Abstract
During an arbuscular mycorrhiza fungal spore survey on a primary coastal sand-dune system in Goa on the west coast of India, entrophosporoid spores tightly covered with a dense hyphal mantle were recovered. When intact, the spores, at first sight, seemed to be identical in morphology to those of Sacculospora baltica (originally described as Entrophospora baltica) extracted from Polish maritime sand dunes and, to date, the sole member of the recently described genus Sacculospora in the new family Sacculosporaceae, phylum Glomeromycota. Later detailed morphological studies indicated that both fungi produce two-walled spores but the structure and phenotypic features of components of the outer spore wall in the novel fungus differ considerably from those of S. baltica. Differences between the fungi were subsequently confirmed in the phylogenetic analysis of SSU–ITS–LSU nrDNA sequences. Consequently, we describe the novel species as Sacculospora felinovii sp. nov.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the ca. 280 described arbuscular mycorrhizal fungal (AMF) species of the phylum Glomeromycota C. Walker & A. Schüßler, only five form spores inside the neck of a sporiferous saccule (Błaszkowski 2012). From one side, the neck is continuous with a mycorrhizal extraradical hypha and from the other side, the neck passes into a globose to ellipsoidal saccule. Contents from both structures are used in spore genesis. In fully developed specimens, the saccule is usually empty and frequently detached from spores and the neck wall is continuous with the outermost spore wall layer, forming the spore surface (Błaszkowski 2012). This mode of spore synthesis was first described by Ames and Schneider (1979) in a species originally named Glomus infrequens I.R. Hall (Hall 1977). Ames and Schneider (1979) subsequently transferred G. infrequens to a newly erected genus Entrophospora R.N. Ames & R.W. Schneid., with E. infrequens (I.R. Hall) R.N. Ames & R.W. Schneid. as type species. The other four species accommodated in Entrophospora were E. baltica Błaszk., Madej & Tadych, E. colombiana Spain & N.C. Schenck, E. kentinensis C.G. Wu & Y.S. Liu and E. schenckii Sieverd. & S. Toro (Schenck et al. 1984; Sieverding and Toro 1987; Wu et al. 1995; Błaszkowski et al. 1998). Such a mode of spore formation and the spores themselves have been named entrophosporoid (Goto and Maia 2006).
From observed differences in the subcellular structure of spores and the phenotypic and histochemical characters of components of spore walls of Entrophospora species, Sieverding and Oehl (2006) transferred three of the species to two newly erected genera, Intraspora Oehl & Sieverd., with I. schenckii (Sieverd. & S. Toro) Oehl & Sieverd. (formerly E. schenckii) in the family Archaeosporaceae J.B. Morton & D. Redecker, and Kuklospora Oehl & Sieverd., with K. colombiana (Spain & N.C. Schenck) Oehl & Sieverd. (formerly E. colombiana) and K. kentinensis (C.G. Wu & Y.S. Liu) Oehl & Sieverd. (formerly E. kentinensis) in the family Acaulosporaceae J.B. Morton & Benny. Kaonongbua et al. (2010) and Schüßler and Walker (2010) rejected Kuklospora and Intraspora, respectively, later substantiated by the phylogenetic analyses of Krüger et al. (2012). Oehl et al. (2011) concluded from phylogenetic analyses of nrDNA sequences that E. infrequens and E. baltica differ at the family level and, therefore, erected Sacculosporaceae Oehl et al. fam. nov., with Sacculospora Oehl et al. gen. nov. and S. baltica (Błaszk., Madej & Tadych) Oehl et al. comb. nov.
To date, the genus Sacculospora has been represented by S. baltica alone. Morphologically, S. baltica differs clearly from all other known species forming entrophosporoid spores. The most conspicuous structure is the hyphal mantle tightly covering spores. The spore subcellular structure, which is complex and difficult to define, further distinguishes the species. According to Błaszkowski et al. (1998), S. baltica spores contain two spore walls: an outer wall with four layers and an inner three-layered wall. Oehl et al. (2011) stated that layer 1 of the inner wall sensu Błaszkowski et al. (1998) clearly separates from the other layers of the wall and, therefore, redefined the subcellular structure of S. baltica spores as three-walled. However, neither Błaszkowski et al. (1998) nor Oehl et al. (2011) performed ontogenetic studies of the species, characterising the subcellular structure of S. baltica spores based only on the spatial grouping of its components in crushed specimens. Importantly, the spatial arrangement of spore subcellular components in AMF may be influenced by the vigour of spore crushing (Błaszkowski, pers. observ.). Study of the ontogeny of S. baltica spores is severely hindered for two reasons. First, the fungus is difficult to grow in culture. In the literature, there is no report that the species has been grown in single-species cultures and numerous efforts to obtain such cultures by J. Błaszkowski failed (Błaszkowski et al. 1998; Błaszkowski 2012). The ontogeny of any AMF may be known with certainty only by the examination of specimens originating from single-species cultures. Second, the disclosure of sequences of differentiation and organisation of the spore subcellular components may be difficult, or impossible, because of the obscuring influence of the dense and coloured hyphal mantle.
Whilst investigating AMF spore density and diversity on an interrupted belt transect across a primary dune system on the coast of Goa, India (73°40′33″ E, 14°53′54″ N), AMF spore specimens were found that seemed identical to those of S. baltica. Detailed morphological studies of the spore subcellular structure, however, and the phenotypic and histochemical characters of components of spore walls of the specimens, indicated that traits in the outer spore wall differed. Unsure if the differences represented intraspecific variability of one organism, a comparison was made using molecular methods. The molecular analyses confirmed the previous conclusions resulting from morphological observations that the novel fungal species differs considerably from S. baltica. Consequently, the fungus is described as Sacculospora felinovii sp. nov.
Materials and methods
Extraction of spores, establishment and growth of trap and single-species cultures
Spores were extracted by the wet-sieving and decanting method (Gerdemann and Nicolson 1963) from field-collected samples of rhizosphere soil of the perennial C4 Zoysia matrella (L.) Merr. (Poaceae) and incorporated into pot trap cultures, 12 cm diam., 9 cm high (1018 cm3). The pots were maintained outdoors at temperatures of 18–30 °C for 10 months at Goa University. Host plants were Z. matrella, rhizomes transferred from the dunes, along with stem cuttings of Solenostemon scutellarioides (L.) R.Br. (Lamiaceae), cultured in unsterilised Z. matrella rhizosphere soil. Plants were watered 2–3 times a week. No nutrients were applied during the growing period. Subsequently, spores (ca. 200) extracted from the trap culture were placed in pots at the base of holes into which stem cuttings of S. scutellarioides were inserted. The medium was sterilised (180 °C for 12 h × 3) dune sand. The plants were raised in a ‘closed’ greenhouse (i.e. no ventilation, reducing risk of outside contamination) at Goa University. Hoaglands solution minus P was applied fortnightly. After 8 months, single-species spore cultures were achieved and harvested. This two-stage method follows that of Dr. B. F. Rodrigues, Goa University (unpublished), demonstrating successful single-species culture where, all too often, novel species culture fails. Nevertheless, only one of two initial cultures sporulated and only one of two subsequent cultures has sporulated but in less abundance. The morphological and histochemical features of mycorrhizal structures revealed following staining in Trypan blue (Phillips and Hayman 1970) testified that they represent one species of AMF. In addition, in the cultures, no other spore morphotypes were found among the characteristic spores of the new species.
Microscopy and nomenclature
The morphological features of spores and the phenotypic and histochemical characters of spore wall layers were determined after examination of at least 100 spores mounted in water, lactic acid, polyvinyl alcohol/lactic acid/glycerol (PVLG; Omar et al. 1979) and a mixture of PVLG and Melzer’s reagent (1:1, v/v). The preparation of spores and mycorrhizal structures for study and photography is described by Błaszkowski (2012) and Błaszkowski et al. (2012). Descriptions of spore wall layers follow definitions from Błaszkowski (2012), Stürmer and Morton (1997) and Walker (1983). Colour names are from Kornerup and Wanscher (1983). Nomenclature of fungi and the authors of fungal names are from the Index Fungorum website http://www.indexfungorum.org/AuthorsOfFungalNames.htm. Voucher specimens were mounted in PVLG and a mixture of PVLG and Melzer’s reagent (1:1, v/v) on slides and deposited at ETH Zurich, Switzerland (Z + ZT; holotype), the Botany Department, Goa University, the Department of Ecology, Protection and Shaping of Environment (DEPSE), West Pomeranian University of Technology, Szczecin, and in the herbarium at Oregon State University (OSC) in Corvallis, Oregon, USA (isotypes).
DNA extraction, polymerase chain reaction, cloning and DNA sequencing
Crude DNA was extracted from eight single spores. Procedures prior to polymerase chain reactions (PCRs), the conditions and primers used in the PCRs to obtain SSU–ITS–LSU nrDNA sequences, and cloning and sequencing were as those described in Błaszkowski et al. (2013). The sequences were deposited in GenBank (KX345938–KX345943).
Sequence alignment and phylogenetic analyses
Comparisons made between sequences of the novel fungus and those listed after BLAST enquiries indicated that the species belongs in the Glomeromycota. The closest relatives are firstly S. baltica, and then a number of Acaulospora spp. and Pacispora scintillans (S.L. Rose & Trappe) Sieverd. & Oehl. A set was, therefore, established comprising six sequences each of the new species and S. baltica and three to four sequences each of seven Acaulospora spp., including two forming entrophosporoid spores, i.e. A. colombiana and A. kentinensis. Pacispora franciscana Sieverd. & Oehl and P. scintillans served as the outgroup taxa. The sequences covered the SSU–ITS–LSU nrDNA segment in all species except for two LSU sequences of A. colombiana, two SSU sequences of P. franciscana and one SSU sequence of P. scintillans. The sequences were aligned and analysed phylogenetically by the method described and justified by Błaszkowski et al. (2015a, b). In Bayesian (BI) phylogenetic analysis, GTR + G and two-parameter Markov (Mk2 Lewis) models were applied for the established nucleotide partitions and indel matrices, respectively. GTR + G was estimated as the best-fit model by Modeltest 3.7 (Posada and Buckley 2004). Maximum likelihood (ML) analysis was carried out with the raxmlGUI (Silvestro and Michalak 2012) implementation of RAxML (Stamatakis 2006), with GTRGAMMA for DNA and the default set for binary (indel) characters. The generated phylogenetic trees were visualised and edited in MEGA6 (Tamura et al. 2013).
Results
General data and phylogeny
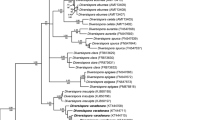
The established set of SSU–ITS–LSU nrDNA sequences, including those of the putative new species, had a length of 1929 characters, 100 % of which were informative. The six PCR-generated new-species sequences showed only 1 % variability. Bayesian and ML phylogenetic analyses of the sequence set fully supported the morphological indication that the fungus is a glomeromycotean species not previously described, and the closest relative is S. baltica (Fig. 1). The clade with the new species described below as S. felinovii and the clade with S. baltica, as well as the node linking them, received full (1.0 and 100 %) supports. The node linking the Sacculosporaceae and the Acaulosporaceae clades was also fully supported in both analyses. The trees generated following the BI and ML analyses had identical topologies.
50 % majority rule consensus phylogram inferred from a Bayesian analysis of SSU–ITS–LSU rDNA sequences of Sacculospora felinovii among seven known Acaulospora spp. and S. baltica. Pacispora spp. served as the outgroup. Sequences of S. felinovii are in bold and are followed by their GenBank accession numbers. The Bayesian posterior probabilities ≥0.50 and ML bootstrap values ≥50 %, respectively, are shown near the branches. The bar indicates 0.05 expected change per site per branch
Taxonomy
Sacculospora felinovii Willis, Błaszk., T. Prabhu, Chwat, Góralska, Sashidhar, Harris, J. D’Souza, Vaingankar & Adholeya, sp. nov. Figs. 2 and 3
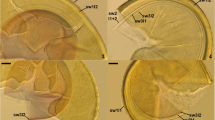
Sacculospora felinovii spores. a Intact spores (sp) with sporiferous saccule (ss) and hyphal mantle (hm). b–d Spore wall 1 layers (sw1l) 1–3, spore wall 2 layers (sw2l) 1–3 and hyphal mantle (hm). e Warts ornamenting the upper surface of spore wall 1 layer 1 (sw1l1) seen in plan view. f Sporiferous saccule wall layers (sswl) 2 and 3 and hole (h) connecting the interior of the spore with the interior of the sporiferous saccule. g Sporiferous saccule wall layers (sswl) 1–3. h Hyphae of the hyphal mantle seen in plan view. A Spores in lactic acid. B, C, E–H Spores in PVLG. D Spores in PVLG + Melzer’s reagent. A–H Differential interference microscopy. Bars: A = 50 μm, B–H = 10 μm
a, b Mycorrhizal structures of Sacculospora felinovii in roots of Solenostemon scutellarioides stained in 0.1 % Trypan blue: arbuscule (a) with trunk (t) and intraradical hyphae (ih). Sacculospora baltica spores: c Spore wall 1 layers (sw1l) 1–4, spore wall 2 layers (sw2l) 1–3 and hyphal mantle (hm). d, e Spore wall 1 layers (sw1l) 1–4, spore wall 2 (sw2) and hyphal mantle (hm). f Spore wall 1 layer 2 (sw1l2) and spore wall 2 layers (sw2l) 1–3. A–F in PVLG, differential interference microscopy, bars = 10 μm
MycoBank No. MB 817258
Holotype: ZT Myc 55704 (Z + ZT), isotypes: 3517–3530 (DEPSE), and OSC 154006, OSC 154007 (OSC).
The holotype and isotype spore specimens come from single-species culture with S. scutellarioides as host plant grown at Goa University, India. The spores were collected on 14/04/2015 by Ms. Prabhu.
Etymology
The specific epithet is respectfully given in honour of Prof. Bernard Felinov Rodrigues, Botany Department, Goa University, India, who has dedicated more than 25 years to the study of AMF.
Sporocarps unknown. Spores are entrophosporoid arising inside the neck of a sporiferous saccule, occurring singly in soil, covered with (less often the saccules) a hyphal mantle (Fig. 2a); pale yellow (4A3) to greyish orange (5B5); globose to subglobose (100–)171(−230) μm diam; rarely ovoid (140–170 × 190–210 μm). Mantle hyaline to brownish yellow (5C8), 15–50 μm thick when seen in cross view, composed of hyaline to pale yellow (4A3) tightly interwoven hyphae; hyphae straight or twisted with numerous branches inclined at different angles, 4.0–8.0 μm wide, walls 1.3–2.3 μm thick (Fig. 2a–c, h). Spore wall structure composed of two walls, spore walls 1 and 2 (Figs. 2 and 3). Spore wall 1 (Fig. 2b–d) consists of three layers (spore wall 1 layers 1–3). Layer 1 permanent, flexible to semi-flexible, pale yellow (4A3) to pastel yellow (4A4), (1.3–)1.5(−1.8) μm thick, the outer surface ornamented with evenly distributed warts, 1.0–4.0 μm wide at the base, 1.8–3.3 μm high; layer 1 loosely associated with layer 2, frequently easily separating from it in crushed spores (Fig. 2b–e). Layer 2 uniform (not divided into visible sublayers), semi-flexible, smooth, (0.8–)1.1(−1.5) μm thick, rarely separating from layer 3 in even vigorously crushed spores (Fig. 2b–d). Layer 3 permanent, uniform, rigid, sometimes cracking in vigorously crushed spores, (1.5–)2.6(−4.0) μm thick, edges almost black in polarised light (Fig. 2b–d). Spore wall 2 (Fig. 2b–d) composed of three uniform, smooth, hyaline layers (spore wall 2 layers 1–3). Layer 1 flexible to semi-flexible, (1.0–)1.3(−1.5) μm thick, loosely associated with layer 2 in intact spores, always separating from it in crushed spores (Fig. 2b–d). Layer 2 laminate, coriaceous, (1.0–)3.5(−5.8) μm thick (Fig. 2). Layer 3 flexible, (1.0–)1.1(−1.3) μm thick, always tightly adherent to the lower surface of layer 2 in intact spores, frequently separating from it in crushed spores (Fig. 2b–d). Sporiferous saccule hyaline to light yellow (4A4); egg-shaped; 104–116 × 123–148 μm; connecting with a spore by a short stalk (Fig. 2a, f, g). Sporiferous saccule wall consists of three layers (sporiferous saccule wall layers 1–3; Fig. 2f, g), of which layer 1 is continuous with the coloured and ornamented spore wall 1 layer 1 and is present only in the first, short, up to 12.5 μm long, part of the sporiferous saccule wall (Fig. 2g), and layers 2 and 3 are probably continuous with spore wall 1 layers 2 and 3; the connection is invisible as it is tightly covered by the hyphal mantle. Layer 2 is hyaline, 1.8–3.5 μm thick at the spore, gradually reducing to no more than ca. 1.0 μm thick (Fig. 2f, g). Layer 3 is pale yellow (3A3) to brownish yellow (5C7), 2.8–4.0 μm thick at the spore, gradually becoming colourless and reducing to ca. 1.0 μm thick distally (Fig. 2f, g). Stalk cylindrical to funnel-shaped, 10.5–25.0 μm wide, surrounding a circular hole, 10.0–22.5 diam. when seen in cross view (Fig. 2f, g); hole closed by a hyphal plug, 4.3–7.8 μm thick (Fig. 2g). It is likely the plug is formed in the final stage of spore development. Connection to mycelium was not visible. Germination shield not observed to date. Spores and hyphal mantles do not react in Melzer’s reagent (Fig. 2d).
Mycorrhizal associations
In single-species cultures with S. scutellarioides as host plant, S. felinovii formed mycorrhiza with arbuscules and intra- and extraradical hyphae (Fig. 3a, b). No vesicles were found. All the structures stained very faintly in 0.1 % Trypan blue and, hence, were difficult to visualise but there probably was a patchy distribution along the examined root fragments.
Distribution and habitat
In the field, S. felinovii was likely associated with roots of Z. matrella. No molecular analysis on DNA extracted from roots was performed to confirm this supposition however. Spores were most abundant (ca. 35 ± 7 100 g−1 soil) in the regions of the transect where Z. matrella was the dominant species, 101–138 m from the strand. BLAST searches revealed no sequence with similarity ≥97 % to SSU–ITS–LSU sequences of S. felinovii.
Discussion
Sacculospora baltica has, so far, been the only member of the recently newly erected monospecific genus Sacculospora and the family Sacculosporaceae (Oehl et al. 2011). Intact S. felinovii entrophosporoid spores, always tightly surrounded by a dense hyphal mantle (Fig. 2a–c, h), are indistinguishable from those of S. baltica (Błaszkowski et al. 1998; Błaszkowski 2012). However, the unique feature of S. felinovii is its spore wall 1 layer 3 that has birefringent properties in polarised light, where the edges of the wall, despite being colourless, turn almost black (Fig. 2b–d). Further, it is comparatively thick, fragile and is covered with a thin layer (spore wall 1 layer 2). This layer rarely separates from the upper surface of spore wall 1 layer 3 in even vigorously crushed spores (Fig. 2b–d).
Morphological differences clearly separating S. felinovii and S. baltica reside in the structure and phenotypic features of spore wall 1. First, spore wall 1 of S. felinovii consists of three layers (Fig. 2b–d; four layers in S. baltica, Fig. 3c–e), lacking the evanescent hyaline spore wall 1 layer 1 of S. baltica (Błaszkowski et al. 1998; Błaszkowski 2012). Secondly, spore wall 1 layer 1 of S. felinovii (Fig. 2b, c, e), where in S. baltica it is spore wall 1 layer 2 (Fig. 3c–f), is permanent and similarly coloured, and on the upper surfaces ornamented with warts. The warts in S. felinovii are 3–4-fold higher and, hence, more visible. Thirdly, in S. felinovii, the main structural layer of spore wall 1 is the thickest innermost layer 3 that is covered with a thin layer 2 (Fig. 2b–d). In S. baltica, layer 3 is also the thickest but, in comparison, it is 1.2–1.5-fold thinner than spore wall 1 layer 3 of S. felinovii and covers a thin innermost layer 4 (Fig. 3c–e). Finally, in polarised light, none of the spore wall 1 layers of S. baltica show the birefringent properties of spore wall 1 layer 3 of S. felinovii.
Further differing structures are spore wall 2 layer 2 and hyphae of the hyphal mantle. Spore wall 2 layer 2 of S. felinovii is 1.3–1.8-fold thicker than that of S. baltica. Hyphae of the hyphal mantle in both species are similar in size and colour, but those of S. felinovii are usually straight and have numerous branches of different inclinations relative to the parent hypha (Fig. 2h), whereas those of S. baltica are generally sinuous (Błaszkowski et al. 1998; Błaszkowski 2012).
The birefringent property of spore wall 1 layer 3 in polarised light is the most important differential feature. Of members of the Glomeromycota, this property has, so far, been found only in Ambispora appendicula (Spain, Sieverd. & N.C. Schenck) C. Walker, Am. fennica C. Walker, Vestberg & A. Schüßler, Am. gerdemannii (S.L. Rose, B.A. Daniels & Trappe) C. Walker, Vestberg & A. Schüßler and Am. granatensis Palenz., N. Ferrol & Oehl of the order Archaeosporales C. Walker & A. Schüßler (Spain et al. 2006; Walker et al. 2007; Palenzuela et al. 2011; Błaszkowski 2012). However, Ambispora spp. produce acaulosporoid spores, i.e. laterally from the sporiferous saccule neck, whereas S. felinovii produces entrophosporoid spores (Fig. 2a, f, g). Furthermore, Ambispora spp. are genetically very distant from the Diversisporales C. Walker & A. Schüßler, to which S. felinovii belongs.
Finally, the uniqueness of S. felinovii is shown in the phylogenetic analyses of SSU–ITS–LSU nrDNA sequences (Fig. 1). The SSU–ITS–LSU nrDNA sequences of S. felinovii differed by an average of 10.1 % from those of S. baltica.
The mycorrhizal structures of S. felinovii grown in single-species culture stained exceptionally faintly (Fig. 3a, b) and many may not have stained at all in 0.1 % Trypan blue. Unfortunately, comparison cannot be made with S. baltica where, as mentioned above, pure culture has not been attained. Therefore, currently, it is possible to only conjecture that the faint or no staining is a family-level synapomorphy, as is likely in members of Ambisporaceae, Archaeosporaceae and Paraglomeraceae (Morton and Redecker 2001; Spain et al. 2006; http://invam.wvu.edu/).
The literature and our observations, and the lack in public databases of uncultured AMF sequences with a similarity of ≤97 % to those of S. baltica and S. felinovii, suggest that these species occur rarely in the world. However, it is likely they are widely distributed on the Earth and are adapted to different soil and climatic conditions, despite S. felinovii being, so far, identified only in one dune site of the west coast of India. In Poland, S. baltica was found in dunes of the Baltic Sea but also in inland dunes of the Błędowska Desert and in the Tatra Mountains, ca. 600 and 700 km away from the sea, respectively (Tadych and Błaszkowski 2000; Błaszkowski et al. 2002; Zubek et al. 2008; Błaszkowski 2012). In addition, S. baltica was recorded at several Alpine elevations in Switzerland and in forest ecosystems near Valdina in southern Chile (Sieverding and Oehl 2006; Oehl et al. 2011). The rare records of both fungi may also result from there being few mycologists dealing with the morphology of AMF and weaknesses of molecular methods applied in recognition of intraradical AMF (Oehl et al. 2010; Wetzel et al. 2014).
References
Ames RN, Schneider RW (1979) Entrophospora, a new genus in the Endogonaceae. Mycotaxon 8:347–352
Błaszkowski J (2012) Glomeromycota. W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków
Błaszkowski J, Madej T, Tadych M (1998) Entrophospora baltica sp. nov. and Glomus fuegianum, two species in the Glomales from Poland. Mycotaxon 68:165–184
Błaszkowski J, Tadych M, Madej T (2002) Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of the Błędowska Desert, Poland. Acta Soc Bot Pol 71:71–85. doi:10.5586/asbp.2002.008
Błaszkowski J, Kovács GM, Gáspár BK, Balázs TK, Buscot F, Ryszka P (2012) The arbuscular mycorrhizal Paraglomus majewskii sp. nov. represents a new distinct basal lineage in Paraglomeraceae (Glomeromycota). Mycologia 104:148–156. doi:10.3852/10-430
Błaszkowski J, Chwat G, Kovács GM, Gáspár BK, Ryszka P, Orłowska E, Pagano MC, Araújo FS, Wubet T, Buscot F (2013) Septoglomus fuscum and S. furcatum, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycologia 105:670–680. doi:10.3852/12-127
Błaszkowski J, Chwat G, Góralska A (2015a) Acaulospora ignota and Claroideoglomus hanlinii, two new species of arbuscular mycorrhizal fungi (Glomeromycota) from Brazil and Cuba. Mycol Prog 14:18. doi:10.1007/s11557-015-1042-2
Błaszkowski J, Chwat G, Góralska A, Ryszka P, Kovács GM (2015b) Two new genera, Dominikia and Kamienskia, and D. disticha sp. nov. in Glomeromycota. Nova Hedwigia 100:225–238. doi:10.1127/nova_hedwigia/2014/0216
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Brit Mycol Soc 46:235–244
Goto BT, Maia LC (2006) Glomerospores: a new denomination for the spores of Glomeromycota, a group molecularly distinct from the Zygomycota. Mycotaxon 96:29–132
Hall IR (1977) Species and mycorrhizal infections of New Zealand Endogonaceae. Trans Br Mycol Soc 68:341–356. doi:10.1016/S0007-1536(77)80186-1
Kaonongbua W, Morton JB, Bever JD (2010) Taxonomic revision transferring species in Kuklospora to Acaulospora (Glomeromycota) and a description of Acaulospora colliculosa sp. nov. from field collected spores. Mycologia 102(6):1497–1509. doi:10.3852/10-011
Kornerup A, Wanscher JH (1983) Methuen handbook of colour, 3rd edn. Eyre Methuen, London
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984. doi:10.1111/j.1469-8137.2011.03962.x
Morton JB, Redecker D (2001) Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93:181–195. doi:10.2307/3761615
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738. doi:10.1016/j.soilbio.2010.01.006
Oehl F, da Silva GA, Sánchez-Castro I, Goto BT, Maia LC, Vieira HEE, Barea J-M, Sieverding E, Palenzuela J (2011) Revision of Glomeromycetes with entrophosporoid and glomoid spore formation with three new genera. Mycotaxon 117:297–316. doi:10.5248/117.297
Omar MB, Bolland L, Heather WA (1979) A permanent mounting medium for fungi. Bull Br Mycol Soc 13:31–32. doi:10.1016/S0007-1528(79)80038-3
Palenzuela J, Barea JM, Ferrol N, Oehl F (2011) Ambispora granatensis, a new arbuscular mycorrhizal fungus, associated with Asparagus officinalis in Andalucía (Spain). Mycologia 103:333–340. doi:10.3852/09-146
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55:158–161
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808. doi:10.1080/10635150490522304
Schenck NC, Spain JL, Sieverding E, Howeler RH (1984) Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76:685–699. doi:10.2307/3793226
Schüßler A, Walker C (2010) The Glomeromycota: a species list with new families and new genera. www.arbuscular-mycorrhiza.net/Schuessler&Walker2010_Glomeromycota.pdf
Sieverding E, Oehl F (2006) Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. J Appl Bot Food Qual 80:69–81
Sieverding E, Toro S (1987) Entrophospora schenckii: a new species in the Endogonaceae from Colombia. Mycotaxon 28:209–214
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337. doi:10.1007/s13127-011-0056-0
Spain JL, Sieverding E, Oehl F (2006) Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97:163–182
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi:10.1093/bioinformatics/btl446
Stürmer SL, Morton JB (1997) Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89:72–81. doi:10.2307/3761174
Tadych M, Błaszkowski J (2000) Arbuscular fungi and mycorrhizae (Glomales) of the Słowiński National Park, Poland. Mycotaxon 74:463–483
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Walker C (1983) Taxonomic concepts in the Endogonaceae; spore wall characteristics in species descriptions. Mycotaxon 18:443–455
Walker C, Vestberg M, Demircik F, Stockinger H, Saito M, Sawaki H, Nishmura I, Schüßler A (2007) Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol Res 111:137–153. doi:10.1016/j.mycres.2006.11.008
Wetzel K, Silva G, Matczinski U, Oehl F, Fester T (2014) Superior differentiation of arbuscular mycorrhizal fungal communities from till and no-till plots by morphological spore identification when compared to T-RFLP. Soil Biol Biochem 72:88–96. doi:10.1016/j.soilbio.2014.01.033
Wu C-H, Liu Y-S, Huang Y-L, Wang Y-P, Chao C-C (1995) Glomales of Taiwan: V. Glomus chimonobambusae and Entrophospora kentinensis, spp. novum. Mycotaxon 53:283–294
Zubek S, Turnau K, Błaszkowski J (2008) Arbuscular mycorrhiza of endemic and endangered plants from the Tatra Mts. Acta Soc Bot Pol 77:149–156. doi:10.5586/asbp.2008.019
Acknowledgments
The study was supported, in part, by the Polish National Centre of Science, grant nos. 2012/05/B/NZ8/00498 and 2012/07/N/NZ8/02363. Ms. J. Vaingankar was supported by the Department of Science and Technology, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Marco Thines
Rights and permissions
About this article
Cite this article
Willis, A., Błaszkowski, J., Prabhu, T. et al. Sacculospora felinovii, a novel arbuscular mycorrhizal fungal species (Glomeromycota) from dunes on the west coast of India. Mycol Progress 15, 791–798 (2016). https://doi.org/10.1007/s11557-016-1208-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1208-6