Abstract
Geoglossum simile is a distinctive species of the earth tongue class Geoglossomycetes, first described in 1873. The taxonomic standing of this species has long been disputed, resulting in nearly 70 years of potential misdiagnoses. Although G. simile was originally described from North America, it has subsequently been reported from several European countries as well as Asia, Australasia, and India. In this study, we report the first records of G. simile from Slovakia and the Czech Republic, examine the morphological and molecular diversity of Northern Hemisphere collections, discuss the taxonomic history and current status of the species, and designate a recent North American collection as the epitype of this widely distributed and conservationally significant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fungal class Geoglossomycetes Schoch et al. (2009) has been a subject of study for mycologists for over three centuries (Hustad et al. 2013). This long history of research has yielded a complicated taxonomy with many synonyms and nomenclatural transfers, resulting in over two hundred published names (www.indexfungorum.org). Currently, the class contains six genera and an estimated fifty species (Kirk et al. 2008, Hustad et al. 2013). Fungi of the class Geoglossomycetes have been included in several studies on the evaluation of European grasslands (Rald 1985, Nitare 1988, Jordal 1997, McHugh et al. 2001, Adamčík and Kautmanová 2005) and are of conservation interest. The genus Geoglossum Pers. contains the earliest described species in the class and is also the most widespread genus with members reported from every continent except Antarctica.
Geoglossum simile Peck was described (Peck 1873) from specimens collected in Sand Lake (New York, USA) by Charles Horton Peck and Ft. Edward (New York, USA) by Elliot Calvin Howe. Peck did not designate either specimen as the holotype and because these specimens are the only ones listed in the protologue, they are regarded as syntypes. At that time Peck was under the erroneous assumption that G. glabrum possesed 3-4 septate ascospores (as reported by Cooke (1871)), and, thus. he believed that his specimen with 7-septate ascospores was a separate and undescribed species. Peck (1878)) reconsidered his original diagnosis of a new species and placed G. simile as a synonym under G. glabrum Pers. Peck made no mention of the distinctive paraphyses in his collections of G. simile in either treatment and may not have compared them with authentic material of G. glabrum from Europe. He did admit (Peck 1878) the “application of the specific name glabrum” to his specimens was “unfortunate and liable to mislead the student, for the stem is covered by a kind of minutely-tufted tomentum of matted septate filaments, which, with the projecting masses of spores from the mature club, give the plant a scarcely less hairy aspect than that of G. hirsutum”. The squamulose stem composed of acute tufts of paraphyses is a characteristic morphological feature observed in dried specimens of G. simile, but is not found in G. glabrum.
Peck’s confusion over the identity of this species influenced subsequent authors for over 70 years. Massee (1897), Durand (1908), and Lloyd (1916) considered Geoglossum simile as a synonym of G. glabrum in their influential early monographs of Geoglossaceae. It was not until Imai (1941) that the species were again recognized as separate. With the exception of Seaver (1951), the concept of G. simile as a species separate from G. glabrum was subsequently accepted by the majority of researchers (e.g., Nannfeldt 1942, Mains 1954, Eckblad 1963, Maas Geesteranus 1965, Ohenoja 2000, Roobeek 2009, Hustad et al. 2011). G. simile is one of the most commonly occurring Geoglossum species in eastern North America (Mains 1954), but many collections made prior to the 1940s may be mislabeled as G. glabrum. Hustad et al. (2013) found G. simile to occur on a well-supported branch in the Geoglossum clade in a four-gene molecular phylogeny of Geoglossomycetes, separate from G. glabrum and sister to G. sphagnophilum.
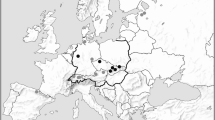
Although Geoglossum simile was originally described from North America, it has subsequently been collected in Asia (Imai 1941), Australasia (Beaton & Weste 1979), Europe (Bille-Hansen 1954), and India (Batra & Batra 1963). Our assumption that the species may occur in Central Europe was confirmed by material collected in Slovakia and the Czech Republic in 2006 (Kučera & Lizoň 2012). Examination of these collections and other European material has raised the question of whether specimens of G. simile from Europe and North America represent the same species or discrete species with convergent morphologies. Members of the genus Geoglossum were thought to be quite rare in Slovakia and the Czech Republic with only a few taxa reported from these countries before recent collections (Kučera et al. 2008, 2010, 2013, Kučera & Gaisler 2012, Kučera & Lizoň 2012, Kučera 2012).
The goals of this study were to: 1) compare European and North American material using both morphological and molecular techniques, and 2) designate an epitype for Geoglossum simile from recently collected North American material to facilitate the interpretation of the lost and aging material examined by Peck.
Materials and methods
Specimens were identified based on morphological characters using pertinent literature and original species descriptions. The macromorphological ascomatal characters were observed in fresh and dried material. Dried ascomata were handsectioned and fragments were examined in water and Melzer’s reagent or 5 % KOH using an Olympus BX51 compound microscope with differential interference microscopy. Permanent slides were made using PVLG (Omar et al. 1979) with material rehydrated in 5 % KOH. Images were captured with a QImaging QColor3 digital camera and processed using Adobe Photoshop v. 7.0 (Adobe Systems Inc., Mountain View California). A minimum of 30 measurements were made for each micromorphological character using NIH Image (National Institutes of Health, Bethesda, Maryland) and standard univariate statistics were performed on these measurements (Table 1). Acronyms for fungaria follow Index Herbariorum (Thiers 2013). Recent collections were georeferenced and the coordinates reported in WGS 84 format.
Total genomic DNA was extracted from dried ascomata using a QIAGEN DNeasy Plant Mini Kit (QIAGEN Inc., Valencia, California) and gene fragments were PCR amplified and sequenced using methods outlined by Promputtha and Miller (2010). The internal transcribed spacer (ITS) region of nuclear ribosomal DNA (nrDNA), consisting of the ITS1, 5.8S, and ITS2 regions, was amplified and sequenced using a combination of the primers ITS1, ITS4, and ITS5 (White et al. 1990). The 28S large subunit (LSU) nrDNA region was amplified and sequenced using primers JS1 (Landvik 1996) and LR3 (Vilgalys & Hester 1990).
Individual gene alignments were created manually in Sequencher v. 5.0.1 (Gene Codes Corp., Ann Arbor, Michigan) and optimized using Muscle v. 3.7 (Edgar 2004) in SeaView v. 4.2 (Galtier et al. 1996). Ambiguous regions were removed from each dataset using Gblocks v. 0.91b (Castresana 2000) under the following parameters: minimum number of sequences for both conserved and flanking regions = 12, maximum number of contiguous non-conserved positions = 4, minimum length of a block = 4, and allowed gap positions in 12 sequences. GTR + I + G was determined by the Akaike Information Criterion (AIC) (Posada & Buckley 2004) to be the best-fit model of evolution by jModelTest v. 0.1.1 (Posada 2008) for both individual gene datasets. Maximum likelihood (ML) analyses were performed with PhyML (Guindon & Gascuel 2003) under the GTR substitution model with six rate classes and invariable sites optimized. An unrooted BioNJ starting tree was constructed and the best of nearest neighbor interchange (NNI) and subtree pruning and regrafting (SPR) tree improvement was implemented during the heuristic search. Nonparametric bootstrap support (BS) (Felsenstein 1985) was determined with 100 replicates. Clades with BS support ≥ 70 % were considered significant and highly supported (Hillis & Bull 1993).
Bayesian inference employing a Markov Chain Monte Carlo (MCMC) algorithm was performed using MrBayes 3.1.2 (Huelsenbeck & Ronquist 2001) on the CIPRES Science Gateway Teragrid (Miller et al. 2010) as an additional method of determining branch support. The GTR + I + G model with six rate classes was employed. Four independent chains of MCMC were run for 10 million generations. Clades with Bayesian posterior probability (BPP) of ≥ 95 % were considered significantly supported (Alfaro et al. 2003). Effective sample size (ESS) was estimated using Tracer v. 1.6 (Rambaut & Drummond 2009).
Individual datasets of ITS and LSU were examined for potential conflict before being concatenated into a single dataset for total evidence analysis (Kluge 1989, Eernisse & Kluge 1993). Individual gene phylogenies were considered to be incongruent if clades with significant ML BS and BPP were conflicting in the individual tree topologies (Wiens 1998, Alfaro et al. 2003, Lutzoni et al. 2004). Since no incongruencies were found among individual datasets, the ITS and LSU datasets were concatenated and final ML and Bayesian analyses were performed on the combined dataset. Alignments and trees are deposited in TreeBASE (http://treebase.org) under submission ID 15095.
A total of 32 specimens of Geoglossum simile, including the Fort Edward lectotype and the newly-designated epitype from Cortland County, New York were examined (Table 1). Twenty-two sequences were newly generated for this study (11 ITS, 11 LSU). These sequences were analyzed together with 20 sequences from previous studies (Hustad et al. 2011, 2013), along with four sequences from GenBank. Sequences from 23 collections, representing twelve collections of G. simile and eleven collections representing eight closely-related outgroup species (Hustad et al. 2013) were included in the analyses (Table 2).
Results
The final combined matrix had an aligned length of 1265 bp, which was reduced to 1173 after the removal of 92 ambiguous characters by Gblocks. Of the 1173 characters used in the phylogenetic analyses, 1019 of the sites were complete (no gaps or missing characters in any of the sequences), 221 of the complete sites were variable across the dataset, and 110 (10.8 %) of these variable sites were informative. We estimated burn-in of 10 % was sufficient to remove the pre-stationary posterior probability distribution using Tracer v.1.6, resulting in an ESS of 212.5797. The most likely tree produced by the PhyML analysis of the concatenated ITS and LSU dataset is shown in Fig. 1. Sequences from Geoglossum simile form a strongly supported clade (BS = 100 %, BPP = 1.0) with G. sphagnophilum occurring as a sister species to G. simile.
PhyML maximum likelihood phylogeny showing the position of Geoglossum simile based on a combined dataset (1173 bp) of ITS and LSU DNA sequences ((-ln)L score = 3866.3). Numbers at nodes indicate significant BS values (≥70 %) based on 100 replicates; thickened branches indicate significant BPP (≥ 95 %). Several species of Geoglossum and Glutinoglossum glutinosum are included as outgroups. Numbers associated with taxon names are fungarium accession numbers or strain numbers obtained from GenBank
Taxonomy
Geoglossum simile Peck.
= Geoglossum glabrum var. simile (Peck) S. Imai, Trans. Myc. Soc. Jap. 3: 52. 1962.
Mycobank MB 162582.
Syntype: USA, New York, Rensselaer County, Sand Lake [as ‘Sandlake’], Adirondack Mountains, C.H. Peck, September 1873 (NYS, lost).
Lectotype: USA, New York, Washington County, Fort Edward, E.C. Howe, 1873 (NYS f2797) – designated here. Fig. 2.
Epitype: USA, New York, Cortland County, Kennedy State Forest, Scutt Hill Road, T.J. Baroni [TJB 9613], 10 August 2003 (CORT 005220; isoepitype ILLS 71160) – designated here. Fig. 3.
Emended description
Ascomata (21-) 39-65 (-80) mm high, solitary, club-shaped, stipitate. Fertile part lanceolate, more or less compressed, (4-) 9.7-19.2 (-35) mm long, (1-) 2-4 (-7) mm thick, apex usually subacute to obtuse, blackish-brown, differentiated from the sterile stipe, sometimes with a narrow median groove. Stipe gracile, cylindrical above, terete, minutely pubescent or glabrous when fresh, forming acute tufts and squamulose when dried (Fig. 3a and c), brownish-black with dark cinnamon brown base, (15-) 26-48 (-60) mm long, 0.5-2 (-3) mm thick. Asci (155-) 175-200 (-220) × (16.5-) 24.5-29.5 (-36) μm, 8-spored, clavate or lanceolate to narrowly tapered towards the apex, the pore bluing in Melzer’s reagent. Ascospores (60-) 74-91 (-108) × (4.7-) 6.6-7.8 (-11) μm, in parallel fascicle, 7-septate (though 8- and 9- septate ascospores rarely seen), dark fuliginous, cylindric to clavate or slightly narrower at one end, slightly curved (Figs. 2b and 3b). Paraphyses filiform, pale brown in upper part, straight or curved, (though often sigmoid at the terminal end) moderately to closely septate in the upper part constricted to form barrel-shaped segmented cells resembling didymospores (often forming chains of three or more such cells in sequence at the terminal end), protruding beyond the asci, the apical cell (7-) 9.7-13.9 (-20) × (4-) 5.3-7.3 (-10) μm (Figs. 2c and 3d).
Habitat
Geoglossum simile occurs in Europe most commonly in peat bogs and wet meadows among mosses. In North America the species is commonly found on moss-covered soil and humus in mixed deciduous forests. According to the literature, it also is found on soil (Nannfeldt 1942, Zhuang and Wang 1997), as well as in swamps, bogs and well-drained soil (Mains 1954), damp pasture (Jørgensen & Vevle 1968, Ohenoja 2000), soil in Cedrus or Quercus forest (Maas Geesteranus 1965), on rotten logs (Nannfeldt 1942), and among Sphagnum (Nannfeldt 1942, Mains 1954) and other mosses (Mains 1954).
Conservation
Earth tongues are widely collected and studied in North America and Europe and their presence in a particular habitat often indicates limited human disturbance. Earth tongue species diversity has been used as a proxy for grassland health in conservation value assessments (Newton et al. 2003, Genney et al. 2009). Geoglossum simile is present on several Red Data Lists throughout Europe, listed as threatened with extinction in Austria (Aron et al. 2005) and Germany (Sonneborn et al. 2005), critically endangered in Denmark (Stoltze & Pihl 1998), vulnerable in The Netherlands (Arnolds & Veerkamp 2008), and near threatened in Norway (Fandnes 2011). In Sweden, G. simile is listed as not threatened (Gärdenfors 2010), even though the species is often found in the same habitat as G. uliginosum, a very rare and endangered species in Sweden according to a National Biodiversity Action Plan from 2007-2011 (Nitare 2007). The species was listed as one of three Ascomycetes believed to be extinct in the provisional Red Data List of British fungi (Ing 1992), although Mackey et al. (2011) recognized G. simile as a characteristic species of High Nature Value Farmland. Though the conservation status of G. simile has not been formally assessed in eastern North America, it should likely be regarded as least concern due to the abundance of the species in second-growth forests.
Distribution
Asia: China (Zhuang and Wang 1997, Zhuang 1998), India (Batra & Batra 1963, Thind & Sing 1964, Maas Geesteranus 1965, Prasher & Sharma 1997), Japan (Imai 1941); Australasia: Australia (Beaton & Weste 1979); Europe: Austria (www.gbif.org), Czech Republic (this paper), Denmark (Bille-Hansen 1954, Ohenoja 2000), Estonia (www.gbif.org), France (Priou 1992, Moingeon & Moingeon 2003), Germany (Benkert 1976, www.gbif.org), Norway (Eckblad 1963, Jørgensen & Vevle 1968, Olsen 1986), Slovakia (this paper), Sweden (Hakelier 1964, Nitare 1988, Turander 2012), Switzerland (www.gbif.org), United Kingdom (www.gbif.org); North America: Canada (Nannfeldt 1942, Mains 1954, Voitk 2013), Greenland (Petersen & Korf 1982), USA (Peck 1873, Nannfeldt 1942, Mains 1954).
Specimens examined
CANADA: Nova Scotia, Annapolis County, Melvern Square Vault, 3. IX. 1973, K.A. Harrison (ILLS 71106).
CZECH REPUBLIC: Jizerské hory Mountains, Nature Reserve “Jizerka peat bog” 500 m N from “Pešákovna” chalet, Q 5158c, 50°49'45.6" N, 15°20'12.6" E, 882 m, at the margin of peat bog with Molinia caerulea and Sphagnum sp., 25. VIII. 2012, J. Gaisler (SAV 10689), (SAV 10686), (SAV 10681), (SAV 10682). – 380 m NW from “Pod Bukovcem” chalet in Jizerka settlement, Q 5158c, 50°48'52.9" N, 15°20'59.1" E, 891 m, moist meadow with Sphagnum sp., Carex spp., Juncus spp., 25. VIII. 2012, J. Gaisler (SAV 10687). – 280 m NE from “Pyramida” chalet in Jizerka settlement, Q 5158c, 50°49'11.2" N, 15°20'58.8" E, 856 m, in peat bog with Pinus mugo and Molinia caerulea, 9. VIII. 2012, J. Gaisler (SAV 10685), (SAV 10679), (SAV 10680). – 50 m N from “Granit” chalet, Horní Černá Studnice village, Q 5257c, 50°42'38" N, 15°13'08" E, 760 m, in moist meadow with Sphagnum sp., Carex spp. and Juncus spp., 29. VII. 2009, Z. Egertová, M. Kříž s.n. (SAV 10683) – 270 m NE from “Pyramida” chalet in Jizerka settlement, in ditch near road, 50°49'10.2" N, 15°21'0.5" E, 857 m, in Sphagnum sp. and Molinia caerulea, V. Kučera, J. Gaisler, V. Kautman (SAV 10731). – 320 m SE from “Javor” chalet in Jindřichov village, Q 5257c, 50°44'47.8" N, 15°11'54.4" E, 581 m, in moist meadow with Sphagnum sp., Carex spp., Juncus spp. and Cirsium palustre, 7. VIII. 2011, J. Gaisler (SAV 10671). – Horní Maxov, 300 m E from “U náhonu” chalet and 40 m from a small pond, Q 5257a, 50°45'59.8" N, 15°11'59.5" E, 711 m, in wet meadow with Sphagnum sp., Carex spp. and Juncus spp., 7. VIII. 2011, J. Gaisler (SAV 10955).
NETHERLANDS: Gelderland, Lochem, in old meadows with Carex sp. and Juncus sp., 28. VII. 2012, C.F. Roobeek (SAV 10956).
SLOVAKIA: Laborecká vrchovina Mts., Natural Reserve Haburské rašelinisko peat bog, ca 5 km NNE from the Habura village, Q 6697a, 49°22'11" N, 21°53‘09" E, 682 m, in Sphagnum sp., 20. IX. 2006, V. Kučera (SAV 9061), (SAV 9070). – Nízke Tatry, village Liptovská Teplička, in peat bog pod Soľankou“, 48°58'44" N, 20°02'52" E, 920 m, 9. IX. 2006, V. Kučera, I. Kautmanová (SAV 9063). – Kysuce, village Raková, peat bog with Sphagnum sp., 42°29'18.59" N, 18°40'44.2" E, 677 m, 10. IX. 2009, M. Zajac (SAV 10136).
SWEDEN: Närke, Sköllersta, ca 1 km S of Hälla, drained swamp, 06. IX. 1964, N. Hakelier (UPS F-122051). – Värmland, Övre Ullerud, slightly damp pasture, 22. VIII. 1991, Bo Jansson (UPS F-599179). – Vadkärr, municipality of Varberg, pasture, in grass, 57°16'23.5'' N, 12°23'08.3'' E, 16. IX. 2012, V. Kučera (SAV 10587).
UNITED KINGDOM: England, Dunsop Valley, Forest of Bowland, wet, acid soil, associated with Poaceae and short grass, 5. X. 1997, I. Ridge (ILLS 71105).
UNITED STATES: Kansas, Sedgwick County, Derby, City Park, 18. VI. 2010, M. Melicharová (SAV 10129). – Michigan, Chippewa County, Detour, Hardwoods, 20. VIII. 1949, H.A. Imshaug (ILLS 3768). – New York, Cortland County, Kennedy State Forest, Scutt Hill Road, 10. VIII. 2003, T.J. Baroni (TJB 9613) (CORT 005220; ILLS 71160). – Hamilton County, Raquette Lake, Long Point, 21. VII. 2007, T. Galante (TG 9) (CORT 005221) – North Carolina, Macon County, Nantahala National Forest, Standing Indian Campground, 35º04’33.79” N, 83º31’42.45” W, mixed deciduous forest, 3. XIII. 2004, A.S. Methven (ASM 10528) (ILLS 67350). – Haywood County, Great Smoky Mountains National Park, Cataloochee, Rough Fork Trail, 35º37’ N, 83º7’15.8” W, 850 m, 14. VIII. 2009, R.E. Baird (ILLS 71108). – South Carolina, Ocone County, Walhalla Fish Hatchery, 34º59.155’ N, 83º04.374’ W, 14. VII. 2008, G. Presley, (ILLS 71104). – Tennessee, Blount County, Great Smoky Mountains National Park, Cades Cove, Junction of Sparks Lane and Cades Cove Loop Trail, on rotten wood, 35º36’23.5” N, 83º47’19.6” W, 533 m, 13. VIII. 2009, V.P. Hustad (ILLS 61039). – Cocke County, Great Smoky Mountains National Park, near Cosby, Madron Bald Trail 35º45’42.1” N, 83º16’15.7” W, 549-914 m, 21. X. 2009, V.P. Hustad (ILLS 71109).
Discussion
Sequences from twelve collections of Geoglossum simile from throughout Europe and North America were shown to be conspecific, occurring in a well-supported clade (100 % BS, 1.0 B.P. in our analyses. Furthermore, all sequenced collections of G. simile possess less than 3 % ITS sequence divergence across the entire ITS region (data not shown), further supporting our hypothesis that these collections are the same species (Hughes et al. 2009). Interspecific ITS variation between G. simile and G. glabrum was 10-11 % (data not shown), further casting doubt on the synonymy of these species.
We have chosen to make our typological changes in Geoglossum simile (designation of Ft. Edward syntype as the lectotype and designation of new material as the epitype for the species) for several reasons: 1) Peck’s original collection of G. simile from Sand Lake has been lost, leaving only the syntype collection by Howe from Ft. Edward as the only known extant material that had been examined by Peck and this collection is designated here as the lectotype; 2) due to the age and deteriorating condition (Fig. 2a) of the Ft. Edward lectotype collection, molecular sequence data cannot be obtained and morphological study is limited, necessitating the designation of material of later provenance as the epitype pursuant to Article 9.8 of the International Code of Nomenclature for algae, fungi, and plants (McNeill 2012); 3) the epitype collection is from New York and from similar forest as Peck’s original material; and 4) the epitype collection consists of several ascomata in good condition (Fig. 3a).
The erroneous synonymy of Geoglossum simile under G. glabrum has likely resulted in inaccurate distribution records of G. simile as the species was not recognized as separate from G. glabrum until 1941, with Seaver considering the species synonymous as late as 1951. Imai later (1962) considered G. simile to be a variety of G. glabrum and designated the species as G. glabrum var. simile, although this synonymy was not accepted in subsequent taxonomic works on Geoglossaceae (Eckblad 1963, Hakelier 1964, Maas Geesteranus 1965, Benkert 1976, Ohenoja 2000, Roobeek 2009). Imai reasoned that the characteristic barrel-shaped terminal cells of the paraphyses of G. simile were also present in G. cookeanum Nannf., G. glabrum var. americanum Mains, G. glabrum var. glabrum sensu Mains, G. glabrum var. inflatum Mains, G. glabrum var. sphagnophilum (Ehrenb.) Fr., and G. japonicum S. Imai. Imai (1962) did not see enough dissimilarity in the varieties of G. glabrum described by Mains to be significantly distinguishable from one another and held to a broad taxonomic concept of G. glabrum with eight varieties of the species.
Geoglossum glabrum and G. simile are distinctive both macroscopically and microscopically. G. glabrum is characterized by a glabrous stipe, ascospores (55-) 65-80 (-90) × 6-8 μm, and paraphyses slightly longer than the asci with short chains of adherent darkened inflated globose cells toward the tips (Imai 1941, Nannfeldt 1942, Mains 1954). Geoglossum simile has a more gracile stem roughened with acute tufts or squamules of paraphyses on the stipe (especially when dry), slightly longer ascospores ((70-) 75-85 (-100)), though the ascospores of the Ft. Edward lectotype were even longer (80-) 82-90 (-94)), and paraphyses characterized by barrel-shaped elements at the terminal cells. Lastly, the habitats of the two species are different with G. glabrum nearly always found associated with Sphagnum, whereas G. simile is found in a diversity of habitats ranging from soil, humus, rotten logs, and associated with Sphagnum.
Durand undertook the most significant study of the original syntype material collected at Sand Lake by Peck. Although he considered Peck’s material to represent Geoglossum glabrum, his illustration of the paraphyses of the Sand Lake collection (Fig. 53, Durand 1908), clearly shows the characteristic barrel-shaped terminal cells of the paraphyses in G. simile, a character that is not depicted in other illustrations of G. glabrum (or any other Geoglossum) in his monograph. Mains (1954) also examined Peck’s Sand Lake collection and reported similar measurements and morphologies to both the Ft. Edward lectotype and our designated epitype.
Other species of Geoglossum have been placed in synonymy with either G. glabrum or G. simile. G. cookeanum is morphologically similar to G. glabrum, with the major distinction being the lack of dark pigmentation at the terminal cells of the paraphyses, weaker adherence of paraphyses, and greater variation in size of the terminal cells. These characters were considered distinctive enough for Nannfeldt (1942) to separate G. cookeanum from G. glabrum, and the treatment of this taxon as a separate species was accepted in nearly every subsequent publication on Geoglossaceae (Bille-Hansen 1954, Maas Geesteranus 1964, Benkert 1976, Ohenoja 2000), with the notable exception of Mains (1954), who believed the differences to represent simple variation and placed G. cookeanum as a synonym under G. glabrum. Spooner (1987) listed G. glabrum as a synonym under G. cookeanum based on the lack of reliable type material for G. glabrum from Persoon’s collections. The validity of synonyms of G. glabrum is of taxonomic relevance and will be addressed in a future study.
Geoglossum japonicum was accepted by Imai (1962) as another variety under G. glabrum. The paraphyses of G. japonicum are more similar to G. cookeanum and differ from G. simile in having end cells which are irregularly and strongly-curved to circinate. The ascospores of G. japonicum were reported by Imai (1941) as 60-97.5 × 6-8 μm, well within the range of G. simile. Although we have not examined the type material of G. japonicum, the distinctive paraphyses of this species seem sufficient to separate this species from both G. simile and G. glabrum.
Imai (1962) also specifically mentioned three other varieties of Geoglossum glabrum (G. glabrum var. americanum, G. glabrum var. inflatum, G. glabrum var. sphagnophilum) as showing the characteristic paraphyses of G. simile. Geoglossum glabrum var. americanum is the most distinct of the three varieties with paraphyses strongly constricted at the septa and producing globose upper cells, which bear little resemblance to G. simile. Geoglossum glabrum var. inflatum has paraphyses that are rarely constricted at the terminal septum, but also rarely produce two-celled elements, a common and often noticed character in nearly all collections of G. simile.
Geoglossum glabrum var. sphagnophilum (= G. sphagnophilum) is currently regarded as a synonym of G. glabrum, based on similarities of paraphyses, ascospores, and habitats for both taxa. However, as indicated in Fig. 1, the specimen identified as G. sphagnophilum does not group with either G. glabrum or G. simile in our analyses. Our data supports the treatment of these taxa as two separate species. The validity of the name G. sphagnophilum and a phylogenetic treatment of this species and other synonyms of the G. glabrum group will be addressed in a future publication.
Confusion over the identity and taxonomical status of Geoglossum simile has resulted from the variability that occurs in its paraphyses and the inadequate amount of material that has been studied. Measurements of asci and ascospore sizes are very similar in all studied specimens, with no significant differences observed between Slovak and Czech specimens, other European material, or from the lectotype specimen and other North American material. Minor differences were observed in the shape of paraphyses and in the size of apical cells of the paraphyses, with variation occasionally noticed in different ascomata of the same collection. Thinner apical cells were observed in collections from the Czech Republic (SAV 10686) and USA (SAV 10126): 8-14 × 5-7 μm and 9-11× 5-6 μm, respectively, while thicker apical cells (7-12 × 9-10 μm) were observed in a collection from Sweden (SAV10587). In one collection from Slovakia (SAV 9063) we observed variable apical cells in the paraphyses, both thin (9-10 × 5-6 μm) and thick (10-15 × 7-10 μm), in the same ascoma. This variability in apical cell diameter appears to be normal variation within the species.
Conclusions
Our analyses indicate that all collections of G. simile we examined from Europe and North America represent a single, widespread species. We observed strong uniformity in morphological characters, which overlapped within one standard deviation of one another, among most European and North American collections. Although variation was observed in the morphology of the paraphyses, specifically the size of the apical terminal cells, there was no correlation between these characters and molecular data. Molecular evidence generated in this study further indicates that all collections of G. simile sampled are conspecific, with less than 3 % intraspecific sequence divergence observed in the ITS gene.
References
Adamčík S, Kautmanová I (2005) Hygrocybe species as indicators of natural value of grasslands in Slovakia. Catathelasma 6:25–34
Alfaro ME, Zoller S, Lutzoni F (2003) Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov Chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Molec Biol Evol 20:255–266
Arnolds E, Veerkamp M (2008) Basisrapport rode lijst paddenstoelen 2008. Nederlandse Mycologische Vereniging, Utrecht
Aron A, Kahr H, Michelitsch S, Pidlich-Aigner H, Prelicz D (2005) Vorläufige Rote Liste gefährdeter Großpilze der Steiermark. Joannea Bot 4:45–80
Batra LR, Batra SWT (1963) Indian discomycetes. Univ Kansas Sci Bull 44:109–256
Beaton G, Weste G (1979) A check list of discomycetes (fungi) collected in Victoria. Victorian Naturalist 96:245–249
Benkert D (1976) Bemerkenswerte Ascomyceten der DDR II. Die Gattungen Geoglossum und Trichoglossum in der DDR. Mykol Mitteilungsbl 20(3):47–92
Bille-Hansen E (1954) The Danish species of Geoglossum and related genera. Bot Tidsskr 5(l):7–18
Castresana J (2000) Selection of conserved blocks from multiple alignments and their use in phylogenetic analysis. Molec Biol Evol 17:540–552
Cooke MC (1871) Handbook of British fungi: With full descriptions of all the species and illustrations of the genera, vol 2. Macmillan and Co., London
Durand EJ (1908) The Geoglossaceae of North America. Ann Mycol 6:387–477
Eckblad FE (1963) Contributions to the Geoglossaceae of Norway. Nytt Mag Bot 10:137–158
Edgar R (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res 32:1792–1797
Eernisse DJ, Kluge AG (1993) Taxonomic congruence versus total evidence, and amniote phylogeny inferred from fossils, molecules, and morphology. Molec Biol Evol 10:1170–1195
Fandnes P (2011) Supplerende kartlegging av kulturlandskap i Sunnhordland 2009-2010. Oppdatering og status. HSH Report 2011/2. Høgskolen Stord, Stord
Felsenstein J (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783–791
Galtier N, Guouy M, Goutier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Compu Appl Biosci 12:543–548
Gärdenfors U (2010) Rödlistade arter i Sverige 2010. Artdatabanken SLU, Uppsala
Genney DR, Hale AD, Woods RG, Wright M (2009) Grassland fungi. In: Anonymous (ed) Guidelines for selection of SSSIs. Rationale, Operational Approach and Criteria. Joint Nature Conservation Committee, Peterborough, UK, Chapter 18a
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:695–705
Hakelier N (1964) Bidrag til Sveriges svampflora. II. Svensk Bot Tidskr 58:387–343
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hughes KW, Petersen RH, Lickey EB (2009) Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species delimitation across basidiomycete fungi. New Phytol 182:279–282
Hustad VP, Miller AN, Moingeon J-M, Priou J-P (2011) Inclusion of Nothomitra in Geoglossomycetes. Mycosphere 2(6):646–654
Hustad VP, Miller AN, Dentinger BTM, Cannon PF (2013) Generic circumscriptions in Geoglossomycetes. Persoonia 31:100–111
Imai S (1941) Geoglossaceae Japoniae. J Fac Agric Hokkaido Univ 45:155–264
Imai S (1962) Fungi of the Geoglossum glabrum group. Trans Mycol Soc Japan 3:51–52
Ing B (1992) A provisional Red Data List of British fungi. Mycologist 6:124–128
Jordal JB (1997) Sopp i naturbeitemarker i Norge. En kunnskapsstatus over utbredelse, økologi, indikatorverdi og trusler i et europeisk perspektiv. Direktoratet for naturforvaltning, Trondheim
Jørgensen PM, Vevle O (1968) Noen Geoglossum-funn fra Vestlandet. Some Geoglossum-records from western Norway. Blyttia 26:72–74
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Dictionary of the Fungi, 10th edn. CAB International, Wallingford
Kluge AG (1989) A concern for evidence and a phylogenetic hypothesis of relationships among Epicrates (Boidae, Serpentes). Syst Biol 38:7–25
Kučera V (2012) Geoglossaceous fungi in Slovakia IV. Geoglossum alveolatum, a new species for the country. Catathelasma 14:11–14
Kučera V, Gaisler J (2012) First record of Geoglossum uliginosum (Ascomycota, Geoglossales) in the Czech Republic. Czech Mycol 64:135–140
Kučera V, Lizoň P (2012) Geoglossaceous fungi in Slovakia III. The genus Geoglossum. Biologia 67:654–658
Kučera V, Lizoň P, Kautmanová I (2008) Geoglossaceous fungi in Slovakia: rare and new taxa for the territory. Biologia 63:482–486
Kučera V, Lizoň P, Kautmanová I (2010) Geoglossoid fungi in Slovakia II. Trichoglossum octopartitum, a new species for the country. Czech Mycol 62:13–18
Kučera V, Nitare J, Lizoň P, Gaisler J (2013) Geoglossaceous fungi in Slovakia V. Geoglossum uliginosum taxonomy and nomenclature. Mycotaxon 124:111–115
Landvik S (1996) Neolecta, a fruit-body producing genus of the basal Ascomycetes, as shown by SSU and LSU rDNA sequences. Mycol Res 100:199–202
Lloyd CG (1916) Mycological Writings 5: The Geoglossaceae. Lloyd, Cincinnati
Lutzoni F, Kauff F, Cox C, McLaughlin D, Celio G et al (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Amer J Bot 91:1446–1480
Maas Geesteranus RA (1964) De fungi van Nederland I. Geoglossaceae – aardtongen. Wentensch Meded Koninkl Nederl Naturhist Verenig 52:1–24
Maas Geesteranus RA (1965) Geoglossaceae of India and adjacent countries. Persoonia 3:81–96
Mackey E, Blake D, McSorley C (2011) Farmland biodiversity: Mapping high value farmland in Scotland. Scottish Natural Heritage, http://www.scotland.gov.uk/Resource/Doc/355629/0120136.pdf
Mains EB (1954) North American species of Geoglossum and Trichoglossum. Mycologia 46:586–631
Massee G (1897) A monograph of the Geoglosseae. Ann Bot (Oxford) 11:225–306
McHugh R, Mitchel D, Wright M, Anderson R (2001) The fungi of Irish grasslands and their value for nature conservation. Biol Environm, Proc Roy Irish Acad 101B:225–242
McNeill J (2012) International code of nomenclature for algae, fungi and plants (Melbourne code): adopted by the Eighteenth International Botanical Congress Melbourne Australia, July 2011. Koeltz Scientific Books, Königstein
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov 2010, New Orleans
Moingeon S, Moingeon JM (2003) Les Geoglossaceae en Franche-Comte. Bull Soc Hist Nat Doubs 89:61–66
Nannfeldt JA (1942) The Geoglossaceae of Sweden. Ark Bot 30A:1–67
Newton AC, Davy LM, Holden E, Silverside A, Watling R, Ward SD (2003) Status, distribution and definition of mycologically important grasslands in Scotland. Biol Conserv 111:11–23
Nitare J (1988) Jordtungor, en svampgrupp på tillbakagång i naturliga fodermarker. Svensk Bot Tidskr 82:341–368
Nitare J (2007) Åtgärdsprogram för sumpjordtunga 2007-2011, Geoglossum uliginosum. Naturvårdsverket, Stockholm
Ohenoja E (2000) Geoglossaceae. In: Hansen L, Knudsen H (eds) Nordic Macromycetes, vol 1. Nordsvamp, Copenhagen, pp 177–183
Olsen S (1986) Jordtunger i Norge. Agarica 7(14):120–168
Omar MB, Bolland L, Heather WA (1979) A permanent mounting medium for fungi. Bull Brit Mycol Soc 13:31–32
Peck CH (1873) Descriptions of new species of fungi. Bull Buffalo Nat Hist Soc 1(2):41–72
Peck CH (1878) 29th Report of the botanist. Report (Annual) New York State Mus Nat Hist 29:53–54
Petersen PM, Korf RP (1982) Some inoperculate discomycetes and plectomycetes from West Greenland. Nordic J Bot 2:151–154
Posada D (2008) jModelTest: Phylogenetic model averaging. Molec Biol Evol 25:1253–1256
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaiki Information Criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Prasher IB, Sharma R (1997) Geoglossum Pers. Geoglossaceae, Leotiales in Eastern Himalayas. In: Chahal SS, Prashar IB, Randhawa HS, Arya S (eds) Achievements and prospects in mycology and plant pathology. International Book Distributors, Dehra Dun, pp 12–19
Priou JP (1992) Contribution aux Geoglossum de France. Cah Mycol Nantais 4:5–9
Promputtha I, Miller AN (2010) Three new species of Acanthostigma (Tubeufiaceae, Dothidiomycetes) from the Great Smoky Mountains National Park. Mycologia 102:574–587
Rald E (1985) Voxhatte som indikatorarter for mykologisk vardifulde overdrevslokaliteter. Svampe 11:1–9
Rambaut A, Drummond AJ (2009) Tracer v.1.5. http://tree.bio.ed.ac.uk/software/tracer. Accessed 5 December 2013
Roobeek CF (2009) Earth tongues in Northern Kennemerland. Roobeek, Bergen
Schoch CL, Wang Z, Townsend JP, Spatafora JW (2009) Geoglossomycetes cl. nov., Geoglossales ord. nov., and taxa above class rank in the Ascomycota tree of life. Persoonia 22:129–138
Seaver FJ (1951) The North American Cup-fungi (Inoperculates). Hafner, New York
Sonneborn I, Sonneborn W, Siepe K (2005) Rote Liste der gefährdeten Großpilze (Makromyzeten) in Nordrhein-Westfalen. In: Loepf H (ed) Rote Liste der gefährdeten Pflanzen und Tiere in Nordhein-Westfalen. Landesanstalt für Ökologie, Recklinghausen, pp 259–294
Spooner BM (1987) Helotiales of Australasia: Geoglossaceae, Orbiliaceae, Sclerotiniaceae, Hyaloscyphaceae. Bibl Mycol 116:1–711
Stoltze M, Pihl S (1998) Rødliste 1997 over planter og dyr i Danmark. Danmarks Miljøundersøgelser og Skov- og Naturstyrelsen, Aarhus
Thiers B (continuously updated) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/. Accessed 31 October 2013
Thind KS, Sing H (1964) The Helotiales of India III. J Indian Bot Soc 43:529–543
Turander F (2012) Sumpjordtunga (Geoglossum uliginosum) och andra jordtungor på Malmbackarna i Värmland. Svensk Mykol Tidskr 33(2):16–20
Vilgalys R, Hester M (1990) Rapid identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Voitk A (2013) Earth tongues of Newfoundland and Labrador. Omphalina 4(5):14–19
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocol: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wiens JJ (1998) Combining data sets with different phylogenetic histories. Syst Biol 47:568–581
Zhuang W (1998) Geoglossaceae. Fl Fung Sinic 8:78–127
Zhuang W, Wang Z (1997) Some new species and new records of discomycetes in China, 7. Mycotaxon 63:307–321
Acknowledgments
The authors wish to thank the following for graciously providing specimens for study and analysis: RE Baird, AS Methven, I Ridge, CF Roobeek, and the fungaria ILLS, MICH, NYS, and SAV for the loan of specimens and assistance. Lastly, we acknowledge the following sources of funding: American Society of Plant Taxonomists, Discover Life in America, Mycological Society of America, Slovak Academy of Sciences (grants VEGA 2/0150/12 and VEGA 2/0088/13), and a grant from the Czech Republic Ministry of Agriculture (MACR 0002700604).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hustad, V.P., Kučera, V., Rybáriková, N. et al. Geoglossum simile of North America and Europe: distribution of a widespread earth tongue species and designation of an epitype. Mycol Progress 13, 857–866 (2014). https://doi.org/10.1007/s11557-014-0969-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-014-0969-z