only twelve have been reported from hardwoods. One of these only, L. calophylli (Wiehe) J.F. Webber et al., is considered a significant pathogen, causing a serious wilting disease of Calophyllum trees in the Seychelles and Mauritius (Wiehe 1949; Webber et al. 1999). The fungus is vectored by the bark beetle Cryphalus trypanus Sampson (Wainhouse et al. 1998). These examples of pathogenic Leptographium spp. illustrate the risks that Leptographium spp. can pose in a changing climate and when introduced into new environments.
Among the above examples and in recent literature (e.g. Linnakoski et al. 2012; Huang and Chi-Y 2014; De Errasti et al. 2016), the use of the genus names Leptographium and Grosmannia might seem inconsistent. The reason for this is that for many years the taxonomy of the Ophiostomatales was defined based on morphological features of their sexual and asexual states. Under the dual nomenclature system, Leptographium accommodated asexual morphs, while species with known sexual states were treated in Grosmannia (Jacobs and Wingfield 2001; Zipfel et al. 2006). Applying the ‘one fungus one name’ principles adopted in the International Code of Nomenclature (ICN) for Algae, Fungi and Plants (Hawksworth 2011), De Beer and Wingfield (2013) re-evaluated the taxonomy of Leptographium and Grosmannia based on ribosomal DNA sequences. They recognized nine species complexes within Leptographium sensu lato, showing that the type species for Leptographium and Grosmannia grouped in different complexes. They thus suggested that a more comprehensive study using sequences of more gene regions is needed to resolve the status of the two genera, and that for the interim, species names should not be changed and that new species should be treated in Leptographium.
During a recent survey of ophiostomatoid fungi on hardwood trees in Norway and Poland, an unidentified Leptographium species was isolated from Betula verrucosa Ehrh. infested with the bark beetle Scolytus ratzeburgi Jans. In addition, another unknown species of Leptographium was isolated from various tree species infested with the wood-boring beetle Trypodendron domesticum (L.) and the bark beetle Dryocoetes alni (Georg). Two isolates of a third species were also obtained from Tilia cordata Mill. trees. The aim of this study was to identify these fungi by comparing their morphology and DNA sequences to those of known species.
Materials and methods
Isolations, fungal isolates and herbarium specimens
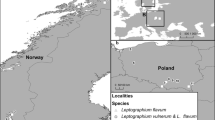
Isolations were made from the bark beetle S. ratzeburgi and its galleries established in decaying trees of B. verrucosa, and from the wood-boring bark beetle T. domesticum and its galleries in F. sylvatica logs. Strains were collected at two localities in southern Poland during March–July 2011–2013 (Fig. 1). In Norway, isolations were done from the bark beetles T. domesticum and D. alni in Alnus incana (L.) Moench, Betula pubescens Ehrh., Fraxinus excelsior L. and Quercus robur L. In A. incana branches, T. domesticum coexisted with D. alni. The bark beetles were collected at three localities in southern Norway (Fig. 1). Isolates from T. cordata were made from stained wood from wounds in the trees during July 2016 in Poland (Fig. 1; Table 1).
Origin of isolates used in this study: 1—Nes, Ullershov, Norway (59°41′20.79″N, 10°45′10.18″E); 2—Ås, Syverud, Norway (59°41′20.79″N, 10°45′10.18″E); 3—Larvik, Norway (59° 3′37.00″N, 10° 4′6.68″E); 4—Myszyniec, Poland (53°23′5.89″N, 21°22′58.08″E); 5—Krzeszowice, Poland (50°9′20.46″N, 19°42′8.06″E; 6—Muszyna, Poland (49°21′5.97″N, 20°52′47.83″E); 7—Higashidori, Aomori, Japan (41°16′3.05″N, 141°20′24.07″E); 8—Iwate, Morioka, Japan (39°56′58.81″N, 141°18′49.38″E)
Small pieces of the gallery tissue (4 × 4 mm2) were removed with a sterile scalpel and placed on 2% malt extract agar (MEA) (20 g Biocorp malt extract, 20 g agar, 1000 ml distilled water), containing cycloheximide (200 mg, Aldrich-Sigma, St. Louis, Co. LLC.) and tetracycline sulfate (200 mg, Polfa, Tarchomin SA), incubated at 22 °C and later examined for fungal growth. Isolations directly from beetles were made by crushing them onto the surface of the same medium as above. In Norway, each bark beetle was divided in three parts, elytra, head and the rest, before placing the parts in three different Petri dishes with 2% MEA without any cycloheximide.
All isolates used in the study are listed in Table 1. These isolates were maintained in the culture collection of the Department of Forest Pathology, Mycology and Tree Physiology, University of Agriculture in Krakow, Poland. The Norwegian isolates are kept at the culture collection of Norwegian Institute of Bioeconomy. Ex-type isolates of new species described in this study were deposited in the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, the Netherlands, and in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa. Type specimens were deposited in the TUR Fungus collections of the Turku University, Finland and in the Mycological Herbarium, Natural History Museum, University of Oslo, Norway.
Cultures of Leptographium pruni Masuya and M.J. Wingf. were sourced from the culture collection of the Tohoku Research Center of Forestry and Forest Products Research Institute, Nabeyashiki, Shimi-Kuriyagawa, Morioka, Japan (Hayato Masuya), and the ex-type isolate of L. pruni from the Japan Collection of Microorganisms (JCM). Some isolates of L. pruni were also deposited in the CBS collection. Two isolates of G. grandifoliae (R.W. Davidson) Zipfel et al., which is closely related to L. pruni were also included for the morphological and sequence analysis (Table 1). These isolates were also deposited in the CBS collection. Taxonomic descriptions and nomenclatural data were registered in MycoBank (www.MycoBank.org) (Robert et al. 2013).
DNA extraction, PCR and sequencing
Fungal isolates were grown on 2% malt extract agar [MEA: 20 g malt extract L−1 (Biocorp™, Warszawa, Poland), 20 g Biocorp™ agar−1and 1 L distilled water] in 60 mm plastic Petri dishes for 1–2 weeks prior to DNA extraction. DNA was extracted using the Genomic Mini AX Plant Kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s protocol.
Three gene regions were amplified for sequencing and phylogenetic analyses, including ITS2-LSU, βT and TEF1-α. The following primers were used: ITS3 and LR3 (White et al. 1990) for ITS2-LSU, T10 (O’Donnell and Cigelnik 1997) and Bt2b (Glass and Donaldson 1995) for βT, and EF1-F and EF2-R (Jacobs et al. 2004) for TEF1-α.
Amplification of the gene regions was performed under the following conditions: a denaturation step at 98 °C for 30 s followed by 35 cycles of 5 s at 98 °C, 10 s at 52–64 °C (depending on the type of primer and fungal species) and 30 s at 72 °C, and a final chain elongation at 72 °C for 8 min. Gene fragments were amplified in a 25 µL reaction mixture containing 0.25 µL of Phusion High-Fidelity DNA polymerase (Finnzymes, Espoo, Finland), 5 µL Phusion HF buffer (5×), 0.5 µL of dNTPs (10 mM), 0.75 µL DMSO (100%) and 0.5 µL of each primer (25 µM). Amplification reactions were performed in the LabCycler Gradient (Sensoquest Biomedical Electronics GmbH, Germany). The PCR products were visualized under UV light on a 2% agarose gel stained with Midori Green DNA Stain (Nippon Genetic, Europe).
Amplified products were sequenced with the BigDye® Terminator v 3.1 Cycle Sequencing Kit (AB Applied Biosystems, Foster City, CA, USA) and ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, USA), at the DNA Research Centre (Poznań, Poland) using the same primers that were used for the PCR. The sequences (Table 1) were deposited in NCBI GenBank and compared with those in GenBank using the BLASTn algorithm.
Sequence analyses
BLAST searches using the BLASTn algorithm were performed to retrieve similar sequences from GenBank (http://www.ncbi.nlm.nih.gov). Accession numbers of these sequences are presented in the corresponding phylogenetic trees (Figs. 2, 3, 4). The DNA sequences obtained in this study were deposited in GenBank and their accession numbers are presented in the Table 1.
Phylogram obtained from ML analyses of the ITS2-LSU region showing the placement of isolates obtained from Poland and Norway in Leptographium s. l. Sequences obtained during this study are presented in bold type. The phylogram was obtained from Maximum Likelihood (ML) analyses. Bootstrap values >75% for ML and posterior probabilities >75% obtained from Bayesian (BI) analyses are presented at nodes as follows: ML/BI. *Bootstrap values <75%. Ⓗ species from hardwood hosts
Phylogram obtained from the βT sequences of Leptographium spp. and Grosmannia spp. related to the Polish and Norwegian isolates. Sequences obtained during this study are presented in bold type. The phylogram was obtained from Maximum Likelihood (ML) analyses. Bootstrap values >75% for ML and posterior probabilities >75% obtained from Bayesian (BI) analyses are presented at nodes as follows: ML/BI. *Bootstrap values < 75%
Phylogram obtained from the TEF1-α sequences of Leptographium spp. and Grosmannia spp. related to the Polish and Norwegian isolates. Sequences obtained during this study are presented in bold type. The phylogram was obtained from Maximum Likelihood (ML) analyses. Bootstrap values >75% for ML and posterior probabilities >75% obtained from Bayesian (BI) analyses are presented at nodes as follows: ML/BI. *Bootstrap values <75%
Individual data sets for the ITS2-LSU, the βT and the TEF1-α gene regions were used for phylogenetic analyses. Data sets were compiled and edited in Molecular Evolutionary Genetic Analysis (MEGA) v6.06 (Tamura et al. 2013). The ITS2-LSU sequences of the isolates of each new species and L. pruni that were most closely related to them (Table 1), were compared with those of 72 other species in Leptographium s. l. obtained from GenBank to show the placement of the species within the genus. Two protein coding gene regions (βT and TEF1-α) for 23 isolates (Table 1) were sequenced for the delineation of closely related species.
Sequence alignments were performed using the online version of MAFFT v7 (Katoh and Standley 2013). The ITS, βT and TEF1-α data sets were aligned using the E-INS-i strategy with a 200PAM/κ = 2 scoring matrix, a gap opening penalty of 1.53 and an offset value of 0.00. Aligned data sets of the protein-coding genes were compared to gene maps constructed by Yin et al. (2015) to determine the presence or absence of introns and confirm that introns and exons were appropriately aligned (Table 2). For ML and Bayesian analyses, the best-fit substitution models for each data set were established using the corrected Akaike Information Criterion (AICc) in jModelTest 2.1.10 (Guindon and Gascuel 2003; Darriba et al. 2012).
Phylogenetic analyses were performed for each of the data sets using two different methods: maximum likelihood (ML) and Bayesian inference (BI). Maximum likelihood (ML) searches were conducted in PhyML 3.0 (Guindon et al. 2010), via the Montpelier online server (http://www.atgc-montpellier.fr/phyml/) with 1000 bootstrap replicates. BI analyses based on a Markov Chain Monte Carlo (MCMC) were carried out with MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003). The MCMC chains were run for 10 million generations using the best-fit model. Trees were sampled every 100 generations, resulting in 100,000 trees from both runs. The burn-in value for each dataset was determined in Tracer v1.4.1 (Rambaut and Drummond 2007).
Morphological characterization
Morphological characteristics for selected isolates and herbarium specimens chosen to represent the type specimens were examined. Cultures were grown on 2% MEA with and without host tree twigs to induce potential ascocarp formation. The autoclaved twigs with bark were placed in the middle of the agar plates. Fungi were grown as single isolate cultures, and crossings were made following the technique described by Grobbelaar et al. (2010). Cultures were incubated at 25 °C and inspected regularly for fruiting structures.
Morphological characteristics were examined by mounting the sexual and asexual fruiting structures in 80% lactic acid on glass slides, and these were observed using Nikon Eclipse 50i microscope (Nikon® Corporation, Tokyo, Japan) and Invenio 5S digital camera (DeltaPix®, Maalov, Denmark) to capture photographic images. Measurements were made of 50 each of the taxonomically relevant structures where this was possible with Coolview 1.6.0 software (Precoptic®, Warsaw, Poland). Averages, ranges and standard deviations were computed for the measurements. The measurements are presented in the format ‘(min–max) (mean–SD)’.
Culture characteristics
Growth characteristics of isolates in pure culture were considered for four representative isolates of each of the studied species (Table 1). Four replicate plates per isolate were used for each temperature (5, 10, 15, 20, 25, 30 and 35 °C). Agar disks 5 mm diam. were cut from actively growing margins of colonies of each isolate to be tested, and placed at the center of plates containing 2% MEA. Colony diameters (three measurements per plate) were determined 7 and 14 days after inoculation and growth rates were calculated as mm/d.
Results
Morphological characteristics
Despite their close phylogenetic relationship, the two unknown Leptographium spp. were morphologically distinct from each other as well as from the closely related L. pruni and G. grandifoliae. They formed olivaceous leptographium-like asexual states and sporothrix- or hyalorhinocladiella-like synanamorphs. Morphological differences between the two new taxa and the other species are discussed in the Notes provided for the new species descriptions in the “Taxonomy” section. The optimal growth temperature for all the isolates was 25 °C.
DNA sequence analyses
The amplified DNA fragments were approximately 860–918 bp long for the 5.8S-ITS2-LSU region, 372–545 bp long for the partial βT, and 805–834 bp long for the TEF1-α genes. The aligned data set for the ITS2-28S region gene region included 97 taxa and 629 characters (with gaps). The βT data set consisted of 50 taxa and 484 characters (with gaps), and included the partial exon 3, intron 3, exon 4, intron 4, exon 5, intron 5 and partial exon 6. The TEF1-α data set consisted of 49 taxa and 708 characters (with gaps), including partial intron 4, exon 5, intron 5, and partial exon 6. The BI and ML analyses for each data set produced trees with similar topologies (Figs. 2–4). The best-fitting substitution model selected for ML/BI analyses was HKY+I+G for all data sets.
In the ITS2-LSU tree (Fig. 2), the two unidentified species from Poland grouped with L. pruni and G. grandifoliae in a well-supported monophyletic lineage adjacent to the G. penicillata complex. In addition, the two isolates from Tilia grouped with the ex-type isolate of G. grandifoliae. However, the ITS2-LSU data did not distinguish clearly between L. pruni and the Polish isolates.
Analyses of the partial βT gene distinguished clearly between G. grandifoliae, including the two isolates from Tilia, and L. pruni, and separated the other isolates from Poland in two well-supported clades, Taxa A and B. Taxon A includes three isolates from Norway (Fig. 3). Together, the four taxa again formed a monophyletic clade with good support. In the βT data set, 35/384 (9.1%) positions were variable. Little or no intraspecific sequence variation was found within each of the four species in the complex. Intraspecific variability of the βT and TEF1-α genes was only detected for L. trypodendri in two positions i.e. 70, 175 and six positions i.e. 94, 124, 131, 158, 451 and 502, respectively (Table 2).
The phylogram based on the TEF1-α data resolved G. grandifoliae, L. pruni and Taxa A and B, with the latter three grouping closely together (Fig. 4). Together, the four species again formed a well-supported monophyletic lineage.
Taxonomy
Based on DNA sequences and morphological differences, Taxa A and B from Poland and Norway could be distinguished from L. pruni and G. grandifoliae, and are thus described below as novel species.
Taxon A
Leptographium trypodendri R. Jankowiak, B. Strzałka and R. Linnakoski, sp. nov. Fig. 5 MB 821669.
Morphological characteristics of Leptographium trypodendri sp. nov. (CBS 142,724). a Conidiophore. b Conidiophores arising in loosely arranged groups on spiral hyphae. c Conidiogenous apparatus with two primary branches. d Conidia. e, f Hyalorhinocladiella-like synanamorph. g Chlamydospore-like structures. h Culture on MEA
Etymology: trypodendri refers to the fungus being collected mainly from Trypodendron.
Sexual state not observed. Conidiophores macronematous, arising laterally from hyphae, single solitary or often also in loosely arranged groups on spiral hyphae, without rhizoidal hyphae at the bases (Fig. 5a, b). Stipes erect, light to dark brown, 1–7 septa, 31–130 (mean 74.9 ± 19.8) μm long and 2.5–5 (mean 3.6 ± 0.6) μm wide at base. Conidiogenous apparatus 39.5–86 (mean 62.7 ± 11) μm long (excluding conidial mass) consisting of (1–) 2 (–3) series of branches-type B (more than two branches) (Jacobs and Wingfield 2001) (Fig. 5c). Primary branches dark olivaceous, cylindrical, smooth, 8.5–26 × 2.5–6 μm. Conidiogenous cells hyaline, tapering from base to apex, 3.5–31 (mean 23 ± 6) × 1–2 (mean 1.6 ± 0.2) μm. Conidia hyaline, oblong to elliptical, sometimes allantoid or obovoid, 2.5–4.5 (mean 3.4 ± 0.43) × 1–2 (mean 1.6 ± 0.2) μm, accumulating around the conidiogenous apparatus in a hyaline mucilaginous mass (Fig. 5 d).
Hyalorhinocladiella-like micronematal asexual state present (Fig. 5e, f). Conidiogenous cells arising directly from hyphae, 5–53 (mean 23.3 ± 10.7) × 0.5–2.0 (mean 1.2 ± 0.4) μm; conidia hyaline, cylindrical, obovate or pyriform, 3–7.5 (mean 4.7 ± 0.9) × 0.5–3.5 (mean 2 ± 0.7) μm. Chlamydospores present in young and older cultures, 16–71.5 (mean 37.5 ± 10.6) μm in diameter (Fig. 5g).
Culture characteristics colonies on 2% MEA at first light gray, becoming darker (Fig. 5h). Aerial mycelium abundant, laniferous, often forming subvisible “annular zones” and later “cloud-like structures”. Aerial mycelium hyaline to pale brown, sometimes brown, 1.5–3 (mean 2.3 ± 0,4) μm thick, occurring singly, rarely aggregated in strands of 2–10 hyphae. Optimal growth temperature at 25 °C. Culture growth rates 2.2 mm/day (±0.1) at 20 °C and 2.4 mm/day (±0.1) at 25 °C. No growth observed at 5 °C and at 35 °C.
Type material POLAND, Krzeszowice, from Trypodendron domesticum beetle infesting Fagus sylvatica, 12 April 2013, R Jankowiak, holotype TUR http://mus.utu.fi/TFU.206896, culture ex-holotype CBS 142724 = CMW 43182; POLAND, Krzeszowice, from Trypodendron domesticum beetle infesting Fagus sylvatica, 12 April 2013, R Jankowiak, paratype TUR http://mus.utu.fi/TFU.206894, culture ex-paratype CBS 142722 = CMW44156; POLAND, Krzeszowice, from Trypodendron domesticum beetle infesting Fagus sylvatica, 12 April 2013, R Jankowiak, paratype http://mus.utu.fi/TFU.206897, culture ex-paratype CBS14725 = CMW43185; POLAND, Krzeszowice, from Trypodendron domesticum beetle infesting Fagus sylvatica, 12 April 2013, R. Jankowiak, paratype OF-304919, culture ex-paratype CBS 142729; NORWAY, Ås, from Dryocoetes alni beetle infesting Alnus incana, 7 February 2016, A Truls, paratype OF-304920, culture ex-paratype CBS 142730; NORWAY, Larvik, from Trypodendron domesticum beetle infesting Alnus incana, 4 April 2016, A Truls.
Host trees: Alnus incana, Betula pubescens, Fagus sylvatica, Fraxinus excelsior and Quercus robur
Insect vectors: Dryocoetes alni, Trypodendron domesticum
Known distribution: Norway, Poland
Notes Isolates of L. trypodendri grouped close to L. pruni in all phylogenetic analyses (Figs. 2–4), and can clearly be separated from the latter species based on sequences of two protein-coding genes (Figs. 3, 4). These two species differ in 35 bp in β-tubulin and 15 bp in TEF1-α (Table 2).
Morphologically, L. trypodendri differs from L. pruni in having shorter conidiophores, longer conidiogenous apparatus, chlamydospore-like structures, and with no growth observed at 35 °C on MEA. The only other Leptographium spp. known to produce chlamydospores are L. piriforme Greif et al. (Greif et al. 2006) and L. chlamydatum (Jacobs et al. 2010), both unrelated to the species treated in this study. Furthermore, L. trypodendri has a hyalorhinocladiella-like synanamorph versus a sporothrix-like form of L. pruni with more pronounced denticles. In turn, the closely related G. grandifoliae forms perithecia and has larger conidiophores having distinct rhizoids. Leptographium trypodendri differs from L. betulae in having shorter stipes, longer conidiogenous apparatuses and larger conidia. However, the most obvious distinguishing characteristic of these taxa is the presence of different synanamorphs: L. trypodendri is associated with a hyalorhinocladiella-like synanamorph, while L. betulae has a sporothrix-like synanamorph.
Leptographium trypodendri was found respectively on 43, 63 and 77% of T. domesticum beetles from A. incana on the three sample plots in south-eastern Norway. It was isolated more rarely (25%) on F. excelsior and Q. robur, but fewer bark beetles were collected on these tree species. Only one D. alni beetle carried L. trypodendri on each of the three sample plots, and on those places D. alni was collected from the same branches as T. domesticum. Similarly, to the Norwegian results, L. trypodendri was often recorded in association with T. domesticum in Poland. It was isolated from 52% of the beetles collected from F. sylvatica logs.
Taxon B
Leptographium betulae R. Jankowiak, B. Strzałka & R. Linnakoski, sp. nov. Fig. 6 MB 821670.
Etymology: betulae refers to the fungus being collected only from Betula verrucosa.
Sexual state not observed. Conidiophores macronematous, arising laterally from hyphae, solitary or often also in loosely arranged groups on hyphae, without rhizoidal hyphae at the bases (Fig. 6a, b). Stipes erect, pale brown to dark brown, 1–6-septate, 54.5–423 (mean 166.4 ± 70.9) μm long and 2–7 (mean 3.9 ± 1) μm wide at base. Conidiogenous apparatus 27–70 μm (mean 49.4 ± 9.7) long (excluding conidial mass) consisting of 1–3 branches (branch type B) (Jacobs and Wingfield 2001) (Fig. 6c). Primary branches olivaceous, cylindrical, smooth, 11–29.5 × 3.5–5 μm. Conidiogenous cells hyaline, tapering from base to apex, 7.5–13.5 (mean 10 ± 2) × 1.5–3 (mean 2.2 ± 0,4) μm. Conidia hyaline, obovate, elliptical, sometimes allantoid, 2–4 (mean 2.9 ± 0,3) × 1.0–1.5 (mean 1.3 ± 0.2) μm, accumulating around the conidiogenous apparatus in a hyaline mucilaginous mass (Fig. 6d).
Sporothrix-like micronematal asexual state present. Conidiophores micronematous sporothrix-like, arising individually from undifferentiated hyphae (Fig. 6e, f). Conidiogenous cells cylindrical, variable in shape and size, usually widest at the basal part and slightly tapering, having distinct appendages at their tip, 21–74 (mean 43 ± 13.5) μm long, 1–3 μm wide at the base. Conidia hyaline, 1-celled, oblong to oval, 3–7 (mean 5 ± 1) × 1.5–3.5 (mean 2.5 ± 0.6) μm, sometimes developing into larger ramoconidia, producing distinct denticles and secondary conidia. Ramoconidia hyaline, 1-celled, clavate, 5.0–12.0 (mean 7.5 ± 1.9) × 1–3 (mean 2 ± 0.5) μm. The secondary conidia hyaline, 1-celled, ellipsoidal, 2–4 (mean 3 ± 0.6) × 1–2.5 (mean 1.6 ± 0.4) μm.
Culture characteristics colonies on 2% MEA initially hyaline, whitish, later light gray, with abundant aerial mycelium (Fig. 6g). Mycelium laniferous, without “annular zones”. Hyphae 2–3 (mean 2.4 ± 0.4) μm thick, hyaline to light gray, sometimes pale brown, occurring singly or aggregated in strands of 2–10 hyphae. Macronematous and micronematous conidiophores present. Optimal growth temperature at 25 °C. Culture growth rates 2.4 mm/day (±0.1) at 20 °C, 2.6 mm/day (±0.1) at 25 °C, and 0.9 mm/day (±0.1) at 35 °C. No growth observed at 5 °C.
Type material POLAND, Myszyniec, from galleries of Scolytus ratzeburgi infesting Betula verrucosa, 24 July 2011, R Jankowiak, holotype TUR http://mus.utu.fi/TFU.206905, culture ex-holotype CBS 142734 = CMW 43191; POLAND, Myszyniec, from galleries of Scolytus ratzeburgi infesting Betula verrucosa, 24 July 2011, R Jankowiak, paratype TUR http://mus.utu.fi/TFU.206901, culture ex-paratype CBS 142731 = CMW43190; POLAND, Myszyniec, from galleries of Scolytus ratzeburgi infesting Betula verrucosa, 24 July 2011, R Jankowiak, paratype TUR http://mus.utu.fi/TFU.206903, culture ex-paratype CBS 142733 = CMW44157; POLAND, Myszyniec, from galleries of Scolytus ratzeburgi infesting Betula verrucosa, 24 July 2011, R Jankowiak.
Host tree: Betula verrucosa
Insect vectors: Scolytus ratzeburgi
Known distribution: Poland
Notes Isolates of L. betulae grouped close to but distinct from L. pruni in phylogenetic analyses (Fig. 2), and can clearly be separated from the latter based on sequences of the two protein-coding gene regions (Figs. 3, 4). Leptographium betulae differs from L. pruni in 8 bp in β-tubulin, and 16 bp in TEF1-α (Table 2). Morphologically, it differs from L. pruni in having smaller conidia and lower optimal growth temperature on MEA. Leptographium betulae appears to be S. ratzeburgi-specific on B. verrucosa in Poland, occurring only in galleries of this beetle species (7%), always together with another S. ratzeburgi-associated fungus, O. karelicum Linnakoski, Z.W. de Beer & M.J. Wingf. (Jankowiak et al., unpublished data).
Discussion
Two new species of Leptographium, as well as two Polish isolates of G. grandifoliae, were discovered from European hardwoods in this study. These three species, together with L. pruni, formed the first well-supported lineage in Leptographium s.l. consisting only of hardwood-infecting species. The species in this complex are characterised by relatively small conidiophores arranged in loosely groups and synanamorphs of the hyalorhinocladiella- to sporothrix-like forms. The oldest known species in the complex, G. grandifoliae, is only known by a sexual stage. The two new species are potential symbiotic associates of the European hardwood ambrosia beetle, T. domesticum, and the European birch bark beetle, S. ratzeburgi. These results are unusual as by far, the majority of Leptographium spp. are known as associates of conifer-infesting bark beetles, especially root-feeding species.
In comparison with conifer-infesting bark beetles, the interactions between ophiostomatoid fungi and bark beetle species occurring on hardwood trees have been poorly studied. For this reason, the nine well-defined Leptographium s.l. species complexes recognized to date all include only conifer-infecting species. The G. penicillata complex includes among its more than 20 species three species from hardwoods: L. hughesii K. Jacobs et al. from Parashorea in Borneo and Vietnam (Jacobs et al. 1998), L. eucalyptophylum K. Jacobs et al. from Eucalyptus spp. in West Africa (Jacobs et al. 1999), and L. pistaciae Paciura et al. from Pistacia trees in China (Paciura et al. 2010). Of the nine species in the L. procerum complex (Yin et al. 2015), only L. profanum K. Jacobs et al. comes from the roots of various hardwoods in the USA (Jacobs et al. 2006). The phylogenetic placement of three hardwood-infesting species remains uncertain (De Beer et al. 2013; De Beer and Wingfield 2013): L. calophylli, G. francke-grosmanniae (R.W. Davidson) Zipfel et al. in association with Elateroides dermestoides (L.) beetles (Lymexylidae) from oak in Europe (Davidson 1971), and L. brevicolle K. Jacobs and M.J. Wingf. from Trypodendron retusus (Le Conte) galleries on aspen in the USA (Davidson 1958; Jacobs and Wingfield 2001). Three more species from hardwoods are placed peripheral to or between other species complexes in Leptographium s.l.: G. leptographioides (R.W. Davidson) Zipfel et al. from Quercus in the USA (Davidson 1942; Linnakoski et al. 2012), L. verrucosum (Gebhardt, R. Kirschner and Oberw.) Z.W. de Beer & M.J. Wingf. from Xyleborus dryographus (Ratzeburg) (Scolytinae) from Quercus in Germany (Gebhardt et al. 2002; Musvuugwa et al. 2015), and L. globosum Y.T. Huang and Chi Y. Chen from fallen hardwood in Taiwan (Huang and Chi-Y 2014).
The two remaining species from hardwoods, G. grandifoliae from stained Fagus grandifoliae Ehrh. wood in the USA (Davidson 1976), and L. pruni from Polygraphus ssiori Niijima attacking Prunus in Japan (Masuya et al. 2004), grouped close to each other, but not in a supported lineage, in Leptographium s.l. in the LSU phylogeny of De Beer and Wingfield (2013). The two species also formed an unsupported clade, sister to a new species, L. gestamen De Errasti & Z.W. de Beer from galleries of Gnathotrupes ambrosia beetles on Nothofagus in Argentina (De Errasti et al. 2016). Although L. gestamen also grouped with G. grandifoliae, L. pruni and the two newly described species from the present study in a lineage with some support in the ITS2-LSU region (Fig. 1), it did not form part of a monophyletic lineage with these species in the β-tubulin (Fig. 2) and TEF1-α (Fig. 3) trees. We thus restrict the newly defined species complex to include G. grandifoliae, L. pruni, L. betulae and L. trypodendri, and name it after the species that was first described, G. grandifoliae (Davidson 1976). Our study also represents the first report of G. grandifoliae from outside the USA and from another host than Fagus.
Apart from morphological differences between L. betulae and L. trypodendri described in the taxonomy section, the two species also differ with regards to their host range and beetle vectors. Leptographium trypodendri seems to be very common on T. domesticum attacking A. incana in south-eastern Norway, and F. sylvatica in Poland, and less often from F. excelsior and Q. robur in Norway. It was found on rare occasions also on D. alni. Our results suggest that L. betulae is only associated with S. ratzeburgi attacking B. verrucosa.
While the majority of previous studies have focused on conifer-associated beetle species and their fungal associates, the diversity of hardwood-associated ophiostomatoid fungi has yet to be explored and understood in more detail in Europe and elsewhere. The results of the present study clearly demonstrate that the species diversity of hardwood-infesting Leptographium species occurring in Europe might be underestimated. Therefore, it will be important to expand these surveys to cover larger geographic areas including more host tree and insect vector species in Europe.
References
Alamouti SM, Wang V, Di Guistini S, Six DL, Bohlmann J, Hamelin RC, Feau N, Breuil C (2011) Gene genealogies reveal cryptic species and host preferences for the pine fungal pathogen Grosmania clavigera. Mol Ecol 20:2581–2602. doi:10.1111/j.1365-294X.2011.05109.x
Cobb FW (1988) Leptographium wageneri, cause of black stain root disease; a review of its discovery, occurrence and biology with emphasis on pinyon and ponderosa pine. In: Harrington TC, Cobb FW (eds) Leptographium root disease in conifers. APS Press, St Paul, pp 41–62
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772. doi:10.1038/nmeth.2109
Davidson RW (1942) Some additional species of Ceratostomella in the United States. Mycologia 34:650–662
Davidson RW (1958) Additional species of Ophiostomataceae from Colorado. Mycologia 50:661–670
Davidson RW (1971) New species of Ceratocystis. Mycologia 63:5–15
Davidson RW (1976) Sapwood staining fungi from two tree species. Mem N. Y. Bot Gard 28:45–49
De Beer ZW, Wingfield MJ (2013) Emerging lineages in the Ophiostomatales. In: Seifert KA, De Beer ZW, Wingfield MJ (eds) The Ophiostomatoid fungi: expanding frontiers. CBS Biodiversity Series, The Netherlands
De Beer ZW, Seifert KA, Wingfield MJ (2013) A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. In: Seifert KA, De Beer ZW, Wingfield MJ (eds) The ophiostomatoid fungi: expanding frontiers. CBS Biodiversity Series, The Netherlands
De Errasti A, de Beer ZW, Coetzee MPA, Roux J, Rajchenberg M, Wingfield MJ (2016) Three new species of Ophiostomatales from Nothofagus in Patagonia. Mycol Prog 15:17. doi:10.1007/s11557-016-1158-z
Gebhardt H, Kirschner R, Oberwinkler F (2002) A new Ophiostoma species isolated from the ambrosia beetle Xyleborus dryographus (Coleoptera: Scolytidae). Mycol Prog 1:377–382. doi:10.1007/s11557-006-0034-7
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Greif MD, Gibas CF, Currah RS (2006) Leptographium piriforme sp. nov., from a taxonomically diverse collection of arthropods collected in an aspen-dominated forest in western Canada. Mycologia 98:771–780. doi:10.3852/mycologia.98.5.771
Grobbelaar J, de Beer ZW, Bloomer P, Wingfield MJ, Wingfield BD (2010) Ophiostoma tsotsi sp. nov., a wound- infesting fungus of hardwood trees in Africa. Mycopathologia 169:413–423. doi:10.1007/s11046-009-9267-8
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704. doi:10.1080/10635150390235520
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi:10.1093/sysbio/syq010
Harrington TC (1988) Leptographium species, their distribution hosts and insect vectors. In: Harrington TC, Cobb FW Jr (eds) Leptographium root diseases on conifers. APS Press, St. Paul, pp 1–39
Harrington TC, Cobb FW (1987) Leprographium wageneri var. pseudotsugae var. nov. cause of black stain root disease on Douglas-fir. Mycotaxon 30:501–507
Hawksworth DL (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. MycoKeys 1:7–20. doi:10.3897/mycokeys.1.2062
Hicke JA, Logan JA, Powell J, Ojima DS (2006) Changing temperatures influence suitability for modeled mountain pine beetle (Dendroctonus ponderosae) outbreaks in the western United States. J Geophys Res 111:G02019. doi:10.1029/2005JG000101
Huang Y-T, Chi-Y Chen (2014) Leptographium globosum sp. nov., a new species with globose conidia. Mycol Prog 13:841–848. doi:10.1007/s11557-014-0967-1
Jacobs K, Wingfield MJ (2001) Leptographium species: tree pathogens, insect associates, and agents of blue-stain. The American Phytopathological Society Press, St. Paul
Jacobs K, Wingfield MJ, Crous PW, Harrington TC (1998) Leptographium engelmannii, a synonym of Leptographium abietinum, and description of Leptographium hughesii sp. nov. Can J Bot 76:1660–1667. doi:10.1139/cjb-76-9-1660
Jacobs K, Wingfield MJ, Roux J (1999) Leptographium eucalyptophilum, a new species from Eucalyptus in the Congo. S Afr J Bot 65:388–391
Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA, Bright DE, Wingfield BD (2004) Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol Res 108:411–418. doi:10.1017/S0953756204009748
Jacobs K, Eckhardt LG, Wingfield MJ (2006) Leptographium profanum sp. nov., a new species from hardwood roots in North America. Can J Bot 84:759–766. doi:10.1139/b036-030
Jacobs K, Krokene P, Solheim H, Wingfield MJ (2010) Two new species of Leptographium from Dryocetes authographus and Hylastes cunicularius in Norway. Mycol Prog 9:69–78
Jankowiak R (2012) Ophiostomatoid fungi associated with Ips sexdentatus on Pinus sylvestris in Poland. Dendrobiology 68:43–54
Jankowiak R, Bilański P (2013a) Association of the pine-infesting Pissodes species with ophiostomatoid fungi in Poland. Eur J For Res 132:523–534. doi:10.1007/s10342-013-0693-2
Jankowiak R, Bilański P (2013b) Diversity of ophiostomatoid fungi associated with the large pine weevil, Hylobius abietis and infested Scots pine seedlings in Poland. Ann For Sci 70:391–402
Jankowiak R, Bilański P (2013c) Ophiostomatoid fungi associated with root-feeding bark beetles in Poland. For Pathol 43:422–428. doi:10.1111/efp.12049
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi:10.1093/molbev/mst010
Kendrick WB (1962) The Leptographium complex Verticicladiella Hughes. Can J Bot 40:771–797
Kirisits T (2004) Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans H (eds) Bark and wood boring insects in living trees in Europe, A Synthesis. Kluwer, Dordrecht, pp 185–223
Linnakoski R, De Beer ZW, Duong TA, Niemelä P, Pappinen A, Wingfield MJ (2012) Grosmannia and Leptographium spp. associated with conifer-infesting bark beetles in Finland and Russia including Leptographium taigense sp. nov. Antonie Van Leeuwenhoek 102:375–399. doi:10.1007/s10482-012-9747-6
Lu M, Zhou XD, de Beer ZW, Wingfield MJ, Sun JH (2009) Ophiostomatoid fungi associated with the invasive pine infesting bark beetle, Dendroctonus valens, in China. Fungal Divers 38:133–145
Lu M, Wingfield MJ, Gillette NE, Mori SR, Sun JH (2010) Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytol 187:859–866. doi:10.1111/j1469-8137.2010.03316.x
Lu M, Wingfield MJ, Gillette NE, Sun JH (2011) Do novel genotypes drive the success of an invasive bark beetle-fungus complex? Implications for potential reinvasion. Ecology 92:2013–2019. doi:10.1890/11-0687.1
Malloch D, Blackwell M (1993) Dispersal biology of ophiostomatoid fungi. In: Wingfield MJ, Seifert KA, Webber JF (eds) Ceratocystis and Ophiostoma: taxonomy, ecology, and pathology. APS Press, St. Paul, pp 195–206
Masuya H, Wingfield MJ, Kubono T, Ichihara Y (2004) Leptographium pruni, sp. nov. from bark beetle-infested Prunus jamasakura in Japan. Mycologia 96:548–557. doi:10.2307/3762174
Musvuugwa T, de Beer ZW, Duong TA, Dreyer LL, Oberlander KC, Roets F (2015) New species of Ophiostomatales from Scolytinae and Platypodinae beetles in the Cape Floristic Region, including the discovery of the sexual state of Raffaelea. A van Leeuw J Microb 108:933–950. doi:10.1007/s10482-015-0547-7
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogent Evol 7:103–116. doi:10.1006/mpev.1996.0376
Paciura D, de Beer ZW, Jacobs K, Zhou XD, Ye H, Wingfield MJ (2010) Eight new Leptographium species associated with tree-infesting bark beetles in China. Persoonia 25:94–108. doi:10.3767/003158510X551097
Rambaut A, Drummond AJ (2007) Tracer v1.4 http://beast.bio.ed.ac.uk/Tracer)
Robert V, Vu D, Amor AB et al (2013) MycoBank gearing up for new horizons. IMAFungus 4:371–379. doi:10.5598/imafungus.2013.04.02.16
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Six DL, Wingfield MJ (2011) The role of phytopathogenicity in bark beetle-fungus symbioses: a challenge to the classic paradigm. Annu Rev Entomol 56:255–272. doi:10.1146/annurev-ent-120709-144839
Solheim H, Krokene P (1998) Growth and virulence of mountain pine beetle associated blue-stain fungi, Ophiostoma clavigerum and Ophiostoma montium. Can J Bot 76:561–566
Taerum SJ, Hoareau TB, Duong TA, de Beer ZW, Jankowiak R, Wingfield MJ (2017) Putative origins of the fungus Leptographium procerum. Fungal Biol 121:82–94. doi:10.1016/j.funbio.2016.09.007
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Wainhouse D, Murphy S, Greig B, Webber J, Vielle M (1998) The role of the bark beetle Cryphalus trypanus in the transmission of the vascular wilt pathogen of takamaka (Calophyllum inophyllum) in the Seychelles. For Ecol Manag 108:193–199. doi:10.1016/S0378-1127(98)00234-5
Webber JF, Jacobs K, Wingfield MJ (1999) A re-examination of the vascular wilt pathogen of takamaka (Calophyllum inophyllum). Mycol Res 103:1588–1592. doi:10.1017/S0953756299001021
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wiehe PO (1949) Wilt of Calophyllum inophyllum L. var. tacamaha (Willd.) R.E.V. caused by Haplographium calophylli sp. nov. in Mauritius. Mycol Pap 29:1–12
Wingfield MJ, Garnas JR, Hajek A, Hurley BP, de Beer ZW, Taerum SJ (2016) Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biol Invasions 18:1045–1056. doi:10.1007//s10530-016-1084-7
Yin M, Duong TA, Wingfield MJ, Zhou XD, De Beer ZW (2015) Taxonomy and phylogeny of the Leptographium procerum complex, including L. sinense sp. nov. and L. longiconidiophorum sp. nov. Antonie Van Leeuwenhoek 107:547–563. doi:10.1007/s10482-014-0351-9
Zipfel RD, De Beer ZW, Jacobs K, Wingfield BD, Wingfield MJ (2006) Multi-gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud Mycol 55:75–97. doi:10.3114/sim.55.1.133
Acknowledgements
This work was supported by the National Science Centre, Poland (contract No. UMO-2014/15/B/NZ9/00560). The work in Norway is partly financed by Norwegian biodiversity information centre and is part of a Master thesis for T.A.
Conflict of interest
The authors declare that have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jankowiak, R., Strzałka, B., Bilański, P. et al. Two new Leptographium spp. reveal an emerging complex of hardwood-infecting species in the Ophiostomatales. Antonie van Leeuwenhoek 110, 1537–1553 (2017). https://doi.org/10.1007/s10482-017-0905-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-017-0905-8