Abstract
Morphological observations of spores and mycorrhizal structures of three arbuscular mycorrhizal fungi (Glomeromycota) prompted, and subsequent phylogenetic analyses of SSU–ITS–LSU nrDNA sequences confirmed, that they are undescribed species of the genus Diversispora. Morphologically, the first species, here named D. varaderana, is most distinguished by its relatively small (≤90 μm diam when globose) and yellow-coloured spores with a simple spore wall consisting of two layers, of which layer 1, forming the spore surface, is short-lived and usually completely sloughed in most spores. The distinctive features of the second species, D. peridiata, are the occasional formation of spores in clusters and peridium-like hyphae covering the clusters and single spores, and especially the permanent and relatively thick spore wall layer 1, which is the only coloured component of the two-layered spore wall of the yellow-coloured and relatively small spores (≤100 μm diam). The third species, D. slowinskiensis, is most characterized by its spore wall layer 1 that is the only coloured component of the three-layered spore wall and frequently is covered with blister-like swellings. All the three species were grown in single-species cultures established from spores extracted from trap cultures inoculated with rhizosphere soils of plants growing in maritime sand dunes: D. varaderana from those located near Varadero on the Hicacos Peninsula, Cuba, and the two others from those of the Słowiński National Park, northern Poland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Diversispora C. Walker & A. Schüssler of the family Diversisporaceae C. Walker & A. Schüssler and the order Diversisporales C. Walker & A. Schüssler belongs to the phylum Glomeromycota C. Walker & A. Schüssler that comprises arbuscular mycorrhizal fungi (AMF) (Schüßler et al. 2001). These fungi have a world-wide distribution and form symbiosis with ca. 70–90 % of vascular land plants (Smith and Read 2008; van der Heijden et al. 2015) and some plants whose roots are permanently flooded (Sudová et al. 2015).
The Diversisporales and Diversisporaceae were originally designated in the Glomeromycota based on phylogenetic analyses of sequences of the small subunit (SSU) rRNA gene of three former Glomus spp. (Schüßler et al. 2001), but validly published 3 years later (Walker and Schüßler 2004). At that time, the Diversisporaceae contained only the genus Diversispora with the type species D. spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & A. Schüssler. Currently, the Diversisporaceae still comprises the genera Corymbiglomus Błaszk. & Chwat, Redeckera C. Walker & A. Schüssler, Otospora Oehl, Palenz. & N. Ferrol and Tricispora Oehl et al. (Redecker et al. 2013). Like Diversispora spp., species of Corymbiglomus and Redeckera form glomoid spores, which develop blastically at the tip of a sporogenous hypha (Redecker et al. 2007; Schüßler and Walker 2010; Błaszkowski 1995, 2012). The genera Otospora and Tricispora are represented by single species, whose mode of spore formation differs substantially from those of Diversispora spp. Otospora bareae Palenz., N. Ferrol & Oehl produces acaulosporoid spores laterally from the neck of a sporiferous saccule (Palenzuela et al. 2008), and the entrophosporoid spores of T. nevadensis arise inside the neck of a sporiferous saccule (Oehl et al. 2011b).
Identification and classification of Diversispora spp. from their spore morphology is uncertain and difficult, even for experienced specialists. The spore wall structure and the phenotypic and histochemical traits of its components are similar or identical to those of species of other clades of the Glomeromycota. According to Oehl et al. (2011a), Diversispora spp. are most distinguished by characters of their subtending hyphae at the spore base, which are cylindrical and have a colourless or light-coloured wall abruptly passing into a coloured spore wall in coloured spores. However, the colour change is difficult to observe in, for example, D. gibbosa (Błaszk.) Błaszk. & Kovács, which produces very light-coloured spores or it is not visible at all in D. clara Oehl et al., whose spores are colourless, as those of many species of other genera of the Glomeromycota with cylindrical subtending hyphae (Estrada et al. 2011; Błaszkowski 2012).
Meanwhile, literature data suggest that the vast majority of AMF existing in the world remain unnamed and among them are many representatives of the genus Diversispora (Gamper et al. 2009; Schüßler et al. 2011). Thus, considering the difficulties of morphological identification of Diversispora spp. characterized above, certain recognition of Diversispora spp. and determination of the phylogenetic position of yet unnamed species among described relatives of the genus have to take into account their morphological and molecular traits, as Gamper et al. (2009) suggested, provided that the resolution of molecular data allows to separate even very closely related taxa.
Sequence data retrieved from the conserved SSU rRNA gene, used in the erection of the Glomeromycota (Schüßler et al. 2001) and many other studies of AMF (Walker et al. 2007; Krüger et al. 2012), frequently poorly recognized and resolved some taxa of AMF, especially those of the genus Diversispora (Gamper et al. 2009). Also, information coming from other regions of DNA, as the more variable internal transcribed spacer 1 (ITS1), the 5.8S rRNA gene, ITS2 rDNA (hereafter named ITS) and the large subunit (LSU) rRNA gene, the genes for mitochondrial LSU rRNA (Börstler et al. 2010; Sýkorová et al. 2012), β-tubulin (Msiska and Morton 2009) or H + −ATPase (Corradi et al. 2004; Sokolski et al. 2010), as well as the largest subunit of RNA polymerase II (RPB1; Stockinger et al. 2014) gene either occasionally were insufficient to know the identity of some AMF or sequences collected in public data bases regard only few named species, making certain identification of most closely related species impossible. For example, in the recently published set of sequences of the RPB1 gene the genus Diversispora is represented by one sequence of D. epigaea (B.A. Daniels & Trappe) C. Walker & A. Schüssler (Stockinger et al. 2014).
Krüger et al. (2012) proved and our numerous phylogenetic analyses (Błaszkowski et al. 2012, 2014, 2015a, b, c) also indicated that the sequences best resolving even morphologically and molecularly very closely related species are those spanning the SSU–ITS–LSU nrDNA region. In addition, the sequences concern a large proportion of described species of AMF, for example, 69 % of Diversispora spp. sensu Oehl et al. (2011a).
Our morphological observations of spores of three AMF extracted from single-species cultures prompted they are undescribed Diversispora spp. Subsequent phylogenetic analyses of SSU–ITS–LSU nrDNA sequences of the fungi confirmed the conclusion and revealed their closest molecular relatives. The fungi are described below as D. varaderana sp. nov., D. peridiata sp. nov., and D. slowinskiensis sp. nov.
Materials and methods
Establishment and growth of trap and single-species cultures, extraction of spores, and staining of mycorrhizal structures
Spores were first extracted from pot trap cultures that were established from the rhizosphere soils and roots of sampled plants mixed with autoclaved coarse grained sand and then grown in conditions described previously (Błaszkowski et al. 2012). The sampled plants are mentioned in the “Mycorrhizal associations” sections regarding each species of AMF (see below). Single-species cultures of each of the AMF species described below were also established and grown as given in Błaszkowski et al. (2012). The cultures were successfully established after inoculation of host plant roots with 10–20 spores per pot and their growing in conditions characterized in Błaszkowski et al. (2012). The host plant in both trap and single-species cultures was Plantago lanceolata L. Spores for morphological and molecular analyses and roots for studies of mycorrhizal structures were collected from 5-month-old cultures. Spores were extracted from trap and single-species cultures by the method described recently by Błaszkowski et al. (2015c). Roots were stained as Błaszkowski (2012) described.
Microscopy and nomenclature
Morphological features of spores and the phenotypic and histochemical characters of spore wall layers were determined after examination of at least 100 spores mounted in water, lactic acid, polyvinyl alcohol/lactic acid/glycerol (PVLG; Omar et al. 1979), and a mixture of PVLG and Melzer’s reagent (1:1, v/v). The preparation of spores and mycorrhizal structures for study and photography were as those described previously (Błaszkowski 2012; Błaszkowski et al. 2012). Types of spore wall layers are those defined by Błaszkowski (2012), Stürmer and Morton (1997) and Walker (1983). Colour names are from Kornerup and Wanscher (1983). Nomenclature of fungi and the authors of fungal names are from the Index Fungorum website http://www.indexfungorum.org/AuthorsOfFungalNames.htm. Voucher specimens were mounted in PVLG and a mixture of PVLG and Melzer’s reagent (1:1, v/v) on slides and deposited at the common mycological herbarium of the University and ETH of Zurich, Switzerland (Z + ZT; holotypes), the Department of Ecology, Protection and Shaping of Environment (DEPSE), West Pomeranian University of Technology in Szczecin, Szczecin, Poland, and in the herbarium at Oregon State University (OSC) in Corvallis, Oregon, USA (isotypes).
DNA extraction, polymerase chain reaction, cloning, and DNA sequencing
Crude DNA of each species was extracted from four single spores. The procedures with the spores prior to polymerase chain reactions (PCR), the conditions and primers used in the PCRs to obtain SSU–ITS–LSU nrDNA sequences, as well as cloning and sequencing were as those described in Błaszkowski et al. (2013). The sequences were deposited in GenBank (KT444708–KT444721).
Sequence alignment and phylogenetic analyses
Comparisons of sequences of our three AMF with sequences listed after BLAST queries showed that they all represent undescribed species of the Glomeromycota. Subsequent pilot analyses of the yet undescribed AMF and randomly selected published sequences of all named species with glomoid spores of known molecular phylogenies proved that all our AMF belong in the genus Diversispora. Then we established a set of sequences comprising 4–5 sequences each of the three new Diversispora spp. and 2–5 sequences each of all previously described Diversispora spp. of known phylogenies. Corymbiglomus corymbiforme Błaszk. & Chwat served as outgroup. All the sequences regarded the SSU–ITS–LSU nrDNA segment. The set of sequences was aligned with MAFFT v. 7 (Katoh and Standley 2013) using the auto option (http://mafft.cbrc.jp/alignment/server/). To improve phylogenetic resolution (Nagy et al. 2012) indels were coded by means of the simple indel coding algorithm (Simmons et al. 2001) as implemented in GapCoder (Young and Healy 2003) and this binary character set was added to the nucleotide alignment. Bayesian (BI) analyses were carried out with MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003); SSU–ITS–LSU sequence data plus indel characters were divided into four partitions. GTR + G and two-parameter Markov (Mk2 Lewis) models were used for the nucleotide partitions and indels, respectively. Four Markov chains were run for 10,000,000 generations, sampling every 1,000 steps, with a burn in at 3,000 sampled trees. Convergence of the MCMC Bayesian phylogenetic inference was checked by AWTY online (Nylander et al. 2007).
Maximum likelihood (ML) phylogenetic analyses were carried out with the raxmlGUI (Silvestro and Michalak 2012) implementation of RAxML (Stamatakis 2014) with GTRGAMMA for DNA and default set for binary (indel) characters. Rapid bootstrap analysis with 1,000 replicates was used to test the support of the branches. The generated phylogenetic trees were visualized and edited in MEGA6 (Tamura et al. 2013).
Results
General data
Our phylogenetic analyses of SSU–ITS–LSU nrDNA sequences of three likely yet unnamed AMF confirmed our supposition and proved that they belong in the genus Diversispora and indicated their closest named relatives (Fig 1). The overall phylogenetic relations of the Diversispora species showed in our tree are in correspondence with previous phylogenetic analyses of the genus. Although species and some clades in the tree have strong support, relative position of some lineages and the intra-group topology of some clades could not be resolved.
50 % majority rule consensus phylogram inferred from a Bayesian analysis of SSU–ITS–LSU rDNA sequences of Diversispora varaderana, D. peridiata, and D. slowinskiensis among 11 known Diversispora spp. and an undescribed Diversispora sp. EE1. Corymbiglomus corymbiforme served as outgroup. Sequences of the three new Diversispora spp. are in boldface and are followed by GenBank accession numbers. The Bayesian posterior probabilities ≥0.50 and ML bootstrap values ≥50 % are shown above and below the branches, respectively. Bar indicates 0.5 expected change per site per branch
Taxonomy
Diversispora varaderana Błaszk., Chwat, Kovács & Góralska, sp. nov. Figs. 2–8.
Diversispora varaderana spores. 2. Intact spores with subtending hypha (sh). 3–5. Spore wall layers (swl) 1 and 2; in Fig. 5, swl1 is highly deteriorated and incorporated sand grain (sg). 6. Spore wall layer 2 (swl2); swl1 is completely sloughed. 7 and 8. Subtending hyphal wall layers (shwl) 1 and 2 continuous with spore wall layers (swl) 1 and 2 and septum (s) continuous with swl2. Fig. 9. Mycorrhizal structures of D. varaderana in roots of Plantago lanceolata stained in 0.1 % trypan blue: arbuscule (a) and intraradical hyphae (ih). Fig. 2. Spores in lactic acid. Figs. 3, 8. Spores in PVLG. Figs. 4–7. Spores in PVLG + Melzer’s reagent. Fig. 9. Mycorrhizal structures in PVLG. Figs. 2–9, differential interference microscopy. Bars: Fig. 2 = 20 μm, Figs. 3–9 = 10 μm
MycoBank No. MB 814172
Holotype: ZT Myc 55234 (Z + ZT), isotypes: 3460–3471 (DEPSE), and OSC 153622, OSC 153623 (OSC). Data on the origin of D. varaderana are given in Table 1.
Etymology
Latin, varaderana, referring to the city Varadero, Cuba, near where the species was found.
Sporocarps unknown
Spores formed singly in soil (Fig. 1); develop blastically at the tip of sporogenous hyphae. Spores yellow (3A6) to dark yellow (4C8); globose to subglobose; (60–)75(−90) μm diam; rarely ovoid; 75–80 × 85–92 μm (Figs. 2–8). Spore wall consists of two layers (Figs 3–5, 7). Layer 1, forming the spore surface, evanescent, short-lived, hyaline to yellowish white (2A2), (1.0–)1.3(−1.5) μm thick, usually highly deteriorated or completely sloughed in most spores (Figs. 3–5, 7). Layer 2 laminate, smooth, yellow (3A6) to dark yellow (4C8), (3.3–)6.2(−7.5) μm thick (Figs. 3–8). None of layers 1 and 2 stains in Melzer’s reagent (Figs 5–7). Subtending hypha hyaline to yellowish white (2A2); straight or recurved, cylindrical to funnel-shaped, sometimes slightly constricted at the spore base; (4.5–)6.4(−8.5) μm wide at the spore base (Figs 2, 7, 8). Wall of subtending hypha hyaline to yellowish white (2A2); (1.3–)2.2(−2.6) μm thick at the spore base; continuous with spore wall layers 1 and 2; subtending hyphal wall layer 1 usually completely sloughed in most spores (Figs 7, 8). Pore (1.3–)1.9(−2.5) μm diam, occluded by a straight or slightly curved septum continuous with spore wall layer 2 (Figs 7, 8); septum positioned at or up to 1.8 μm below the spore base. Germination unknown.
Mycorrhizal associations
In the field D. varaderana was likely associated with roots of P. dactylifera growing near Varadero on the Hicacos Peninsula, Cuba. However, the presence of the fungus inside the sampled roots was not examined using molecular methods.
The mycorrhiza of D. varaderana formed in single-species cultures with P. lanceolata as host plant consisted of arbuscules and intra- and extraradical hyphae (Fig. 9). The structures were uniformly distributed along the root fragments examined. Intraradical hyphae usually were straight, rarely slightly curved, and (2.8–)4.7(−5.8) μm wide. They frequently formed ellipsoidal coils, 13.3–26.5 × 39.0–45.2 μm. In 0.1 % trypan blue arbuscules stained very pale [violet white (16A2) to pale violet (16A3)], and the other structures pale violet (16A3) to lilac (16B4).
Phylogenetic position
Phylogenetic analyses proved that the closest relative of D. varaderana is D. insculpta (Błaszk.) Oehl, G.A. Silva & Sieverd. and the clades of these sister groups were strongly supported (Fig. 1).
Distribution and habitat
Spores of D. varaderana were found in only one trap culture inoculated with the rhizosphere soil and roots of P. dactylifera growing near Varadero on the Hicacos Peninsula. The presence of spores of the fungus in the mixture was not examined. Diversispora varaderana was not found in ca. 2500 other field-collected soils and ca. 2700 trap cultures (personally examined by J. Błaszkowski) that represented different cultivated and uncultivated sites of Africa, Asia, Europe, and USA.
However, BLAST queries indicated that D. varaderana sequences were similar in ≥97 % to nine SSU–ITS–LSU sequences (HG425941–45, HG425948, HG425951–53) obtained from roots of six plant species growing in Central Bohemia, Czech Republic and four SSU–ITS–LSU sequences (FR686946–49) coming from a spore cluster of a fungus named Diversispora sp. W5257 found in the United Kingdom. These similarities suggest D. varaderana is widely distributed in the world.
Notes
Morphologically, D. varaderana is most distinguished by its relatively small and yellow-coloured spores with a simple spore wall consisting of two layers, of which layer 1 is short-lived and usually completely sloughed in most spores (Figs. 2–8).
The phylogenetically closest relative of D. varaderana is D. insculpta (Fig. 1), whose spores are similar in colour, size, and also have a spore wall with two layers (Błaszkowski et al. 2004; Błaszkowski 2012). However, the phenotypic features of the layers in the two species differ considerably. While spore wall layer 1 of D. varaderana is short-lived and usually completely sloughed in most spores extracted from ca. 5-month-old cultures (Figs. 3–8), that of D. insculpta is permanent and always present and intact in spores coming from cultures even older than 2 years (Błaszkowski, pers. observ.). Spore wall layer 2 in D. varaderana is smooth on its upper and lower surfaces (Figs. 3–8), and in D. insculpta the lower surface of this layer is ornamented with evenly distributed pits. In addition, at the spore base the subtending hypha of D. insculpta is 1.2–1.5-fold narrower and has a wall 1.4–1.7-fold thinner.
Another Diversispora sp. closely related morphologically to D. varaderana is D. celata C. Walker, Gamper & A. Schüssler, whose spores are similar in colour and spore wall comprises an evanescent outer layer 1 and a laminate inner layer 2 (Gamper et al. 2009; Błaszkowski 2012). In contrast to the very short-lived spore wall layer 1 of D. varaderana (Figs. 3–8) that of D. celata is much longer-lived and usually present in even the darkest (likely the oldest) spores. This is probably because the layer thickness is 1.3–1.7-fold thicker and, therefore, deteriorates slower. In addition, in D. celata the laminate spore wall layer 2 is clearly thinner (up to 1.7-fold), and its subtending hypha at the spore base is ca. 1.6-fold wider and has a wall up to 1.5-fold thicker than in D. varaderana. Finally, D. varaderana and D. celata differ molecularly and the difference is large (Fig. 1).
Diversispora peridiata Błaszk., Chwat, Kovács & Góralska, sp. nov. Figs. 10–17.
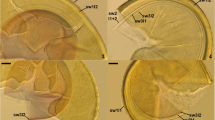
Diversispora peridiata spores. 10. Cluster of intact spores without peridium-like hyphae. 11, 12. Cluster of intact spores (sp, Fig. 11) and single spore (Fig. 12) covered with peridium-like hyphae (plh). 13–15. Coloured layer 1 (swl1) and colourless layer 2 (swl2) of the spore wall. 16. Spore wall layers (swl) 1 and 2, one-layered subtending hyphal wall (shwl1) continuous with swl1 and septum (s) of the subtending hypha continuous with swl2 at the spore base. 17. Spore wall layers (swl) 1 and 2 continuous with subtending hyphal wall layers (shwl) 1 and 2 and septum (s) located below the spore base and continuous with swl2. Figs. 10, 11, 13, 16. Spores in PVLG. Figs. 12, 14, 15, 17. Spores in PVLG + Melzer’s reagent. Figs. 10–17, differential interference microscopy. Bars: 10–12 = 20 μm, Figs. 13–17 = 10 μm
MycoBank No. MB 814173
Holotype: ZT Myc 55235 (Z + ZT), isotypes: 3473–3495 (DEPSE) and OSC 153624, OSC 153625 (OSC). Data on the origin of D. peridiata are given in Table 1.
Etymology: Latin, peridiata, referring to the peridium-like hyphae occasionally formed by the species.
Sporocarps
Unknown. Spores formed in soil, sometimes also inside roots. Hypogeous spores produced singly, more rarely in loose to compact clusters with 3–21 spores (Figs. 10–17); spores develop blastically at the tip of sporogenous hyphae. Single spores and spores in clusters occasionally covered with hyaline peridium-like hyphae, 2.8–8.5 μm wide (Figs. 11, 12). Spores yellowish white (4A2) to maize yellow (4A6); globose to subglobose; (35–)74(−100) μm diam; rarely ovoid; 70–80 × 90–100 μm (Figs. 10–17). Spore wall consists of two layers (Figs. 13–17). Layer 1, forming the spore surface, permanent, uniform (not divided into visible sublayers), pale yellow (3A3) to brownish yellow (5C8), (0.8–)1.5(−2.3) μm thick, always intact in all spores (Figs. 13–17). Layer 2 laminate, smooth, pliable, hyaline, (3.3–)5.1(−7.5) μm thick, frequently releasing single or groups of laminae in crushed spores (Figs. 13–17). Peridium-like hyphae and spore wall layers 1 and 2 do not stain in Melzer’s reagent (Figs. 12, 14, 15, 17). Subtending hypha pale yellow (3A3) to brownish yellow (5C8) near the spore base, then hyaline; straight or recurved, cylindrical to funnel-shaped, sometimes slightly constricted at the spore base; (5.0–)6.1(−8.3) μm wide at the spore base (Figs. 16, 17). Wall of subtending hypha pale yellow (3A3) to brownish yellow (5C8) near the spore base, then hyaline; (1.4–)1.8(−2.8) μm thick at the spore base; continuous with either spore wall layer 1 only (when spore wall layer 2 started its development at the spore base and forms a septum at the level of its upper surface; Fig. 16) or with spore wall layers 1 and 2 (when spore wall layer 2 started developing below the spore base; Fig. 17). Pore (1.6–)2.7(−3.5) μm diam, occluded by a straight or slightly curved septum continuous with spore wall layer 2 (Figs. 16, 17); septum positioned at the spore base or up to 3.5 μm below the spore base. Germination unknown.
Mycorrhizal associations
In the field D. peridiata was likely associated with roots of A. arenaria growing near a beach of the Baltic Sea and the deflation hollow no. 6 and in the deflation hollow no. 12 of SPN in northern Poland. However, no molecular studies were performed to check if roots A. arenaria harboured D. peridiata. Deflation hollows are depressions produced by wind erosion, during which dry sand is gradually blown away until moist sand is exposed (Piotrowska 1991). Soil of such hollows usually is wet, and its vegetation is frequently periodically under water.
In single-species cultures with P. lanceolata as host plant, the mycorrhiza of D. peridiata consisted of arbuscules and intra- and extraradical hyphae. No vesicles were found. Arbuscules and hyphae were widely dispersed along roots. The intraradical hyphae were (2.8–)5.5(−8.5) μm wide and sometimes formed circular to ellipsoidal coils, 24.8–66.0 × 28.8–66.0 μm, when seen in plan view. The structures stained violet white (15A2) to light violet (17A5) in 0.1 % trypan blue.
Phylogenetic position
Phylogenetic analyses placed D. peridiata sequences in a strongly supported clade with D. gibbosa and D. trimurales (Koske & Halvorson) C. Walker & A. Schüssler (Fig. 1). The sequences of the three species unambiguously separated from each other, but the relative positions of the three species within the clade could not be resolved (Fig 1).
Distribution and habitat
Diversispora peridiata was so far identified in three trap cultures inoculated with the rhizosphere soils and roots of A. arenaria colonizing maritime sand dunes belonging to SPN and was not revealed in ca. 5200 other soils from different sites and regions of the world (see “Distribution and habitat” regarding D. varaderana described above).
BLAST searches showed that, except for D. gibbosa, no other AMF was thus far recognized, whose sequences are similar in ≥97 % to SSU–ITS–LSU sequences of D. peridiata. Thus, D. peridiata may rarely occur in the world.
Notes
Morphologically, D. peridiata is distinguished by the occasional formation of spores in clusters and the peridium-like hyphae covering the clusters and single spores (Figs. 10–12), and especially by the permanent and relatively thick spore wall layer 1, which is the only coloured component of the two-layered spore wall of the yellow-coloured and relatively small-spored species (Figs. 13–17).
Diversispora gibbosa, one of the two closest relatives of D. peridiata in molecular phylogenetic analyses (Fig. 1), sometimes also produces spores in clusters, but they usually contain fewer spores (2–8) and are completely enclosed by a hyphal mantle having a continuous wall, without openings (Błaszkowski 1997, 2012; vs. are partly covered with narrow peridium-like hyphae; Figs. 11, 12). Spores of D. gibbosa may also be lighter (hyaline vs. never are hyaline in D. peridiata), are 1.3–2.3-fold larger when globose and, most importantly, their spore wall comprises four layers, of which an evanescent, hyaline layer 1 and a flexible, hyaline layer 4 are lacking in the spore wall of D. peridiata.
The second species closely related in phylogenetic analyses to D. peridiata is D. trimurales (Fig. 1). However, morphologically the fungi also differ substantially. Diversispora peridiata occasionally forms spores in clusters (vs. only singly in D. trimurales) and peridium-like hyphae covering single spores and their clusters (Figs. 10–12; vs. no such hyphae) and the spores are maize yellow at most [4A6 vs. golden yellow (5B8)] and ca. 1.6–3.1-fold smaller when globose (Błaszkowski et al. 2003; Błaszkowski 2012). Most importantly, the spore wall of D. peridiata consists of two layers (Figs. 13–17) and does not possess layer 1 of the three-layered spore wall of D. trimurales, which, in addition, may be up to 1.9-fold thicker. Finally, in D. peridiata the subtending hypha and its pore at the spore base are 1.2-fold and up to 1.6-fold narrower, respectively.
Diversispora slowinskiensis Błaszk., Chwat, Góralska & Kovács, sp. nov. Figs. 18–25.
Diversispora slowinskiensis spores. 18. Intact spores with subtending hyphae (sh). 19–22. Spore wall layers (swl) 1–3; note swollen swl1 of young spore in Fig. 19. 23, 24. Spore wall layers (swl) 1–3, subtending hyphal wall layers (shwl) 1 and 2 continuous with swl1 and 2 and septum (s) located at the spore base and continuous with swl3; note swollen swl1 of young spore in Fig. 23. 25. Spore wall layers (swl) 1–3 continuous with subtending hyphal wall layers (shwl) 1–3 and septum (s) continuous with swl3. Figure 18. Spores in lactic acid. Figs. 23, 25. Spores in PVLG. Figs. 19–22, 24. Spores in PVLG + Melzer’s reagent. Figures 18–25, differential interference microscopy. Bars: Fig. 18 = 50 μm, Figs. 19–25 = 10 μm
MycoBank No. MB 814174
Holotype: ZT Myc 55236 (Z + ZT), isotypes: 3497–3516 (DEPSE) and OSC 153626, OSC 153627 (OSC). Data on the origin of D. slowinskiensis are given in Table 1.
Etymology. Latin, slowinskiensis, referring to SPN, Poland, in which the species was discovered.
Sporocarps unknown
Spores formed singly in soil (Fig. 18); develop blastically at the tip of sporogenous hyphae. Spores pale yellow (2A3) to greyish yellow (3B5); globose to subglobose; (75–)138(−190) μm diam; rarely ovoid; 70–115 × 105–170 μm (Figs. 18–25). Spore wall consists of three layers (Figs. 19–25). Layer 1, forming the spore surface, evanescent, hyaline to yellowish brown (5E8), (1.0–)2.3(−5.3) μm thick, frequently with blister-like swellings, up to 7.0 μm high, on its upper surface, usually slowly deteriorating with age, rarely completely sloughed in older specimens (Figs. 18–25); in youth plastic, hyaline, with its upper part frequently swelling in PVLG (Figs. 19, 23), then darkening and becoming more compact (Figs. 20–22, 24). Layer 2 uniform (not divided into visible sublayers), smooth, permanent, hyaline, (0.8–)2.3(−4.0) μm thick (Figs. 19–25). Layer 3 laminate, smooth, pliable, hyaline, (2.3–)5.1(−10.0) μm thick, consisting of very thin laminae, <0.5 μm thick, frequently stratifying into groups of laminate, rarely into single laminae in even slightly crushed spores (Figs. 19–25). None of layers 1–3 stains in Melzer’s reagent (Figs. 19–22, 24). Subtending hypha usually hyaline, rarely yellowish brown (5E8) close at the spore base, when subtending hyphal wall layer 1 continuous with spore wall layer 1 remains intact or is slightly deteriorated; straight or recurved, cylindrical to funnel-shaped, sometimes slightly constricted at the spore base; (9.3–)12.3(−15.4) μm wide at the spore base (Figs. 18, 23–25). Wall of subtending hypha hyaline to yellowish brown (5E8); (2.6–)4.5(−7.0) μm thick at the spore base. The wall is continuous with spore wall layers 1 and 2, when spore wall layer 3 started developing at or slightly above the spore base (Figs. 23, 24), or spore wall layers 1–3, when spore wall layer 3 began arising below the spore base (Fig. 25). Pore (2.5–)3.7(−4.5) μm diam, occluded by a straight or slightly curved septum continuous with spore wall layer 3 (Figs. 23–25); septum positioned at the level of the center or the upper surface of spore wall layer 3 (Figs. 23–25). Germination unknown.
Mycorrhizal associations
In the field D. slowinskiensis was likely associated with roots of A. arenaria and Juncus articulatus L. growing in the 12 deflation hollow of SPN. However, the existence of the fungus inside the roots of the plant species was not examined using molecular methods.
In single-species cultures with P. lanceolata as host plant, D. slowinskiensis formed mycorrhiza with arbuscules and intra- and extraradical hyphae. The structures were patchily distributed along root fragments. Intraradical hyphae were straight or slightly curved, (2.8–)6.3(−9.8) μm wide and rarely formed ellipsoidal coils, 21.3–41.5 × 65.0–87.3 μm, when observed in plain view. All of the structures stained violet white (17A2) to greyish violet (17C6) in 0.1 % trypan blue.
Phylogenetic position
In the phylogenetic analyses, sequences of D. slowinskiensis formed a clade with the fully supported monophyletic group of D. arenaria (Błaszk., Tadych & Madej) Oehl, G.A. Silva & Sieverd., D. jakucsiae Błaszk., Balázs & Kovács, and an undescribed Diversispora sp. EE1 (Fig. 1).
Distribution and habitat
Spores of D. slowinskiensis were extracted from 2 of 298 (0.7 %) trap cultures inoculated with field-collected mixtures of the rhizosphere soils and root fragments of A. arenaria and J. articulatus growing in the 12 deflation hollow of SPN.
Searches of BLAST did not indicate any sequence of similarity of ≥97 % to sequences of D. slowinskiensis. This and the lack of D. slowinskiensis spores in the large number of soils and sites so far examined by J. Błaszkowski (see above in “Distribution and habitat” regarding D. varaderana) suggest our new species has a very narrow distribution in the world.
Notes
Two morphological structures distinguish D. slowinskiensis spores. The first is spore wall layer 1, which is the only coloured component of the three-layered spore wall, frequently is covered with blister-like swellings and in youth its upper part often swells in PVLG while its basal part always adherers to the upper surface of spore wall layer 2 (Figs. 19–25). The second is the pliable, laminate, hyaline spore wall layer 3, which consists of loosely associated laminae (Figs. 19–25). This layer either starts its development at the spore base, and then is not a component of the subtending hyphal wall, or arises far below the spore base forming subtending hyphal wall layer 3.
In addition, D. slowinskiensis is unique in molecular phylogeny: sequences of the species grouped in its own clade sister to a clade comprising D. arenaria, D. jakucsiae and an undescribed Diversispora sp. EE1 (Thiery et al. 2012) (Fig. 1).
None of the species mentioned above forms spores ornamented with blister-like swellings like those of D. slowinskiensis spores (Figs. 19–21). In addition, D. arenaria spores may be clearly darker, are 1.4–1.6-fold smaller when globose and at the spore base their subtending hypha is 1.9–2.5-fold narrower and has a 3.7–4.1-fold thinner wall (Błaszkowski et al. 2001; Błaszkowski 2012). Spore wall layer 1 in both species is evanescent, but in D. arenaria it is always hyaline [vs. hyaline to yellowish brown (5E8) in D. slowinskiensis; Figs. 19–25] and 2.0–3.5-fold thinner when intact. Spore wall layer 2 in D. arenaria also is much thinner (up to 2.7-fold) and sloughs with age (vs. it is permanent in D. slowinskiensis; Figs. 19–25), and layer 3 is coloured (vs. hyaline; Figs. 19–25).
Spores of D. jakucsiae also are much darker, ca. 1.3-fold smaller when globose and their subtending hypha may be up to 1.9-fold narrower at the spore base (Balazs et al. 2015). Most importantly, in D. jacuksiae spore wall layer 1 is permanent, smooth and does not give the spores their proper colour (vs. evanescent and the sole coloured component in the spore wall in D. slowinskiensis; Figs. 19–25), layer 2 is laminate and darkest (vs. uniform, hyaline; Figs. 19–25) and 3.6–5.3-fold thicker than that of D. slowinskiensis, and layer 3 is flexible to semi-flexible and up to 1.3 μm thick (vs. laminate, up to 10.0 μm thick; Figs. 19–25).
Diversispora
Sp. EE1 was characterized by molecular phylogenetic methods with no information on morphology of its spores (Thiéry et al. 2012). From microphotographs of crushed spores of the fungus (kindly provided by Drs. Odile Thiéry and Maarja Öpik) we can see that our D. slowinskiensis and Diversispora sp. EE1 differ substantially: spores of the latter fungus are lighter and likely have a two-walled spore wall (vs. three-layered in D. slowinskiensis).
Based on morphology, without molecular evidence, Oehl et al. (2011a) transferred G. pustulatum Koske et al. to the genus Diversispora as a new combination, D. pustulata (Koske et al.) Oehl, G.A. Silva & Sieverd. The upper surface of layer 1 of its three-layered spore wall is covered with a pustulate ornamentation (Koske et al. 1986; Błaszkowski 1994, 2012) like that of D. slowinskiensis (Figs. 19, 21), but in D. pustulata this layer is permanent (vs. evanescent), layer 2 is laminate and coloured (vs. uniform, hyaline; Figs. 19–25), and layer 3 is flexible and <1 μm thick (vs. laminate, up to 10 μm thick; Figs. 19–25). Finally, D. pustulata spores may be much darker [up to deep orange (5A8)], are 1.4–1.7-fold smaller when globose and at the spore base their subtending hypha is 1.3–2.2-fold narrower and has a ca. 3.5-fold thinner wall.
References
Balazs TK, Błaszkowski J, Chwat G, Góralska A, Gaspar BK, Lukacs AF, Kovács GM (2015) Spore-based study of arbuscular mycorrhizal fungi of semiarid sandy areas in Hungary, with Diversispora jakucsiae sp. nov. Mycol Prog 14:1021. 10.1007/s11557-014-1021-z
Błaszkowski J (1994) Polish Glomales 11 Glomus pustulatum. Mycorrhiza 4:201–207. doi:10.1007/BF00206781
Błaszkowski J (1995) Glomus corymbiforme, a new species in Glomales from Poland. Mycologia 87:732–737. doi:10.2307/3760819
Błaszkowski J (1997) Glomus gibbosum, a new species from Poland. Mycologia 89:339–345. doi:10.2307/3761092
Błaszkowski J (2012) Glomeromycota. W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków
Błaszkowski J, Tadych M, Madej T (2001) Glomus arenarium, a new species in Glomales (Zygomycetes). Acta Soc Bot Pol 70:97–101. doi:10.5586/asbp.2001.013
Błaszkowski J, Adamska I, Czerniawska B (2003) Glomus trimurales, an arbuscular mycorrhizal fungus (Glomerales) new for Poland and Europe. Mycotaxon 87:425–436
Błaszkowski J, Adamska I, Czerniawska B (2004) Glomus insculptum, a new arbuscular mycorrhizal species from Poland. Mycotaxon 89:225–234
Błaszkowski J, Kovács GM, Gáspár BK, Balázs TK, Buscot F, Ryszka P (2012) The arbuscular mycorrhizal Paraglomus majewskii sp. nov. represents a new distinct basal lineage in Paraglomeraceae (Glomeromycota). Mycologia 104:148–156. doi:10.3852/10-430
Błaszkowski J, Chwat G, Kovács GM, Gáspár BK, Ryszka P, Orłowska E, Pagano MC, Araújo FS, Wubet T, Buscot F (2013) Septoglomus fuscum and S. furcatum, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycologia 105:670–680. doi:10.3852/12-127
Błaszkowski J, Chwat G, Góralska A, Ryszka P, Orfanoudakis M (2014) Septoglomus jasnowskae and Septoglomus turnauae, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycol Prog 13(4):999–1009. doi:10.1007/s11557-014-0985-z)
Błaszkowski J, Chwat G, Góralska A (2015a) Acaulospora ignota and Claroideoglomus hanlinii, two new species of arbuscular mycorrhizal fungi (Glomeromycota) from Brazil and Cuba. Mycol Prog 14:18. doi:10.1007/s11557-015-1042-2
Błaszkowski J, Chwat G, Góralska A, Ryszka P, Kovács GM (2015b) Two new genera, Dominikia and Kamienskia, and D. disticha sp. nov. in Glomeromycota. Nova Hedwigia 100:225–238. doi:10.1127/nova_hedwigia/2014/0216
Błaszkowski J, Chwat G, Symanczik S, Góralska A (2015c) Dominikia duoreactiva and D. difficilevidera, two new species in the Glomeromycota. Botany. doi:10.1139/cjb-2015-0016
Börstler B, Thiéry O, Sýkorová Z, Berner A, Redecker D (2010) Diversity of mitochondrial large subunit rDNA haplotypes of Glomus intraradices in two agricultural field experiments and two semi-natural grasslands. Mol Ecol 19:1497–1511. doi:10.1111/j.1365-294X.2010.04590.x
Corradi N, Kuhn G, Sanders IR (2004) Monophyly of β-tubulin and H + −ATPase gene variants in Glomus intraradices: consequences for molecular evolutionary studies of AM fungal genes. Fungal Genet Biol 41:262–273. doi:10.1016/j.fgb.2003.11.001
Estrada B, Palenzuela J, Barea J-M, Ruiz-Lozano JM, Alves G, da Silva GA, Oehl F (2011) Diversispora clara (Glomeromycetes) - a new species from saline dunes in the Natural Park Cabo de Gata (Spain). Mycotaxon 118:73–81. doi:10.5248/118.73
Gamper HA, Walker C, Schüßler A (2009) Diversispora celata sp. nov: molecular ecology and phylotaxonomy of an inconspicuous arbuscular mycorrhizal fungus. New Phytol 182:495–506. doi:10.1111/j.1469-8137.2008.02750.x
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. doi:10.1093/Bioinformatics/17.8.754
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi:10.1093/molbev/mst010
Kornerup A, Wanscher JH (1983) Methuen handbook of colour, 3rd edn. Eyre Methuen, London
Koske RE, Friese C, Walker C, Dalpé Y (1986) Glomus pustulatum: a new species in the Endogonaceae. Mycotaxon 26:143–149
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984. doi:10.1111/j.1469-8137.2011.03962.x
Msiska Z, Morton J (2009) Phylogenetic analysis of the Glomeromycota by partial β-tubulin gene sequences. Mycorrhiza 19:247–254. doi:10.1007/s00572-008-0216-z
Nagy LG, Kocsubé S, Csanádi Z, Kovács GM, Petkovits T, Vágvölgyi C, Papp T (2012) Re-mind the gap! Insertion – deletion data reveal neglected phylogenetic potential of the nuclear ribosomal internal transcribed spacer (ITS) of fungi. PLoS ONE 7, e49794. doi:10.1371/journal.pone. 0049794
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2007) AWTY (Are We There Yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583. doi:10.1093/bioinformatics/btm388
Oehl F, da Silva GA, Goto BT, Sieverding E (2011a) Glomeromycota: three new genera and glomoid species reorganized. Mycotaxon 116:75–120. doi:10.5248/116.75
Oehl F, da Silva GA, Sánchez-Castro I, Goto BT, Maia LC, Vieira HEE, Barea J-M, Sieverding E, Palenzuela J (2011b) Revision of Glomeromycetes with entrophosporoid and glomoid spore formation with three new genera. Mycotaxon 117:297–316. doi:10.5248/117.297
Omar MB, Bollan L, Heather WA (1979) A permanent mounting medium for fungi. B Brit Mycol Soc 13:31–32. doi:10.1016/S0007-1528(79)80038-3
Palenzuela J, Ferrol N, Boller T, Azcón-Aquila C, Oehl F (2008) Otospora bareai, a new fungal species in the Glomeromycetes from a dolomitic shrub-land in the natural park of Sierra de Baza (Granada, Spain). Mycologia 100:296–305. doi:10.3852/mycologia.100.2.296
Piotrowska H (1991) The development of the vegetation in the active deflation hollows of the Łeba Bar (N Poland). Frag Flor Geobot 35:173–215
Redecker D, Raab P, Oehl F, Camacho FJ, Courtecuisse R (2007) A novel clade of sporocarp-forming species of glomeromycotan fungi in the Diversisporales lineage. Mycol Prog 6:35–44. doi:10.1007/s11557-007-0524-2
Redecker D, Schüβler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the clasification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531. doi:10.1007/s00572-013-0486-y
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg 180
Schüßler A, Walker C (2010) The Glomeromycota: a species list with new families and new genera. Gloucester, England
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Myc Res 105:1413–1421. doi:10.1017/S0953756201005196
Schüßler A, Krüger M, Walker C (2011) Revealing natural relationships among arbuscular mycorrhizal fungi: culture line BEG47 represents Diversispora epigaea, not Glomus versiforme. PLoS ONE 6(8), e23333. doi:10.1371/journal.pone.0023333
Silvestro D, Michalak I (2012) raxmlGUI: A graphical front-end for RAxML. Org Divers Evol 12:335–337. doi:10.1007/s13127-011-0056-0
Simmons MP, Ochoterena H, Carr TG (2001) Incorporation, relative homoplasy, and effect of gap characters in sequence-based phylogenetic analyses. Syst Biol 50:454–462. doi:10.1080/106351501300318049
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, San Diego
Sokolski S, Dalpé Y, Séguin S, Khasa D, Lévesque A, Pitché Y (2010) Conspecificity of DAOM 197198, the model arbuscular mycorrhizal fungus, with Glomus irregulare: molecular evidence with three protein-encoding genes. Botany 88:829–838. doi:10.1139/B10-050
Stamatakis A (2014) RAxML version 8: a tool phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Stockinger H, Peyret-Guzzon MP, Koegel S, Bouffaud M-L, Redecker D (2014) The largest subunit of RNA polymerase II as a New marker gene to study assemblages of arbuscular mycorrhizal fungi in the field. PLoS ONE 9(10), e107783. doi:10.1371/journal.pone.0107783
Stürmer SL, Morton JB (1997) Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89:72–81. doi:10.2307/3761174
Sudová R, Sýkorová Z, Rydlová J, Čtvrtlíková M, Oehl F (2015) Rhizoglomus melanum, a new arbuscular mycorrhizal fungal species associated with submerged plants in freshwater lake Avsjøen in Norway. Mycol Prog 14:9. doi:10.1007/s11557-015-1031-5
Sýkorová Z, Börster B, Zvolenská S, Fehrer J, Gryndler M, Vosátka M, Redecker D (2012) Long-term tracing of Rhizophagus irregularis isolate BEG140 inoculated on Phalaris arundinaceae in a coal mine spoil bank, using mitochondrial large subunit rDNA markers. Mycorrhiza 1:69–80. doi:10.1007/s00572-011-0375-1
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Thiéry O, Moora M, Vasar M, Zobel M, Öpik M (2012) Inter- and intrasporal nuclear ribosomal gene sequence variation within one isolate of arbuscular mycorrhizal fungus, Diversispora sp. Symbiosis 58:135–147. doi:10.1007/s13199-012-0212-0
van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. doi:10.1111/nph.13288
Walker C (1983) Taxonomic concepts in the Endogonaceae: spore wall characteristics in species descriptions. Mycotaxon 18:443–455
Walker C, Schüßler A (2004) Nomenclatural clarifications and new taxa in the Glomeromycota. Mycol Res 108:979–982. doi:10.1017/S0953756204231173
Walker C, Vestberg M, Demircik F, Stockinger H, Saito M, Sawaki H, Nishmura I, Schüβler A (2007) Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol Res 111:137–153. doi:10.1016/j.mycres.2006.11.008
Young ND, Healy J (2003) GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4:6. doi:10.1186/1471-2105-4-6
Acknowledgments
This study was supported in part by Polish National Centre of Science, grants no. 2012/05/B/NZ8/00498 and 2012/07/N/NZ8/02363. Gábor M. Kovács is supported by a János Bolyai Research Scholarship of the Hungarian Academy of Sciences. We thank the assistant manager of the Institute of Ecology and Systematic, Havana, Cuba and the manager of the Słowiński National Park, Poland for permitting us to collect rhizosphere soil samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Marco Thines
Rights and permissions
About this article
Cite this article
Błaszkowski, J., Furrazola, E., Chwat, G. et al. Three new arbuscular mycorrhizal Diversispora species in Glomeromycota. Mycol Progress 14, 105 (2015). https://doi.org/10.1007/s11557-015-1122-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-015-1122-3