Abstract
A survey was conducted in Brazil to collect fungi on ferns. Based on morphology and inferred phylogeny from DNA sequences of two loci, namely the internal transcribed spacer (ITS) regions and the large subunit nuclear ribosomal RNA gene (LSU), several species belonging to chalara-like genera and lachnoid fungi were recognized. Eighteen fungal isolates, collected from five host species, representing 10 different localities were studied. Three novel genera (Lachnopsis, Scolecolachnum and Zymochalara), and six novel species (Bloxamia cyatheicola, Lachnopsis catarinensis, Lachnopsis dicksoniae, Scolecolachnum pteridii, Zymochalara lygodii and Zymochalara cyatheae) are introduced. Furthermore, two new combinations (Erioscyphella euterpes and Erioscyphella lushanensis) are proposed. Two novel taxa (Lachnopsis catarinensis and Lachnopsis dicksoniae) may be included in the list of potentially endangered fungal species in Brazil, if proven to be restricted to their tree-fern host, Dicksonia sellowiana, which is included in the official list of endangered plant species in Brazil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous fungal taxa have been published from Brazil in recent years. These represent additions to its rich, but rather underexplored, fungal biodiversity. Surveys for Brazilian fungi have followed biome-based approaches such as for the Cerrado (Hernández-Gutiérrez and Dianese 2014; Hernández-Gutiérrez et al. 2014; Armando et al. 2015) and the Caatinga (Almeida et al. 2012; Fiuza et al. 2015; Izabel et al. 2015), crop-based approaches such as for Eucalyptus (Cândido et al. 2014; Rodrigues et al. 2014; Alfenas et al. 2015; Oliveira et al. 2015), and weed-based approaches (Guatimosim et al. 2015a; Macedo et al. 2013, 2016), among others. A plethora of mycological novelties emerged from such systematic surveys, particularly when these involved groups of host-plants that were poorly studied by mycologists. An example of a poorly studied niche for fungi is the tropical Brazilian fern flora. Ferns are members of Pteridophyta (= ‘Monilophyta’), and represent some of the oldest lineages of vascular plants (Smith et al. 2008). In recent classifications (e.g., Smith et al. 2008), the division includes 37 families, approximately 300 genera and more than 9000 species. Around 48 fungal species have been recorded on ferns from Brazil (Farr and Rossman 2015; Mendes and Urben 2015). This is a very small number of species, especially considering that the number of fern species in Brazil is estimated to be more than 1110 (Forzza et al. 2015). If the postulated 5–6 unique fungal species per plant species (Hawksworth 1991) holds true for ferns, thousands of undescribed fungi may wait to be named from this group of hosts. In a recent study focused on cercosporoid fungi causing frond diseases on Brazilian ferns, 1 new genus, 15 new species, 11 new combinations and 9 new host records were published (Guatimosim et al. 2016), confirming that this group of plants harbors a highly diverse and overlooked mycobiota.
The present work also aimed at contributing to the field of fungal conservation in Brazil, representing an expansion of a theme covered in two previously published studies (Rocha et al. 2010; Silva et al. 2016). Throughout the survey, collections were systematically made of fungal pathogenic, or seemingly pathogenic, to Dicksonia sellowiana. This large and slow-growing tree fern species (known in Brazil as ‘xaxim’ or ‘samambaiaçu’) used to be a common component of the Brazilian Atlantic rainforest, but progressively became rare, owing to biome destruction and unsustainable exploitation by the vase and substrate industry (Windisch 2002). It is included in the official list of Brazilian species threatened with extinction (Pillar et al. 2009). This and previous publications involved the search of unique, specialized and potentially host-specific fungi, therefore under threat of co-extinction simultaneously with their sole host-species. As in the previous studies, it was recognized that, before any attempt to include such organisms in a novel list of endangered fungi from Brazil, surveying the fungi associated with the selected hosts and clarifying their taxonomy would be critical.
Lachnoid fungi are members of the Hyaloscyphaceae sensu lato, which is considered the largest family in Helotiales, comprising about 933 species belonging to 74 genera (Kirk et al. 2008). Species in this family are small discomycetes, with brightly colored apothecial ascomata that are ornamented with more or less conspicuous hairs along the apothecial margins and the outside of the receptacle (Han et al. 2014). Earlier studies by Cantrell and Hanlin (1997) suggested that the family was probably monophyletic, and, based on this premise, mycologists have considered the presence of hairs on the ascomata as a synapomorphic character (Han et al. 2014).
Based on morphology, Hyaloscyphaceae was subdivided into three tribes: Arachnopezizeae, Hyaloscypheae, and Lachneae (Nannfeldt 1932). Arachnopezizeae includes species with apothecia formed on a well-developed subiculum or in a false subiculum-like hyphal layer. Hyaloscypheae has species with tiny apothecia bearing hairs that have highly diverse shapes, and mostly cylindrical paraphyses, while Lachneae includes species with relatively large apothecia, multiseptate, usually granulate hairs, and lanceolate paraphyses (Nannfeldt 1932).
Raitviir (2004) employed morphological characters to elevate Lachneae to familial level—Lachnaceae, Baral (2015) elevated Arachnopezizeae to familial level—Arachnopezizaceae, and Hosoya et al. (2010), using morphology and multi-locus DNA sequence data, confirmed this hypothesis. The latter authors, however, acknowledged that low taxon sampling was a barrier to an adequate understanding of the taxonomy of Lachanaceae. In the most recent work dealing with taxonomy of Hyaloscyphaceae sensu lato, Han et al. (2014) examined the morphological characteristics in the context of multi-locus DNA sequence data and, based on 70 species belonging to each of the formerly accepted tribes, demonstrated Hyaloscyphaceae to be polyphyletic; they rejected the presence of hairs as a synapomorphic feature for the family. Additionally, Hyaloscyphaceae sensu stricto was tentatively restricted to the genus Hyaloscypha, although a more extensive sampling within this family is recognized as required for further confirmation of this hypothesis (Han et al. 2014).
Chalara asexual morphs are relatively poor in distinctive features that would allow a natural subdivision of this polyphyletic group (Cai et al. 2009). The monograph by Nag Raj and Kendrick (1975) is just a first step in its resolution. Since DNA sequencing became available to properly evaluate the evolutionary relationships among fungi, only some representative taxa of the chalara-like complex have been thoroughly studied (Réblová 1999; Coetsee et al. 2000). The segregation of the Ceratocystis-related taxa (Microascales) has become well established (Paulin-Mahady and Harrington 2000; Paulin-Mahady et al. 2002), while the bulk of the genus in the Helotiales is still poorly resolved, and the type species of the genus is not yet available in culture.
Species within Bloxamia are sporodochial, having scattered or gregarious, black, disciform sporodochia, with pale brown superficial stromata composed of subhyaline to pale brown cells, arranged in dense palisades, from which the conidiophores emerge and produce catenulate, hyaline conidia (Nag Raj and Kendrick 1975). The genus is based on Bl. truncata occurring on dead decorticated wood of Ulmus sp. from England (Pirozynski and Morgan-Jones 1968). Presently seven species are recognized within Bloxamia, as summarized in Table 2 (below).
A morphological and phylogenetic-based study involving inference of two DNA regions (ITS and LSU) was performed on chalara-like genera and lachnoid species collected on ferns in Brazil, and the results are presented here.
Materials and methods
Specimens and isolates
Frond samples of five fern species bearing fungal colonies were collected in Brazil from different biomes, including the Amazon, Atlantic rainforest, Caatinga and Cerrado between 2011 and 2014. These were examined under a Nikon SMZ1500 stereo-microscope (Nikon Instruments, Tokyo, Japan) and later dried in a plant press. Conidia were scraped from a single frond spot, and single-conidial colonies were established on potato carrot agar (PCA; Crous et al. 2009). Ascospore-grown colonies were obtained by excising fragments from selected ascomata and fastening them on the inner side of Petri dish lids. Such dishes contained PCA and were inverted. Ascospores shot onto the surface of the medium were transferred individually onto fresh plates after germination, and observed under an Olympus SZX7 stereo microscope (Olympus, Tokyo, Japan). Freehand sections of fungal colonies were prepared and fungal structures mounted in water, clear lactic acid, lactofuchsin, Melzer᾿s reagent and Lugol. Whenever necessary, sections were made using a Microm HM520 freezing microtome (Microm, Neuss, Germany). Observations and images were made with a Nikon Eclipse 80i (Nikon Instruments) compound microscope with differential interference contrast illumination fitted with a Nikon DS-Fi1 camera. Images were processed with NIS-Elements imaging software (Nikon Instruments). Colony descriptions were based on observations of colonies formed on potato dextrose agar (PDA; Crous et al. 2009) and PCA, incubated at 25 °C in the dark, and under a 12-h light regime. After 30 days, the colony diameter was measured and the colony color was described following the terminology of Rayner (1970). Representative fungarium specimens were deposited at the Fungarium of the Universidade Federal de Viçosa (VIC). Axenic cultures were deposited at the working collection of P.W. Crous (CPC), housed at the CBS-KNAW Fungal Biodiversity Centre, and at the Coleção Octávio de Almeida Drumond (COAD, Universidade Federal de Viçosa). A complete list of the isolates used in this study is presented in Table 1.
Scanning electron microscopy
Samples of dried material containing fungal structures were mounted on stubs with double-sided adhesive tape and gold-coated using a Balzer’s FDU 010 sputter coater (Optics Balzers, Neugrüt, Liechtenstein). A LEO VP 1430 scanning electron microscope (SEM; Carl-Zeiss, Jena, Germany) was used to analyze and generate images from the samples.
DNA isolation, amplification and sequencing
Isolates were grown on 2 % malt extract agar (MEA; Crous et al. 2009) for 20 days at 25 °C on the laboratory bench. Genomic DNA was extracted from mycelium scraped from colonies of each isolate using the Wizard® Genomic DNA Purification Kit (Promega, WI, USA) following the manufacturer’s instructions. For Bloxamia species, fronds harboring fertile stromata were examined under a dissecting microscope to check for possible contamination by other fungi, including yeasts. The fronds were then soaked in sterile water for 1 h in order to hydrate the specimens and facilitate removal of the stromata. Thirty fertile stromata were removed from the fronds with a sterile fine-pointed needle, and placed into a microcentrifuge tube (1.5 mL). Total genomic DNA was extracted as described above in addition to the steps described by Pinho et al. (2012). The DNA samples were subsequently diluted 50–100 times in preparation for further DNA amplification reactions. Three partial nuclear genes were targeted for PCR amplification and sequencing, namely, the 18S nrRNA gene (SSU), the 28S nrRNA gene (LSU) and the internal transcribed spacer regions and intervening 5.8S nrRNA gene (ITS) of the nrDNA operon. The primer pair NS1 + NS4 (White et al. 1990) was used to amplify and sequence the SSU locus, the primer pair LR0R + LR5 (Vilgalys and Hester 1990) was used to amplify and sequence the LSU locus, whereas the ITS locus was amplified and sequenced with the primer pair ITS5 + ITS4 (White et al. 1990). The PCR amplifications were performed in a total volume of 12.5 μL solution, containing 10–20 ng of template DNA, 1× PCR buffer, 0.63 μL DMSO (99.9 %), 1.5 mM MgCl2, 0.5 μM of each primer, 0.25 mM of each dNTP, 1.0 U BioTaq DNA polymerase (Bioline, Luckenwalde, Germany). PCR conditions were set as follows: an initial denaturation temperature of 95 °C for 5 min, followed by 35 cycles of denaturation temperature of 95 °C for 30 s, primer annealing at 52 °C for 30 s, primer extension at 72 °C for 1 min, and a final extension step at 72 °C for 1 min. The resulting fragments were sequenced using the PCR primers and the BigDye Terminator Cycle Sequencing Kit v.3.1 (Applied Biosystems, Foster City, CA, USA) following the protocol of the manufacturer. DNA sequencing amplicons were purified through Sephadex® G-50 Superfine columns (Sigma Aldrich, St. Louis, MO, USA) in MultiScreen HV plates (Millipore, Billerica, MA, USA). Purified sequence reactions were run on an ABI Prism 3730xl DNA Sequencer (Life Technologies, Carlsbad, CA, USA).
IDNA sequence data were analyzed in Molecular Evolutionary Genetics Analysis v.6.0 (MEGA; Tamura et al. 2013). Consensus sequences were generated and imported into MEGA for initial alignment and the construction of sequence datasets. Initially, sequences obtained from the datasets of Crous et al. (2014, TreeBASE S16625), Han et al. (2014, TreeBASE S12034), Hosoya et al. (2010), Perić and Baral (2014), from GenBank (www.ncbi.nlm.nih.gov), and the novel sequences generated on this study, were aligned using MAFFT v.7 (http://mafft.cbrc.jp/alignment/server/index.html; Katoh et al. 2002) and, whenever indicated, manually improved in MEGA. After a preliminary analysis, the datasets were trimmed down to Brazilian isolates and their direct neighbors.
Phylogenetic analysis
Appropriate gene models were selected using MrModeltest v.2.3 (Nylander 2004) and applied to each gene partition. Based on the results of MrModeltest, a Bayesian phylogenetic analysis was performed with MrBayes v.3.2.3 applying the GTR+I+G substitution model for ITS and LSU, through Cipres Gateway (Miller et al. 2010). Coccomyces dentatus AFTOL-ID 147 and Graphium fabiforme CMW 30626 served as outgroup for the chalara-like ITS and LSU analyses, respectively, while Saccharomyces cerevisiae DAOM 216365 and Geoglossum uliginosum SAV 10162 served as outgroup for the lachnoid ITS and LSU analyses, respectively. Posterior probabilities were determined by Markov Chain Monte Carlo sampling (MCMC) in MrBayes v.3.2.3 (Ronquist et al. 2012). Six simultaneous Markov chains were run for 10,000,000 generations, trees were sampled every 100th generation, and 10,000 trees were obtained. The first 2000 trees, representing the burn-in phase were discarded, while the remaining 8000 trees were used for calculating posterior probabilities. Bayesian posterior probabilities are presented on the left of each node, on each tree. Sequences derived in this study were lodged in GenBank, the alignment in TreeBASE (http://www.treebase.org; S19782), and taxonomic novelties in MycoBank.
Results
Phylogenetic results
The four datasets consisted of 446 characters, representing 34 taxa (including the outgroup) for the chalara-like ITS tree, 793 characters, representing 48 taxa (including the outgroup) for the chalara-like LSU tree, 477 characters, representing 94 taxa (including the outgroup) for the lachnoid ITS tree, and 788 characters, representing 57 taxa (including the outgroup) for the lachnoid LSU tree.
The respective alignments included 185 and 229 unique site patterns for the chalara-like ITS and LSU trees, respectively, and 226 and 406 unique site patterns for the lachnoid ITS and LSU trees, respectively. After topological convergence of the Bayesian runs, the following numbers of trees were generated and subsequently sampled (using a burn-in fraction of 0.25 and indicated after the slash) in order to generate the four Bayesian phylogenies: 1710/1368, 903/722 for the chalara-like ITS and LSU trees (Figs. 1, 2), respectively, and 2093/1674, 2130/1704 for the lachnoid ITS and LSU trees (Figs. 3, 4), respectively. The resulting phylogenetic trees of the individual datasets could not be concatenated, as sequences for both loci were not available for all isolates. The results are presented below.
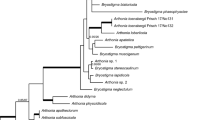
Consensus phylogram (50 % majority rule) of chalara-like species, from a Bayesian analysis of the ITS sequence alignment. Bayesian posterior probabilities are indicated with color-coded branches and numbers (see legend) and the scale bar indicates 0.03 expected changes per site. Isolates from Brazil are indicated in bold. The tree was rooted to Coccomyces dentatus (isolate AFTOL-ID 147)
Consensus phylogram (50 % majority rule) of chalara-like species, from a Bayesian analysis of the LSU sequence alignment. Bayesian posterior probabilities are indicated with color-coded branches and numbers (see legend) and the scale bar indicates 0.02 expected changes per site. Isolates from Brazil are indicated in bold. The tree was rooted to Graphium fabiforme (isolate CMW 30626)
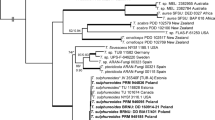
Consensus phylogram (50 % majority rule) of lachnoid species, from a Bayesian analysis of the ITS sequence alignment. Bayesian posterior probabilities are indicated with color-coded branches and numbers (see legend) and the scale bar indicates 0.1 expected changes per site. Isolates from Brazil are indicated in bold. The tree was rooted to Saccharomyces cerevisiae (isolate DAOM 216365)
Consensus phylogram (50 % majority rule) of lachnoid species, from a Bayesian analysis of the LSU sequence alignment. Bayesian posterior probabilities are indicated with color-coded branches and numbers (see legend) and the scale bar indicates 0.03 expected changes per site. Isolates from Brazil are indicated in bold. The tree was rooted to Geoglossum uliginosum (isolate SAV 10162)
Taxonomy
The phylogenetic analyses employed, aiming to distinguish species boundaries of the fungi studied, revealed a rich diversity among the fungi collected on Brazilian ferns. Six isolates of lachnoid and 12 isolates for chalara-like fungi, collected from five host species, representing 10 different localities were studied. The Bayesian analyses resulted in a total of six frond-related taxa, belonging to four genera, including Bloxamia, and three new genera that are introduced below. Additionally, six species are newly described.
Bloxamia cyatheicola Guatimosim, R.W. Barreto & Crous, sp. nov. (Fig. 5).
Bloxamia cyatheicola (VIC 42563, holotype). a Colonized fronds of Cyathea delgadii showing yellowed colonized areas adaxially; b, c sporodochial conidiomata; d, e apothecia; f vertical section of conidioma; g, h conidiophores; i phialoconidia; j vertical section of apothecium; k asci; l ascospores (f, g, k, l in lactofuchsin; h–j in lactic acid). Scale bars (f, j) 100 μm, (g–i, k, l) 10 μm
MycoBank MB813045
Etymology: Name refers to the host tree fern genus Cyathea.
Frond spots amphigenous, irregular, starting as small chlorotic areas, becoming pale brown and necrotic, affecting scattered pinnulae. Internal hyphae not observed. External hyphae absent. Conidiomata sporodochial, hypophyllous, erumpent, either solitary or crowded along the margins of the pinnule, discoid, up to 1000 × 2000 μm, solitary, when wet pulvinate, slimy, amber-colored, when dry, flattened, pulvinate and tough, black. In vertical section, sporodochia with a basal stroma of textura intricata, 190–205 μm deep in the centre of the conidioma, composed of 4–5 μm diam cells, dark brown towards the host tissue, and paler towards the external side. Coniodiophores often reduced to the conidiogenous cells. Phialides arising from the stroma surface in a densely packed palisade, discrete, subcylindrical, 17–41 × 1.5–3.5 μm, rarely 1-septate, pale brown, becoming paler towards the apex, smooth. Phialoconida endogenous, basipetal, extruded in short easily fragmenting chains, cylindrical, truncate at both ends, 2.5–8 × 1–3 μm, aseptate, hyaline, with small guttules, smooth. Ascomata apothecial, hypophyllous, sometimes associated with the conidioma on the same pinnula, erumpent, scattered at the margin of the pinnulae, discoid or cupulate (when dry), up to 500 μm diam and 1900 μm high, solitary, sessile, slimy, tough, black. In vertical section, apothecia with a basal stroma of textura intricata, 103–198 μm deep, composed of 3 μm diam cells. Medullary excipulum of textura epidermoidea, up to 250 μm thick, composed of thin-walled hyphae, 1–1.5 μm diam, sub-hyaline to hyaline. Paraphyses unbranched, filiform, swollen at the tip, 1–2.5 μm wide, septate, hyaline, smooth. Asci unitunicate, subcylindrical or clavate, without croziers, straight to curved, 68–113 × 6.5–14 μm, 8-spored, with small euamyloid apical ring, hyaline, smooth. Ascospores uniseriate, rarely biseriate, fusoid, straight, 10–18 × 4–7 μm, 1-septate, with one cell slightly larger, biguttulate, hyaline, smooth.
Holotype: Brazil, Rio de Janeiro, Nova Friburgo, Macaé de Cima, on fronds of Cyathea delgadii, both morphs, 29 Apr 2012, R.W. Barreto (VIC 42563).
Habitat/Distribution: Known from C. delgadii and C. atrovirens in the states of Minas Gerais, Paraná and Rio de Janeiro, Brazil.
Additional specimens examined: Brazil, Rio de Janeiro, Nova Friburgo, Macaé de Cima, on fronds of C. delgadii, 29 Apr 2012, R.W. Barreto, (VIC 42579) asexual morph; Paraná, Quatro Barras, on fronds of C. atrovirens, 1 Feb. 2012, R.W. Barreto (VIC 42574), sexual morph; Rio de Janeiro, Nova Friburgo, Mury, on fronds of C. delgadii, 29 Jul. 2012, R.W. Barreto (VIC 42584), asexual morph; Minas Gerais, Araponga, Parque Estadual da Serra do Brigadeiro, on fronds of C. delgadii, 23 Feb. 2014, E. Guatimosim (VIC 42460), asexual morph.
Notes: The genus Bloxamia includes seven species, and among them, only Bl. foliicola is known as a pathogen, causing disease on Oxyspora paniculata (Melastomataceae) in China (Liu and Zhang 1998). Bloxamia foliicola differs from Bl. cyatheicola by having its conidiophores organized in synnemata (Liu and Zhang 1998). The other species that have sporodochial conidiomata are not known from ferns (Table 2). Based on morphology, Bl. cyatheicola is rather similar to Bl. cremea recorded on dead wood from Argentina (Arambarri et al. 1992), and Bl. truncata recorded on decorticated wood of Ulmus sp. from England (Pirozynski and Morgan-Jones 1968). Bloxamia cremea has white to creamy conidiomata instead of amber-colored to black in Bl. cyatheicola, and dark brown, well-developed and larger conidiophores (24–26 × 2.5–3 μm) as compared with Bl. cyatheicola (17–41 × 1.5–3.5 μm). Additionally, phialoconidia in Bl. cremea are formed in long and slimy chains, whereas in Bl. cyatheicola these are short and easily fragmented (Arambarri et al. 1992). Bloxamia truncata differs from Bl. cyatheicola by having more or less cuboid phialoconidia, which are produced in endogenous chains (of up to six) within the conidiophore (Pirozynski and Morgan-Jones 1968; Minter and Holubová-Jechová 1981) instead of cylindrical phialoconidia as in Bl. cyatheicola. All attempts to isolate the fungus in culture were unsuccessful.
Erioscyphella Kirschst. Annales Mycologici 36: 384. 1938.
Notes: The genus Erioscyphella was reinstated to accommodate long-spored lachnoid tropical taxa (Haines and Dumont 1984) that cluster rather remotely from other genera of Hyaloscyphaceae, often having a yellow hymenium due to the presence of carotenoids, absence of croziers, hairs with partly light brown wall, never capitate at the apex, and often distantly septate (Perić and Baral 2014). Based on a Neighbor-joining analysis of the ITS region of selected species of Hyaloscyphaceae, Perić and Baral (2014) formally proposed the new combinations of E. abnormis, E. brasiliensis, E. lunata, E. sclerotii, and additionally, concluded that Lachnum euterpes S. A. Cantrell & J. H. Haines and Lachnum lushanense Zhuang & Wang, should also be considered as members of Erioscyphella. In the present ITS phylogenetic analysis (Fig. 3, part 1), we have expanded Erioscyphella by including the two aforementioned species (L. euterpes and L. lushanense) and other phylogenetically related isolates.
Erioscyphella euterpes (S.A. Cantrell & J.H. Haines) Guatimosim, R.W. Barreto & Crous, comb. nov.
Basionym: Lachnum euterpes S.A. Cantrell & J.H. Haines, Mycological Research 101: 1081. 1997.
MycoBank: MB817303
Description and Illustration — Cantrell and Haines (1997).
Holotype: Puerto Rico, Caribbean National Forest, Luquillo Experimental Forest, El Yunque, on fronds of Prestoea montana (= Euterpe globosa), 5 Jun 1970, J. H. Haines et al. (PR 30, NYS-f-4891, isotype PRM).
Specimen examined: Puerto Rico, Adjuntas, Guilarte Trail, on fronds of Prestoea montana (= Euterpe globosa), 3 Dec 1994, S. A. Cantrell (PR 147, GAM, epitype designated here, MBT 371982).
Notes: Perić and Baral (2014) recently indicated that E. euterpes was a likely member of the genus Erioscyphella. Unfortunately the only specimen of E. euterpes from which DNA is available (PR 147; Cantrell and Hanlin 1997) is not the holotype (PR-30; Cantrell and Haines 1997). However, this specimen is cited under the description of L. euterpes as being identical to the type (Cantrell and Haines 1997). On the present study, we have thus decided to designate this specimen as epitype for E. euterpes, and based on the phylogenetic inference, also propose a new combination.
Erioscyphella lushanensis (W.Y. Zhuang & Z. Wang) Guatimosim, R.W. Barreto & Crous, comb. nov.
Basionym: Lachnum lushanense W.Y. Zhuang & Z. Wang, Mycotaxon 66: 429. 1998.
MycoBank: MB817304
Description and Illustration — Zhuang and Wang (1998).
Holotype: China, Lushan Mountains, Jiangxi Province, on dead leaf sheath at stem base of an unknown grass (Gramineae), 18 Oct 1996, W.Y. Zhuang and Z. Wang (1462, HMAS 71903)
Specimen examined: China, Changjiang County, Hainan Province, on stems of Miscanthus sp. (Gramineae), 6 Dec 2000, Z.H. Yu and W.Y. Zhuang (3631, HMAS 81575, epitype designated here, MBT 372554).
Notes: As for E. euterpes, the only specimen of E. lushanensis from which DNA is available (HMAS 81575; Zhao and Zhuang 2011), it is not the holotype (HMAS 71903; Zhuang and Wang 1998), but this specimen shares the same characteristics of the type (Zhuang, personal communication). Therefore, we decided to designate an epitype for E. lushanensis and, based on the phylogenetic inference, propose this new combination.
Lachnopsis Guatimosim, R.W. Barreto & Crous, gen. nov.
MycoBank MB817300
Type species: Lachnopsis catarinensis Guatimosim, R.W. Barreto & Crous
Etymology: Resembling Lachnum, but phylogenetically distinct.
Ascomata apothecial, superficial, scattered or gregarious, usually stipitate, plane or concave, white or pigmented, clothed with hairs. Hairs cylindrical or tapering towards the apex, obtuse, straight or curved, sometimes apically clavate, thin- or thick-walled, multiseptate, hyaline or pigmented, granular throughout their length and sometimes also bearing resinous or crystalline matter. Asci 8-spored, without croziers, cylindrical or cylindric-clavate, apex conical, with euamyloid pore. Ascospores fusoid or filiform, rarely ellipsoid, 0- to multiseptate, hyaline smooth. Paraphyses lanceolate subcylindrical, sometimes with pointed apex, and frequently exceeding the asci in length. The genus Lachnopsis is only distinguishable from Lachnum based on DNA sequence data. ITS as well as LSU sequence data (and SSU, data not shown), can easily differentiate between these genera.
Notes: Lachnopsis is morphologically a typical species of Lachnum s.l., but it is phylogenetically distinct from that genus. Presently, no asexual morphs are known, and the only distinguishing characters from Lachnum are to be found in its DNA sequences.
Lachnopsis catarinensis Guatimosim, R.W. Barreto & Crous, sp. nov. (Fig. 6).
Lachnopsis catarinensis (VIC 42507, holotype). a, b Frond blight on Dicksonia sellowiana; b apothecia; c colony on PDA; d squashed apothecium; e detail of paraphyses with obtuse apices, longer than asci; f roughened hairs, with hyaline rod-shaped granules; g, h asci; i ascospores; j ascospores germinating at both ends (d–j in lactofuchsin). Scale bars (d) 100 μm, (e–j) 10 μm
MycoBank MB813047
Etymology: Name refers to the Brazilian state of Santa Catarina where the fungus was first found.
Frond blight irregular, starting as small necrotic areas and leading to necrosis of the pinnulae, affecting the apex of pinnulae. Ascomata apothecial, hypophyllous, scattered, discoid, 0.23–0.25 mm, opened, when wet, closed and narrowly campanulate, when dry, short-stipitate, stipe 52 × 48 μm, entirely white. Receptacle concolorous with the disc, densely clothed with hyaline hairs. Ectal excipulum of textura prismatica, composed of 8–10 × 4–5 μm large thin-walled cells, becoming intricate towards the base, hyaline, smooth. Hairs subulate or acerose, straight, 56–94 × 3–5 μm at the widest point, 3–4-septate, tapering toward obtuse apex, hyaline, thin-walled, densely roughened with hyaline, rod-shaped granules, non-amyloid. Asci unitunicate, clavate, straight or curved, short-pediculate, without croziers, 45–58 × 9–13 μm, 8-spored, with small euamyloid apical ring. Ascospores uniseriate, overlapping, subcylindrical or narrowly fusoid-acicular, straight to slightly curved, 32–46 × 1–2 μm, 3-septate, tapering towards each subacute end, guttulate, hyaline, smooth, germinating from both ends. Paraphyses cylindric-clavate, straight or slightly curved, unbranched, 55–60 μm long, 4–5 μm wide at the widest point, 3–4-septate, apex rounded, slightly longer than asci, hyaline, smooth. Asexual morph: not observed.
Culture characteristics: Colonies on PDA and PCA very slow-growing, 16 mm diam after 28 days; circular, dome-shaped, radially striate with lobate margins, centrally yeast-like, initially ochreous, passing to umber with age, aerial mycelium sparse to absent, salmon towards the periphery; reverse buff. Cultures sterile.
Holotype: Brazil, Santa Catarina, Urubici, roadside, on dead pinnulae of living fronds of Dicksonia sellowiana, 15 Apr. 2013, E. Guatimosim (VIC 42507, culture ex-type CPC 24723, COAD 2006).
Habitat/Distribution: Known from Dicksonia sellowiana in southern Brazil.
Additional specimens examined: Brazil, Santa Catarina, Luizinho, Highway to São José dos Ausentes, roadside, on fronds of D. sellowiana, 16 Apr. 2013, E. Guatimosim (VIC 42478, culture CPC 24713); ibid., (VIC 42481, cultures CPC 24714, COAD 2003).
Notes: Following the dichotomous key of Spooner (1987), Lachnopsis catarinensis does not fit into any previously described species. Based on the key of Haines and Dumont (1984), Lachnopsis catarinensis could be compared to Lachnum cyphelloides (Pat.) Haines and Dumont, a rare and poorly known species described on twigs and stems of dicotyledonous trees, which presents pale-colored discoid apothecia and long needle-shaped spores (as in Lachnopsis catarinensis), and distinctively covered with bright, white hairs ornamented with white to pale brown resinous or crystalline matters (Haines and Dumont 1984), absent in Lachnopsis catarinensis. According to the keys of Haines (1980, 1992), and Zhuang and Hyde (2001), Lachnopsis catarinensis is close to Lachnum macrosporum (Penz. & Sacc.) Haines – a well-known species distinguished from other species of Lachnum from tropical ferns, by its long fusiform spores – as in Lachnopsis catarinensis (Haines 1992). However, Lachnum macrosporum differs from Lachnopsis catarinensis by having cylindrical to narrowly ellipsoid asci, distinctly obclavate in the latter and aseptate or with one single medianly septate ascospores, 3-septate in the latter (Haines 1992). Additionally, Lachnum macrosporum is only known from an unidentified fern from Java and Guyana, without any information on living cultures derived from either collections (Haines 1980, 1992). Until a proper reassessment of Lachnum macrosporum has been made, with the generation of reliable ex-type cultures and DNA data, we prefer to maintain it as a separate taxon.
Lachnopsis dicksoniae Guatimosim, R.W. Barreto & Crous, sp. nov. (Fig. 7).
Lachnopsis dicksoniae (VIC 44526). a Frond blight on Dicksonia sellowiana; b apothecium; c colony on PDA; d squashed apothecium; e roughened hairs, with hyaline rod-shaped granules; f asci intermixed with narrowly lanceolate or subcylindrical paraphyses; g ascospores (d in lactic acid; e–g in lactofuchsin). Scale bars (d) 100 μm, (e–g) 10 μm
MycoBank MB817301
Etymology: Name refers to the tree fern host genus Dicksonia.
Frond blight irregular, starting as small pale to dark brown areas, becoming necrotic, where ascomata are formed, affecting random parts or entire pinnulae. Ascomata apothecial, hypophyllous, scattered, discoid, 0.18–0.26 mm, stipitate, stipe 40–315 × 35–290 μm, cream to ochre. Receptacle concolorous with the disc, densely clothed with hyaline hairs. Ectal excipulum of textura prismatica, composed of 9–11 × 3–5 μm large thin-walled cells, becoming intricate towards the base, pale brown, smooth. Hairs acerose, straight, 40–70 × 2.5–5 μm, 3–4-septate, gradually tapering toward the obtuse apex, hyaline, thin-walled, roughened with hyaline rod-shaped granules, more crowded towards the apex, non-amyloid. Asci unitunicate, cylindrical, straight, 52–61 × 6–8 μm, 8-spored, with a tapered base, without croziers, and subconical apex, with small euamyloid apical ring. Ascospores uniseriate, overlapping, fusiform, 13–19 × 4–6 μm, 1-septate, tapering towards acute ends, guttulate, hyaline, smooth, germination not seen. Paraphyses narrowly lanceolate or subcylindrical, straight, unbranched, 47–87 × 2–4.5 μm, 1-septate at the base, tapering at the apex, slightly longer than asci, hyaline, smooth. Asexual morph: not observed.
Culture characteristics: Colonies on PDA and PCA very slow-growing, 9 mm diam after 28 days; circular, dome-shaped, margin fimbriate, aerial mycelium centrally sparse to absent, velvety, white; reverse buff. Cultures sterile.
Holotype: Brazil, Minas Gerais, Araponga, Parque Estadual da Serra do Brigadeiro, Serra das Cabeças, atlantic rainforest, on fronds of Dicksonia sellowiana, 27 Apr. 2013, P.B. Schwartsburd (VIC 44526, culture ex-type CPC 24742, COAD 1429).
Notes: When the dichotomous keys of Haines (1980, 1992) are followed, Lachnum pteridophylli (Rodway) Spooner (as ‘pteridophyllum’) appears as the closest option. Nevertheless, significant morphological differences for shapes and sizes of asci and ascospores indicate the distinction between Lachnum pteridophyllli and the fungus on D. sellowiana. The key of Spooner (1987) leads to Lachnum pinnicola Spooner – described from dead pinnae of Dicksonia antarctica from Australia. In this species, apothecia are also superficial, cupulate, stipitate, covered with white hairs, and its ascospores are hyaline, fusoid and have acute ends. Nevertheless, Lachnum pinnicola differs from Lachnopsis dicksoniae by having thinner (3.5–4 μm) and 3-septate ascospores (Spooner 1987), which are 4–6 μm, and only 1-septate in the latter. Although distribution on lachnoid fungi should not be considered as an important feature related to species boundaries (Haines 1992), the absence of living cultures or DNA data related to the Australian species prevents further considerations about whether or not Lachnum pinnicola should be placed in Lachnopsis.
Possible additional members of Lachnopsis
Lachnopsis cf. varians
Notes: Lachnum varians (Rehm) Spooner was described on dead stems of an unidentified fern from Brazil (Haines 1980; Spooner 1987). It represents the most common and widespread discomycete inhabiting decaying remains of tropical ferns and has been collected in localities in northern and western South America, Australia, Hawaii, Japan, New Guinea and New Zealand, on members of Cyatheaceae, Dicksoniaceae (including Dicksonia), and Gleicheniaceae (Haines 1980; Spooner 1987; Hosoya et al. 2010). Despite the large number of collections, only a single isolate from Japan (TNS-F-7631; Hosoya et al. 2010), has had its DNA assessed, and clearly represents an entirely different clade (Hosoya et al. 2010; Perić and Baral 2014). Based on both ITS and LSU phylogenies (Figs. 3 and 4), this isolate clusters within the newly proposed genus Lachnopsis. Nevertheless, at this stage TNS-F-7631 cannot be proposed as an epitype for Lachnum varians given the source of the specimen widely differing from that of the type material. A confirmation of the placement of Lachnum varians within Lachnopsis and the proposal of a new combination depends on recollecting suitable material to be designated as epitype for this taxon.
Lachnopsis cf. pteridophylli
Specimens examined: China, Yunnan, Xishuangbanna, on rotten herbaceous stems, 19 Oct. 1999, W.Y. Zhuang and Z.H. Yu (3155, HMAS 78572), marked as L. cf. pteridophylli. Puerto Rico, Guilarte Trail, Adjuntas, on fronds of an unidentified fern, 3 Dec. 1994, S.A. Cantrell (PR 148, GAM 18397).
Notes: Lachnum pteridophylli was originally described on a dead stipe of Dicksonia antarctica from Tasmania, but later it was widely collected on ferns from tropical areas like Australia, Colombia, Jamaica, Mexico, Panama, Peru, Puerto Rico, New Guinea, New Zealand, and Venezuela. It was found associated with different species of Cyatheaceae, Dicksoniaceae and Gleicheniaceae (Haines 1980; Spooner 1987). Two specimens of Lachnum pteridophylli had their DNA assessed, one from Puerto Rico (SAC PR148; Cantrell and Hanlin 1997), and the other from China (HMAS 78572; Zhao and Zhuang 2011), but the latter was marked as cf., suggesting that the authors were not confident about its identification. Perić and Baral (2014) have already demonstrated that these isolates are not related to each other. The Chinese material would be related to Erioscyphella, whereas the Puerto Rican material belongs to an entirely different clade.
Based on both ITS and LSU phylogenies (Figs. 3 and 4), the Puerto Rican isolate clusters within the newly proposed genus Lachnopsis. Haines (1980) and Spooner (1987) studied Lachnum pteridophylli and Lachnum varians extensively, and concluded that they are closely related, as reflected by the inferred phylogeny in the present study. Haines (1980) studied a large number of collections, including the holotype from Tasmania, and several specimens from a range of ferns from the Neotropics. Because SAC PR 148 cannot be considered as an epitype, given the different locality of this collection, it is hereby maintained as possibly related to Lachnum pteridophylli, and based on the inferred phylogeny, placed within the new genus Lachnopsis.
Scolecolachnum Guatimosim, R.W. Barreto & Crous, gen. nov.
MycoBank MB817302
Type species: Scolecolachnum pteridii Guatimosim, R.W. Barreto & Crous
Etymology: Name refers to a combination of the shape of the ascospore - which is long and narrow – and the overall morphological similarity with fungi belonging to the genus Lachnum.
Ascomata apothecial, hypophyllous, scattered, discoid, sessile, campanulate, pale to cream or white. Receptacle concolorous with the disc. Medullary excipulum perpendicular to the host tissue, composed of hyaline textura angularis. Ectal excipulum of hyaline textura epidermoidea, becoming intricate toward the base. Hairs cylindrical, aseptate, hyaline, smooth. Asci unitunicate, sub-cylindrical, straight, short-pedicellate, 8-spored, without croziers, with small euamyloid apical ring. Ascospores parallel in a bundle, filiform or slightly clavate, straight, 0–3-septate, guttulate, hyaline, smooth. Paraphyses filiform, flexuous, unbranched, septate, as long as the asci, hyaline, smooth.
Notes: Although the fungus found on bracken (Pteridium) in Brazil is morphologically similar to Lachnum, attempts at determining its identity with the dichotomous keys of Haines (1980, 1992), Haines and Dumont (1984), Spooner (1987), and Zhuang and Hyde (2001) have shown that it does not fit in Lachnum or any other described genus. Additionally, it clearly differs morphologically from other genera of lachnoid fungi related to tropical ferns by having distinctly longer (>20 μm) ascospores which are clavate and aseptate when immature, becoming sub-cylindrical and septate at maturity, by its hairs which are cylindrical, aseptate, hyaline and smooth, and its paraphyses which are as long as the asci, hyaline and smooth. Phylogenetically (Figs. 3 and 4), the newly proposed genus is shown to be an entirely separated clade from Lachnum on both ITS and LSU analyses, having Hyphodiscus as its sister clade. The SSU phylogeny (data not shown) also support Scolecolachnum as a separate genus.
Scolecolachnum pteridii Guatimosim, R.W. Barreto & Crous, sp. nov. (Fig. 8).
Scolecolachnum pteridii (VIC 42921, holotype). a, b Hypophyllous apothecia (wet) on Pteridium arachnoideum; c SEM image of apothecium (note the smooth hairs, typical of the genus); d vertical section through apothecium; e, f asci; g ascospores; h colony on PDA (d, f, g in lactofuchsin; e in lactic acid). Scale bars (c) 20 μm, (d–f) 50 μm, (g) 10 μm
MycoBank MB813048
Etymology: Name refers to Pteridium, the generic name of its host genus.
Frond spots amphigenous, irregular, starting as pale brown areas, becoming necrotic, affecting individual pinnulae. Ascomata apothecial, hypophyllous, scattered, discoid, 150–270 μm diam and 260–310 μm high, narrowly campanulate, with elevated margins – when wet, closing as insect egg-like bags – when dry, cream centrally and white at periphery, sessile. Receptacle concolorous with the disc. Medullary excipulum oriented perpendicularly to the host tissue, composed of hyaline textura angularis, cells 4–10 μm diam, thin-walled. Ectal excipulum of hyaline textura epidermoidea, cells 1–2.5 μm diam, thin-walled, becoming intricate toward the base. Hairs cylindrical, 13–16 × 5–6.5 μm, aseptate, hyaline, smooth, thin-walled, non-amyloid. Asci unitunicate, subcylindrical, straight, short-pedicellate, 54–100 × 11–18 μm, 8-spored, without croziers, apex conical to somewhat umbonate, slightly thickened, with a small euamyloid apical ring. Ascospores parallel in a bundle, filiform, initially somewhat clavate, becoming subcylindrical, straight, 44–57 × 2–3 μm, initially aseptate, becoming 3-septate, apex rounded, tapering toward a subacute base, guttulate, hyaline, smooth, germination not seen. Paraphyses filiform, flexuous, unbranched, up to 1 μm wide, septate, apex rounded, as long as the asci, hyaline, smooth. Asexual morph: not observed.
Culture characteristics: Colonies on PCA, slow-growing 11–12 × 15–23 mm after 30 day; circular, dome-shaped, margins entire, aerial mycelium dense, cottony, white to buff; reverse salmon. Cultures on PDA irregular, undulate, margins entire, aerial mycelium dense, cottony, centrally lavender-grey, ochreous in the outer region; reverse luteous. Cultures sterile.
Holotype: Brazil, Pernambuco, Taquaritinga do Norte, trilha do Mirante, Serra da Taquara, on fronds of Pteridium arachnoideum, 9 Jul. 2014, D.J. Soares (VIC 42921, cultures ex-type CPC 25778, COAD 1796).
Habitat/Distribution: Known from P. arachnoideum in the states of Pernambuco and Rio de Janeiro, Brazil.
Additional specimen examined: Brazil, Rio de Janeiro, Nova Friburgo, on fronds of Pteridium arachnoideum, 13 Jun. 2011, R.W. Barreto (VIC 42544, culture CPC 24666).
Notes: Based on both phylogenetic analyses (Figs. 3 and 4), S. pteridii has Hyphodiscus as its sister clade. Scolecolachnum and Hyphodiscus, however, are clearly morphologically distinct genera having different ascospore shape and size (subcylindrical, long and septate in the former, rather ellipsoid, small, and aseptate in all described species belonging to the latter; Zhuang 1988; Hosoya 2002), and hairs (smooth in the former and warted-tuberculate in the latter; Hosoya 2002). Additionally, the genus Hyphodiscus is known as having a gelatinous ectal excipulum (Hosoya 2002; Untereiner et al. 2006) a feature found to be absent in Scolecolachnum.
Zymochalara Guatimosim, R.W. Barreto & Crous, gen. nov.
MycoBank MB815563
Type species: Zymochalara cyatheae Guatimosim, R.W. Barreto & Crous
Etymology: indicating a combination of the yeast-like growth of colonies in pure culture, with a chalara-like morphology.
Conidiophores reduced to phialides. Phialides scattered, solitary, unbranched, lageniform, subulate or subcylindrical, aseptate, brown to cinnamon-brown, paler towards the apex, smooth; venter subcylindrical or ellipsoid, pedicellate or not; collarette cylindrical, transition from venter to collarette gradual. Phialoconidia endogenous, basipetal, extruded singly or in somewhat long and easily fragmenting chains, cylindrical, truncate at both ends, aseptate, hyaline, biguttulate, smooth. Yeast-like in culture.
Notes: Zymochalara is morphologically similar to Chalara, but distinct from fungi in that genus by producing a yeast-like colony in pure culture, instead of having filamentous growth as in Chalara (Nag Raj and Kendrick 1975). Chalara is known to be polyphyletic (Cai et al. 2009). Several genera have a chalara-like morphology (Coetsee et al. 2000; Paulin-Mahady et al. 2002; de Beer et al. 2014). Our phylogenetic analyses clearly place Zymochalara as a separate taxon with Bloxamia cyatheicola as sister clade (Figs. 1 and 2).
Zymochalara cyatheae Guatimosim, R.W. Barreto & Crous, sp. nov. (Fig. 9).
MycoBank MB815126
Etymology: Name refers to the tree fern host genus Cyathea.
Frond spots amphigenous, somewhat angular, starting as small necrotic areas along the margins of the pinnulae, increasing in size (up to 2.5–4 × 1.5–3 mm), coalescing and leading to blight of entire pinnulae. Internal hyphae not observed. External hyphae absent. Stromata absent. Conidiophores reduced to the conidiogenous cells. Phialides hypophyllous, scattered, solitary, erumpent through the cuticle, unbranched, subulate or subcylindrical, 32–50 μm long, 5–8.5 μm wide at the base, aseptate, brown to cinnamon-brown, becoming paler towards the apex, smooth; venter subcylindrical or ellipsoid, 12–26 × 3–7 μm; collarette cylindrical, 15–23 × 2–3.5 μm, transition from venter to collarette gradual. Phialoconidia endogenous, basipetal, extruded singly or in long and easily fragmenting chains, cylindrical, truncate at both ends, 6–10 × 1.5–3 μm, aseptate, hyaline, biguttulate, smooth.
Culture characteristics: Colonies on PDA slow growing, 2.2–3.5 cm diam after 30 day; circular, flat, centrally either yeast-like (in PCA), white with some central random tiny dots of aerial mycelium, dark mouse-grey, dry; or with felty aerial mycelium rosy-buff, becoming white and finally buff at periphery (on PDA); reverse either centrally hazel, passing to honey, passing to buff towards the periphery or equivalent to colors at surface. Cultures sterile in PDA, sporulating abundantly on PCA.
Holotype: Brazil, Rio de Janeiro, Nova Friburgo, Limeira, on fronds of Cyathea delgadii, 13 Jun. 2011, R.W. Barreto (VIC 42543, culture ex-type CPC 24665, COAD 1092).
Habitat/Distribution: Known from C. delgadii in the states of Minas Gerais and Rio de Janeiro, Brazil.
Additional specimens examined: Brazil, Rio de Janeiro, Nova Friburgo, Macaé de Cima, on fronds of C. delgadii, 29 Apr. 2012, R.W. Barreto (VIC 42562, culture CPC 24690); Minas Gerais, Araponga, Parque Estadual da Serra do Brigadeiro, on fronds of C. delgadii, 23 Feb. 2014, E. Guatimosim (VIC 42518, culture CPC 24735); ibid. (VIC 42462, cultures CPC 24736, COAD 2013); Rio de Janeiro, Nova Friburgo, Macaé de Cima, on fronds of C. delgadii, 1 Jun. 2014, R.W. Barreto (culture CPC 25072, COAD 1758).
Note: See the notes for Z. lygodii.
Zymochalara lygodii Guatimosim, R.W. Barreto & Crous, sp. nov. (Fig. 10).
MycoBank MB813046
Etymology: Name refers to the host genus Lygodium.
Frond blight irregular, starting as small, vein-delimited, pale brown to cinnamon-brown spots, close to the main vein of the pinnulae and spreading towards the apex. At later stages, becoming dark, necrotic and distorting the pinnulae, sometimes causing necrosis of the entire pinnulae, affecting mostly the upper pinnulae. Internal hyphae not observed. External hyphae absent. Stromata absent. Conidiophores reduced to the conidiogenous cells. Phialides hypophyllous, scattered, solitary, erumpent through the cuticle, unbranched, lageniform, 29–38 μm long, 5.5–9 μm wide at the base, brown to cinnamon-brown, paler towards the apex, smooth; venter subcylindrical or ellipsoid, pedicellate, 13–16 × 5–6.5 μm; collarette cylindrical, 16–21 × 3–4 μm, transition from venter to collarette gradual. Phialoconidia endogenous, basipetal, extruded in easily fragmenting chains, cylindrical, truncate at the base, apex rounded, 6.5–12 × 1.5–3 μm, aseptate, hyaline, biguttulate, smooth.
Culture characteristics: Colonies on PDA slow-growing, 15 mm diam after 28 days; circular to irregular, convex with papillate surface, margin crenate, aerial mycelium velvety, leaden-black intermixed with umber, and white hyphal tufts, mouse-grey at periphery; reverse leaden-black. Colonies on PCA umbonate, radially striate with lobate margins, yeast-like, mostly rosy-buff and buff at periphery; reverse buff. Cultures sterile.
Holotype: Brazil, Minas Gerais, Viçosa, Cristais, on fronds of Lygodium volubile, 6 Mar. 2013, E. Guatimosim (VIC 42470, culture ex-type CPC 24710, COAD 2001).
Habitat/Distribution: Known from L. volubile in the states of Minas Gerais and Rio de Janeiro, Brazil.
Additional specimens examined: Brazil, Rio de Janeiro, Lumiar, on fronds of L. volubile, 2 May 2013, R.W. Barreto (VIC 42600, culture CPC 24699, COAD 1992).
Notes: The morphology of Z. lygodii is similar to that of Chalara fungorum, but differs from it by having phialides with wider bases (5.5–9 μm in the former and 3–6.5 μm in the latter), and larger phialoconidia (6.5–12 μm long in the former and up to 8 μm in the latter) (Nag Raj and Kendrick 1975). Additionally, C. fungorum is only known attacking the following hosts: Abies lasciocarpa, Fagus sylvatica, Ilex pernyl, Laurus nobilis, Pistacia lentiscus, Quercus ilex, and Rhododendron ponticum in Canada, Italy and the United Kingdom (Nag Raj and Kendrick 1975; Farr and Rossman 2015). Conversely, Z. lygodii was found only on the Neotropical liana fern L. volubile in Brazil.
Besides having a different host, Z. lygodii differs from Z. cyatheae by having longer phialides (29–38 μm in the former and 32–50 μm in the latter). The Bayesian analyses generated in this study provide clear evidence that Z. lygodii is distinct from Z. cyatheae by having 15 bp of variable sites for the ITS locus (Fig. 1), and 10 bp of variable sites for LSU (Fig. 2).
Discussion
The present study aimed to determine the potential fungal diversity occurring on ferns in Brazil. Based on the results obtained here focusing on chalara-like and lachnoid fungi, three genera and six species were found to be new. Furthermore, two novel taxa occurred on an endangered host, Dicksonia sellowiana, and should therefore be considered as potentially endangered.
Of the species collected, one was found to represent a new species of Bloxamia. Berthet (1964) reported the development of Bl. truncata (type species of the genus) from cultures of single ascospore isolations of Bisporella sulphurina. Johnston (1998) also obtained evidence of the connection between these sexual and asexual morphs by recovering a Bloxamia asexual morph through isolation of Bisporella discedens from New Zealand in pure culture. However, the latter author did not propose a separate name for the asexual morph. The genus Bisporella is characterised by its bright yellow, sessile to substipitate apothecia, which generally occur on woody substrata in temperate regions. In vertical section, the internal anatomy of the apothecium is characterized by a gelatinised or subgelatinised ectal excipulum, with little or no differentiation of a medullary excipulum; asci 8-spored, 0-1-septate (Carpenter and Dumont 1978; Saccardo 1884). Over the years, this genus was treated as a repository of a large variety of fungi, having significant differences in morphology (e.g., 3-septate ascospores in Bi. triseptata and aseptate ascospores in Bi. calycellinoides, Bi. iodocyanescens and Bi. oritis). It includes 25 species (Kirk et al. 2008) and is likely to be a generic complex. This assumption is strengthened by the fact that Bi. resinicola has an asexual morph residing in Eustilbum (Baranyay and Funk 1969; Seifert and Carpenter 1987), completely different from Bloxamia. In addition, a recently published phylogeny has shown that some of the species recognised as members of Bisporella (namely Bi. citrina, Bi. claroflava, Bi. drosodes, Bi. lactea, and Bi. scolochloae) are in fact members of Calycina, once they grouped with its type species C. herbarum (Baral et al. 2013). This conclusion, however, did not result in synonymizing the whole genus Bisporella, from which its type species has never been studied with molecular tools in the evolutionary context. For the clarification of the true evolutionary relationships within Bisporella it is necessary to recollect and epitypify its type species, Bi. monilifera, and generate DNA data.
Except for Bl. foliicola, all species of Bloxamia were described from dead wood, or from rotting plant material (Table 2), suggesting a saprobic life style. Nevertheless, Bl. cyatheicola was only found on living fronds either seemingly causing frond spots on Cyathea spp. or sporulating without any obvious symptoms on the host tissue. It may be either a weak pathogen or a specialized hemibiotrophic endophyte. Based solely on this ecological evidence (pathogen instead of wood-inhabiting), this is considered by us as insufficient to justify proposing a separate genus to accommodate Bl. cyatheicola.
Thus far, despite the fact that the new species from Brazil had both sexual and asexual morphs, we decided to describe it in Bloxamia, since this genus is morphologically better circumscribed and older than Bisporella (Nag Raj and Kendrick 1975).
The genus Lachnum is widely distributed and characterized by having small, discoid apothecia covered by numerous subcylindrical, septate and granular hairs (Haines and Dumont 1984). The genus includes about 250 species (Kirk et al. 2008). Most of them are not known from molecular data but it has already been shown that the genus is polyphyletic (Han et al. 2014). The present phylogenetic survey (Fig. 3), agrees with Zhao and Zhuang (2011), who demonstrated that the ITS locus is reliable for delimiting species boundaries within Lachnum, having only L. rhytismatis (strains TNS-F-16544 and TNS-F-16545) grouping in a different clade. The present study, in consonance with Perić and Baral (2014), treats Lachnum as a genus-complex, from which, based on the phylogenetic inference, different genera can be separated like Erioscyphella, Lachnopsis and Scolecolachnum.
The topology of both ITS and LSU trees (Figs. 1, 2) suggests that the genus Zymochalara (including Z. lygodii and Z. cyatheae) is related to Chalara, but significantly distant from all the species included in this study, having Bl. cyatheicola as sister clade.
Only three species of Chalara are known from ferns, namely C. crassipes causing frond spots on Pteridium aquilinum in Germany, Ch. parvispora on Cyathea medullaris from New Zealand, and Ch. pteridina on P. aquilinum from Austria, Australia, England, Germany, Poland, and the United Kingdom (Nag Raj and Kendrick 1975; Farr and Rossman 2015). Although DNA information available for these taxa is limited to LSU sequences for C. crassipes and C. parvispora (Cai et al. 2009), it is clear that data from this locus alone are sufficient to separate C. crassipes and C. parvispora from both Z. cyatheae and Z. lygodii (Fig. 2).
Four specimens of fungi were collected on the endangered tree fern D. sellowiana during our surveys. These were found to represent two novel species within the new genus Lachnopsis. Interestingly, these species were not found on any other taxa of tree ferns collected during this study; often occurring in the same habitat. This suggests that these two fungal species are host-specific. Further studies are needed to confirm this conjecture and to demonstrate that these two species found exclusively on D. sellowiana are not capable of colonizing other substrates, confirming the hypothesized risk of co-extinction. These considerations follow the line of previously published work conducted in Brazil focused on the foliar mycobiota of endangered Brazilian plant species. Previous publications covered the leaf mycobiota of the endangered tree species Coussapoa floccosa (Cecropiaceae) and Dimorphandra wilsonii (Fabaceae). Unique fungi were collected on these two endemic trees highlighting the need to preserve endangered plant species from a mycological as well as a botanical viewpoint (Rocha et al. 2010; Silva et al. 2016). The addition of L. catarinensis and L. dicksoniae to the list of potentially endangered Brazilian microfungi raises the total of species with such status to 11, all of which are recently described as new to science. It is expected that further evidence of complete dependency on endangered plant-hosts will translate into them being added to the IUCN Red List of Threatened Species, as well as to their inclusion in the Brazilian list of endangered species.
The present work contributes towards a better understanding of fungi on tropical ferns as well as of the assemblages of lachnoid, and chalara-like genera within the Hyaloscyphaceae sensu lato. The large proportion of taxonomic novelties obtained from the survey in Brazil, as reflected in the present study and that of Guatimosim et al. (2016), confirmed tropical ferns as a rich and poorly investigated fungal niche, deserving further attention by mycologists.
References
Alfenas RF, Lombard L, Pereira OL, Alfenas AC, Crous PW (2015) Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil. Stud Mycol 80:89–130
Almeida DAC, Barbosa FR, Gusmão LFP (2012) Alguns fungos conidiais aquáticos-facultativos do bioma Caatinga. Acta Bot Bras 26:924–932
Arambarri A, Cabello M, Cazau C (1992) A new hyphomycete from Santiago River. V. Bloxamia cremea. Mycotaxon 43:327–330
Armando EAS, Chaves ZM, Dianese JC (2015) Phaeostilbelloides and Velloziomyces – new dematiaceous genera from the Brazilian Cerrado. Mycotaxon 130:757–767
Baral HO (2015) Nomenclatural novelties. Index Fungorum no. 225
Baral HO, De Sloover JR, Huhtinen S, Laukka T, Stenroos S (2009) An emendation of the genus Hyaloscypha to include Fuscoscypha (Hyaloscyphaceae, Helotiales, Ascomycotina). Karstenia 49:1–17
Baral HO, Galán R, Platas G, Tena R (2013) Phaeohelotium undulatum comb. nov. and Phaeoh. succineoguttulatum sp. nov., two segregates of the Discinella terrestris aggregate found under Eucalyptus in Spain: taxonomy, molecular biology, ecology and distribution. Mycosystema 32:386–428
Baral HO, Haelewaters D (2015) Rommelaarsia flavovirens gen. et sp. nov. (Helotiales), a new discomycete on Equisetum with a peculiar asexual state. Ascomycete.org 7:321–330
Baranyay JA, Funk A (1969) Helotium resinicola n.sp. and its Stilbella conidial state. Can J Bot 47:1011–1014
Berthet P (1964) Formes conidiennes de divers discomycetes. Bull Trimest Soc Mycol Fr 80:125–149
Bogale M, Orr MJ, O’Hara MJ, Untereiner WA (2010) Systematics of Catenulifera (anamorphic Hyaloscyphaceae) with anassessmentofthephylogeneticposition of Phialophora hyalina. Fungal Biol 114:396–409
Cai L, Wu WP, Hyde KD (2009) Phylogenetic relationships of Chalara and allied species inferred from ribosomal DNA sequences. Mycol Prog 8:133–143. doi:10.1007/s11557-009-0585-5
Cândido TS, Silva AC, Guimarães LMS, Ferraz HGM, Júnior NB, Alfenas AC (2014) Teratosphaeria pseudoeucalypti on Eucalyptus in Brazil. Trop Plant Pathol 39:407–412
Cantrell SA, Haines JH (1997) New red species of Lachnum from the tropics. Mycol Res 101:1081–1084
Cantrell SA, Hanlin RT (1997) Phylogenetic relationships in the family Hyaloscyphaceae inferred from sequences of ITS regions, 5.8S ribosomal DNA and morphological characters. Mycologia 89:745–755
Carpenter SE, Dumont KP (1978) Los Hongos de Colombia - IV. Bisporella triseptata and its allies in Colombia. Caldasia 12:339–348
Coetsee C, Wingfield MJ, Crous PW, Wingfield BD (2000) Xenochalara, a new genus of dematiaceous hyphomycetes for Chalara-like fungi with apical wall building conidial development. S Afr J Bot 66:99–103
Coppins BJ, Minter DW (1980) A new hyphomycete from Northumberland. Notes R Bot Gard Edinburgh 38:363–365
Crous PW, Verkley GJM, Groenewald JZ, Samson RA (eds) (2009) Fungal biodiversity. CBS laboratory manual series no 1. Centraalbureau voor Schimmelcultures, Utrecht
Crous PW, Quaedvlieg W, Hansen K, Hawksworth DL, Groenewald JZ (2014) Phacidium and Ceuthospora (Phacidiaceae) are congeneric: taxonomic and nomenclatural implications. IMA Fungus 5:173–193
de Beer ZW, Duong TA, Barnes I, Wingfield BD, Wingfield MJ (2014) Redefining Ceratocystis and allied genera. Stud Mycol 79:187–219
Farr DF, Rossman AY (2015) Fungal databases systematic mycology and microbiology laboratory, ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/. Accessed 15 May 2015
Fiuza PO, Gusmão LFP, Castañeda Ruiz RF (2015) Conidial fungi from the semiarid Caatinga biome of Brazil: a new species of Selenosporella from submerged leaves. Mycotaxon 130:601–605
Forzza R, Leitman P, Costa A, Siqueira Filho JA, Martinelli G, Monteiro RF, Santos-Silva F, Saraiva DP, Paixão-Souza B, Louzada RB (2015) Lista de espécies da flora do Brasil. http://floradobrasil.jbrj.gov.br. Accessed 16 Feb 2015
Guatimosim E, Pinto HJ, Pereira OL, Fuga CAG, Vieira BS, Barreto RW (2015) Pathogenic mycobiota of the weeds Bidens pilosa and Bidens subalternans. Trop Plant Pathol 40:298–397
Guatimosim E, Schwartsburd PB, Barreto RW, Crous PW (2016) Novel fungi from an ancient niche: cercosporoid and related sexual morphs on ferns. Persoonia 37:106–141
Haines JH (1980) Studies in the Hyaloscyphaceae I: some species of Dasyscyphus on tropical ferns. Mycotaxon 11:189–216
Haines JH (1992) Studies in the Hyaloscyphaceae VI: the genus Lachnum (ascomycetes) of the Guyana Highlands. Nova Hedwigia 54:97–112
Haines JH, Dumont KP (1984) Studies in the Hyaloscyphaceae. III: the long-spored, lignicolous species of Lachnum. Mycotaxon 19:1–39
Han JG, Hosoya T, Sung G-H, Shin HD (2014) Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biol 118:150–167
Hawksworth DL (1991) The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res 95:641–655
Hernández-Gutiérrez A, Dianese JC (2014) Cercosporoid hyphomycetes on malpighiaceous hosts from the Brazilian Cerrado: new Passalora and Pseudocercospora species on hosts of the genus Banisteriopsis. Mycol Prog 13:365–371
Hernández-Gutiérrez A, Braun U, Dianese JC (2014) Cercosporoid hyphomycetes on malpighiaceous hosts from the Brazilian Cerrado: species of Pseudocercospora on hosts belonging to Byrsonima. Mycol Prog 13:193–210
Hosoya T (2002) Hyaloscyphaceae in Japan (6): the genus Hyphodiscus in Japan and its anamorph Catenulifera gen. nov. Mycoscience 43:47–57
Hosoya T, Sasagawa R, Hosaka K, Sung G-H, Hirayama Y, Yamaguchi K, Toyama K, Kakishima M (2010) Molecular phylogenetic studies of Lachnum and its allies based on the Japanese material. Mycoscience 51:170–181
Hustad VP, Kucera V, Rybarikova N, Lizon P, GaislerJ BTJ, Miller AN (2014) Geoglossum simile of North America and Europe: distribution of a widespread earth tongue species and designation of an epitype. Mycol Prog 13:857–866
Izabel TSS, Almeida DAC, Monteiro JS, Castañeda-Ruiz RF (2015) Anaexserticlava caatingae, a new conidial fungus from the semi-arid Caatinga biome of Brazil. Mycotaxon 130:445–449
James TY, Kauff F, Schoch CL et al (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818–822
Johnston PR (1998) The Bloxamia anamorph of Bisporella discedens. Mycotaxon 31:345–350
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Nucleic Acids Res 30:3059–3066
Kirk PM, Cannon PF, David WM, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI Publishing, Wallingford, UK
Koukol O (2011) New species of Chalara occupying coniferous needles. Fungal Divers 49:75–91
Liu YL, Zhang ZY (1998) A new species of the genus Bloxamia. Mycosystema 17:7–10
Macedo DM, Pereira OL, Wheeler GS, Barreto RW (2013) Corynespora cassiicola f. sp. schinii, a potential biocontrol agent for the weed Schinus terebinthifolius in the United States. Plant Dis 97:496–500
Macedo DM, Pereira OL, Hora Junior BT, Weir BS, Barreto RW (2016) Mycobiota of the weed Tradescantia fluminensis in its native range in Brazil with particular reference to classical biological control. Australas Plant Pathol 45:45–56
Mendes MAS, Urben AF (2015) Fungos relatados em plantas no Brasil, Laboratório de Quarentena Vegetal. Brasília, DF: Embrapa Recursos Genéticos e Biotecnologia. http://pragawall.cenargen.embrapa.br/-aiqweb/michtml/fgbanco01.asp. Accessed 15 May 2015
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proc Gatew Comp Environ Workshop (GCE), New Orleans, LA pp 1–8
Minter DW, Holubová-Jechová V (1981) New or interesting Hyphomycetes on decaying pine litter from Czechoslovakia. Folia Geobot Phytotax 16:195–217
Nag Raj TR, Kendrick B (1975) A monograph of Chalara and allied genera. Wilfrid Laurier University Press, Waterloo
Nannfeldt JA (1932) Studien über die Morphologie und Systematic der nichtlichenisierten inoperculaten Discomyceten. Nova Act Reg Soc Sci Upsal 8:1–368
Nylander J (2004) MrModeltest v2. Program distributed by the author. Evol Biol Cent Uppsala Univ 2:1–2
Oliveira L, Harrington TC, Ferreira MA, Damacena MB, Al-Sadi AM, Al-Mahmooli IHS, Alfenas AC (2015) Species or genotypes? Reassessment of four recently described species of the Ceratocystis wilt pathogen, Ceratocystis fimbriata, on Mangifera indica. Phytopathology 105:1229–1244
Osmundson TW, Robert VA, Schoch CL, Baker LJ, Smith A, Robich G, Mizzan L, Garbelotto MM (2013) Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLoS ONE 8:e62419
Paulin-Mahady AE, Harrington TC (2000) Phylogenetic placement of anamorphic species of Chalara among Ceratocystis species and other ascomycetes. Stud Mycol 45:209–222
Paulin-Mahady AE, Harrington TC, NcNew D (2002) Phylogenetic and taxonomic evaluation of Chalara, Chalaropsis, and Thielaviopsis anamorphs associated with Ceratocystis. Mycologia 94:62–72. doi:10.2307/3761846
Perić B, Baral HO (2014) Erioscyphella curvispora spec. nov. from Montenegro. Mycol Monten 17:89–104
Pillar VD, Müller SC, Castilhos Z, Jacques AVA (2009) Campos Sulinos: Conservação e Uso Sustentável da Biodiversidade. Ministério do Meio Ambiente, Brasilia
Pinho DB, Firmino AL, Ferreira-Junior WG, Pereira OL (2012) An efficient protocol for DNA extraction from Meliolales and the description of Meliola centellae sp. nov. Mycotaxon 122:333–345
Pirozynski KA, Morgan-Jones G (1968) Notes on microfungi. III. Trans Br Mycol Soc 51:185–206
Raitviir A (2004) Revised synopsis of the Hyaloscyphaceae. Scr Mycol 20:1–133
Rayner RW (1970) A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society, Surrey, UK
Réblová M (1999) Teleomorph-anamorph connections in Ascomycetes 2. Ascochalara gabretae gen. et sp. nov. and its Chalara-like anamorph. Sydowia 51:210–222. doi:10.2307/3762067
Rocha FB, Barreto RW, Bezerra JL, Neto JAAM (2010) Foliar mycobiota of Coussapoa floccosa, a highly threatened tree of the Brazilian Atlantic Forest. Mycologia 102:1241–1252
Rodrigues AL, Pinho DB, Lisboa DO, Nascimento RJ, Pereira OL, Alfenas AC, Furtado GQ (2014) Colletotrichum theobromicola causes defoliation, stem girdling and death of mini-cuttings of Eucalyptus in Brazil. Trop Plant Pathol 39:326–330
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Saccardo PA (1884) Conspectus generum Discomycetum hucusque cognitorum. Bot Centralbl 18(213–220):247–255
Schoch CL, Seifert KA, Huhndorf S et al (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 109:6241–6246
Seifert KA, Carpenter SE (1987) Bisporella resinicola comb. nov. and its Eustilbum anamorph. Can J Bot 65:1262–1267
Silva M, Pinho DB, Pereira OL, Fernandes FM, Barreto RW (2016) Naming potentially endangered parasites: foliicolous mycobiota of Dimorphandra wilsonii, a highly threatened Brazilian tree species. PLoS ONE 11:e0147895. doi:10.1371/journal.pone.0147895
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2008) A classification for extant ferns. Taxon 55:705–731
Spooner BM (1987) Helotiales of Australasia: Geoglossaceae, Orbiliaceae, Sclerotiniaceae, Hyaloscyphaceae. Bibl Mycol 116:1–711
Spooren M (2014) A new species of Bloxamia from freshwater in the Netherlands. Mycosphere 5:346–349
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Untereiner WA, Naveau FA, Bachewich J, Angus A (2006) Evolutionary relationships of Hyphodiscus hymeniophilus (anamorph Catenulifera rhodogena) inferred from β-tubulin and nuclear ribosomal DNA sequences. Can J Bot 84:243–253
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Wang Z, Binder M, Hibbett DS (2005) Life history and systematics of the aquatic discomycete Mitrula (Helotiales, Ascomycota) based on cultural, morphological, and molecular studies. Am J Bot 92:1565–1574
White TJ, Bruns T, Lee J, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications: 315–322. Academic, New York, pp 322–514
Windisch PG (2002) Pteridófitas do Brasil: diversidade decrescente. In: Araujo EL, Moura NA, Sampaio EVSB (eds) Biodiversidade, conservação e uso sustentável da flora do Brasil. Universidade Federal Rural de Pernambuco, Recife, pp 196–198
Zhang YH, Zhuang WY (2004) Phylogenetic relationships of some members in the genus Hymenoscyphus (Ascomycetes, Helotiales). Nova Hedwigia 78:475–484
Zhao P, Zhuang WY (2011) Evaluation of ITS region as a possible DNA barcode for the genus Lachnum (Helotiales). Mycosystema 30:932–937
Zhuang WY (1988) Notes on Lachnellula theiodea. Mycotaxon 31:411–416
Zhuang WY, Hyde KD (2001) New species of Lachnum and Perrotia from Hong Kong, China. Mycologia 93:606–611
Zhuang WY, Wang Z (1998) Some new species and new records of discomycetes in China. VIII. Mycotaxon 66:429–438
Acknowledgments
The authors would like to thank Dr Richard T. Hanlin and Dr Wen-Ying Zhuang for their helpful contributions regarding Herbarium information, and Dr Dartanhã José Soares for his collaboration during the field work. Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) are thanked for financial support. Electron microscopy studies were performed at the Núcelo de Microscopia e Microanálise da Universidade Federal de Viçosa (NMM-UFV).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Gerhard Rambold
Rights and permissions
About this article
Cite this article
Guatimosim, E., Schwartsburd, P.B., Crous, P.W. et al. Novel fungi from an ancient niche: lachnoid and chalara-like fungi on ferns. Mycol Progress 15, 1239–1267 (2016). https://doi.org/10.1007/s11557-016-1232-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1232-6