Abstract
Six species recorded from Russia in the genus Bolbitius were studied. Among them, B. bisporus and B. pallidus are described as new in this paper. Comprehensive descriptions of all species, illustrations, photographs and comparisons with similar taxa are provided. Phylogenetic analyses were conducted to aid in taxa delimitation as well as identification of species boundaries in the genus Bolbitius. Molecular phylogenetic reconstructions inferred from maximum likelihood and Bayesian analyses were based on combined datasets: nrITS-nrLSU for the whole set of studied species and nrITS-tef1-mtSSU for B. titubans specimens. The molecular data of three genetic markers indicate the existence of at least three divergent lineages in B. titubans, and each may represent an independent taxonomic unit, which suggests that B. titubans is likely a species complex rather than one widely distributed taxon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Bolbitius Fr. was established by E. Fries (1838) with a type species named Bolbitius vitellinus (Pers.) Fr., which is presently considered a synonym of Bolbitius titubans (Bull.) Fr., with the latter epithet having priority. Species of this genus develop their basidiocarps on a wide range of substrates—e.g. dung, sawdust, humus, decayed wood or soil (Watling 1982; Arnolds 2005)—and grow in different types of habitats, including ruderal places. Since the work of R. Singer (1951), the genus Bolbitius has been considered within the family Bolbitiaceae Singer, and has been separated from closely related genera on the basis of a combination of characters, including fragile, often deliquescent and brightly coloured basidiocarps, viscid to glutinous pileus with striate-plicate margin, free ochraceous to rusty brown lamellae, mainly lageniform or utriform cystidia, the presence of pseudoparaphyses in the hymenium, and a hymeniform pileipellis. Molecular analyses have confirmed the position of Bolbitius and related genera within the agaricoid clade and supported the sister relationship between Cortinariaceae and Bolbitiaceae (Moncalvo et al. 2002; Matheny et al. 2006; Tóth et al. 2013). According to Tóth et al. (2013), the core Bolbitiaceae includes the genera Conocybe, Pholiotina, Bolbitius, Galerella, and Descolea, as well as the sequestrate genus Gastrocybe, and species of Bolbitius form a well-supported clade sister to Pholiotina.
According to the Index Fungorum database (www.indexfungorum.org, up to 14 March, 2015), more than 70 species of Bolbitius are known worldwide. Species delineation within the genus remains unclear, and several authors (Watling 1982; Enderle et al. 1985; Arnolds 2005) have commented on the need to clarify the range of variation and boundaries of individual species.
At present, there are many records of Bolbitius known from Russia, but a limited number of species. During our taxonomic investigations of the genus, based on available herbarium material as well as new collections resulting from the expeditions, some new records of known species were reported and two new species were described. This study, which deals with collections from Russia, proposes the revision of genus diversity in the studied territory based on morphology and molecular phylogenetic analysis.
Materials and methods
Morphological study
Macroscopic descriptions were based on both fresh and herbarium materials as well as photos taken at the site, with colour codes following Kornerup and Wanscher (1978). The micromorphological characters were determined from dried specimens examined under an Axio Scope.A1 (Carl Zeiss Meditec AG, Jena, Germany) and a Micmed-2 (LOMO [Leningrad Optical Mechanical Association], St. Petersburg, Russia) light microscope, using a 5 % potassium hydroxide (KOH) or ammonia Congo red solution. At least 30 basidiospores were measured (n indicates this number; Q indicates the basidiospore length/width ratio, with Qav denoting the average Q of all spores).
All examined specimens are deposited in the Mycological Herbarium of the Komarov Botanical Institute (LE, Saint Petersburg, Russia).
Molecular techniques
DNA was extracted from small fragments of dried basidiocarps using the AxyPrepTM Multisource Genomic DNA Miniprep Kit (Axygen Biosciences, Inc., Union City, CA, USA) and the NucleoSpin® Plant II Kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany), according to manufacturer protocols, without modification. The following primers were used for amplification and sequencing: ITS1F-ITS4B (Gardes and Bruns 1993) for the ITS1-5.8S-ITS2 fragment; LROR-LR5 (White et al. 1990) for the nrLSU gene; EF1-983F and EF1-1567R for approximately 500 base pairs (bp) of tef1 (Rehner and Buckley 2005); and MS1 and MS2 for mtSSU (White et al. 1990). PCR products were sequenced using the BigDye v3.1® Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA), following manufacturer recommendations, with the same primers. Products were purified using the ethanol precipitation method and sequenced using the ABI 3130 Genetic Analyzer (Applied Biosystems). Sequencing Analysis Software v5.3.1 (Applied Biosystems) was used for processing the raw data. The sequences obtained were then assembled and manually adjusted in MEGA 6 (Tamura et al. 2013).
In addition to the sequences generated here, 17 nrITS, nine nrLSU, and one tef1 sequence, including outgroups, were retrieved from the GenBank database using the BLAST application. The taxonomic identities of these sequences are given as they appear in GenBank. The final datasets were aligned in the Mafft v7 web tool (Katoh and Toh 2008; http://mafft.cbrc.jp/alignment/server/) using the Q-INS-i strategy. Ambiguously aligned regions in the nrITS region were identified and excluded from the alignment for subsequent analyses using TrimAl software (Capella-Gutierrez et al. 2009). The alignments were further corrected manually, as necessary, using MEGA 6. The individual alignment files for the combined datasets were concatenated in MEGA 6. Two separate datasets were assembled for phylogenetic analysis: combined nrITS-nrLSU for all studied taxa and combined nrITS-tef1-mtSSU for the B. titubans species complex.
Phylogenetic analysis
Phylogenetic reconstructions were performed individually for the two datasets (nrITS-nrLSU and nrITS-tef1-mtSSU) using the maximum likelihood (ML) and Bayesian inference (BI) methods of analysis. Prior to analysis, the best-fit substitution models for the alignment were estimated for each dataset using the Akaike information criterion (AIC) in the FindModel web server (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html). In both BI and ML analyses, the GTR + G model was employed for the nrITS-nrLSU dataset and the TrN + G model for the nrITS-tef1-mtSSU dataset. ML analyses were run in the PhyML server, version 3.0 (http://www.atgc-montpellier.fr/phyml/), under a GTR model and gamma-distributed parameter with 1000 rapid bootstrap replicates. BI was performed with MrBayes 3.1 software (Ronquist and Huelsenbeck 2003), under the described DNA models. Analyses were performed with two parallel searches and four chains, with ten million generations for the nrITS-nrLSU dataset and four million generations for the nrITS-tef1-mtSSU dataset, and a sampling frequency of every 100th generation. A clade was considered strongly supported if it received bootstrap support (BS) greater than 50 % and/or posterior probability (PP) equal to or greater than 0.95.
Pairwise distance between nrITS sequences was calculated using MEGA 6, under the GTR model.
Results and discussion
Newly generated sequences
Eighty-five new sequences were generated for this study: 30 nrITS, 29 nrLSU, 13 tef1 and 13 mtSSU. All sequences were deposited in GenBank, with corresponding accession numbers (Table 1). Alignments were deposited in TreeBASE (S17566 for the nrITS-nrLSU dataset, S17617 for the nrITS-tef1-mtSSU dataset).
Phylogeny
Combined nrITS-nrLSU dataset
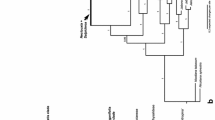
This dataset includes 45 specimens of Bolbitius species as well as Galeropsis desertorum (voucher PR 154181) and Conocybe apala (strain NL-1012) as outgroup taxa: 36 are represented by both nrITS and nrLSU sequence data, and nine by only nrITS data. The final dataset comprised 1513 characters, including gaps, after the exclusion of ambiguously aligned regions. The overall topologies of the ML and BI trees were almost congruent. The main clades recovered were well supported in both analyses. The resulting ML tree is shown in Fig. 1. The phylogenetic tree indicates the existence of ten divergent lineages or phylogenetic taxa within the group of Bolbitius species analysed. These phylogenetic taxa correspond to eight accepted morphological species: B. subvolvatus Hauskn., Contu & Krisai, Bolbitius lacteus J.E. Lange, B. titubans (Bull.) Fr., B. coprophilus (Peck) Hongo, B. callistus (Peck) Watling, B. muscicola (G. Stev.) Watling, B. reticulatus (Pers.) Rick., B. excoriatus Dähncke, Hauskn., Krisai, Contu & Vizzini, and two newly discovered species (described below). The species-level clades received high or 100 % support in all analyses. Phylogenetic lineages, however, only partially coincided with morphologically identified specimens, due to the considerable intraspecific morphological variability (see Fig. 3).
Best tree from ML analyses of the combined nrITS-nrLSU dataset of Bolbitius species. Support values (BS ≥ 50 %/PP ≥ 0.95) are given above the branches. Sequences represented by the nrITS-nrLSU region are followed by the specimen voucher/strain numbers in parentheses. Sequences represented by only one genetic marker are followed by GenBank accession numbers. Scale bar indicates expected changes per site
In ML analysis, two of our collections (LE 303557 and LE 234343) formed a group with moderate support (BS = 69 %), sister to the B. reticulatus/B. muscicola clade, which was not supported in Bayesian inference. However, these specimens were characterised by distinct morphological features that allowed them to be separated from similar taxa and to be considered as a new taxon (described below as B. pallidus).
Another collection (LE 303558), originating from the Primorye Territory, together with one sequence retrieved from GenBank (JF907770), demonstrated some molecular deviation from other studied specimens of B. reticulatus, and this delimitation received high support (BS = 100 %, PP = 1) in all analyses. This phylogenetic data was confirmed by morphological observation, and thus we describe a new species Bolbitius bisporus.
Significant intraspecific variability within the B. titubans group was revealed by subdivision into at least four subclades. For more reliable detection of phylogenetic heterogeneity of this species complex, we carried out additional phylogenetic analysis of a multigene dataset with genetic markers providing significant resolution on both the species and subspecies levels (nrITS-tef1-mtSSU).
Combined nrITS-tef1-mtSSU dataset
This dataset includes 14 specimens from the B. titubans species complex: 13 are represented by all three loci, and one (isolate AFTOL-ID 730 from GenBank) by only nrITS-tef1 data. The final dataset comprised 1855 characters, including gaps.
Four subclades were revealed, as in the analysis of the nrITS + nrLSU dataset, but with greater statistical support (BS = 100 % and PP = 1). The phylogram based on Bayesian analysis is shown in Fig. 2.
Fifty percent majority-rule consensus phylogram inferred from Bayesian analysis of the combined nrITS-tef1-mtSSU dataset of the representatives of the Bolbitius titubans species complex. The tree is midpoint-rooted. Support values (BS ≥ 50 %/PP ≥ 0.95) are given above the branches. Sequences are followed by the specimen voucher/strain numbers in parentheses
One of the subclades (subclade I) corresponds to B. titubans in its narrow interpretation (Watling 1982), as indicated by the presence of specimens with vividly coloured lemon-yellow or orange-yellow deliquescent basidiocarps, although the collection with pure white basidiocarps and small basidiospores (LE 202345) is also included here. The second subclade (subclade II) includes some Russian specimens previously identified as B. lacteus. However, the morphological features of the studied collections belonging to this subclade are consistent with the description of B. lacteus only in terms of basidiocarp colouration; the size of basidiospores can reach 16–18 μm in length and 10 μm in width. The third sequence group (subclade III), which includes only two studied collections (together with two collections from GenBank in previous analysis), has uncertain taxonomic connections: the sequences retrieved from GenBank are all labeled as B. variicolor. and one specimen from Russia (LE 214359) was also previously identified as B. variicolor. Morphological examination showed that pileus colouration was the only character separating these collections from the specimens of B. titubans. One specimen (LE 303576) from subclade IV consistently occupies an independent position in all phylogenetic reconstructions (Figs. 1 and 2).
Thus we are confronted with the fact that phylogenetic studies only confirm the taxonomic confusion around the B. titubans complex and closely related species. It is becoming increasingly apparent that B. titubans represents a species complex rather than one widely distributed taxon. The species delimitation within this complex clearly requires additional study of collections from around the world, using both morphological and molecular data. At this time, in the absence of clear morphological and molecular evidence, we cannot consider subclades revealed in our study as independent entities and treat them as independent taxonomic units. However, we found it useful to present separate morphological descriptions for each of our subclades in order to highlight the presence or absence of morphological differences that may have taxonomic significance in the future (see "Taxonomy").
Taxonomy
Bolbitius titubans (Bull.) Fr., Epicrisis Systematis Mycologici: 254, 1838.
Subclade I
Pileus 15–60 mm broad and 15–25 mm high, oblong-ovoid or ellipsoid when young, then conical or conico-convex becoming applanate, often with broad low umbo or slightly depressed at center; rapidly deliquescent; surface usually viscid with separable sticky pellicle, more rarely dry, glabrous, sometimes wrinkled at center, not hygrophanous, pale yellow (1A2), vivid yellow (2A6-8, 3A7-8), lemon-yellow (3B8) to straw-yellow (3B4), often with darker orange-yellow (4A7, B7) center and pale-coloured ochraceous or beige margin (4B3-4); margin translucently striate to sulcate-striate in mature basidiocarps. Lamellae free or almost free, crowded, thin, soon deliquescent, pale yellow or lemon-yellow (3A3, 3B8) at first, then hazel (6E8), ochre-brown, cinnamon or olive-brown (4D8, E8), with flocculose concolorous edge. Stipe 45–100 × 2–8 mm, cylindrical or uniformly broadened towards base but without bulb, fistulose, fragile, white to pastel yellow, pale lemon-yellow or ochre-yellow (1A3-4, 2A3-6), entirely pruinose, often with moire pattern of white floccules at base. Context thin, fragile, yellowish. Smell and taste not distinctive.
Basidiospores (12)13–16(18.5) × (7)8–9.5(10.5) μm, Q = 1.43–1.84(1.94), Qav. = 1.67, n = 30, narrowly to broadly ellipsoid-oblong, ovoid-oblong, sometimes weakly angular or lentiform, slightly flattened or phaseoliform in side view, yellow-brown to dark rusty brown in KOH, thick-walled, with wide (2–2.5 μm) central or slightly eccentric germ pore. Basidia predominantly 4-spored, 18–30 × 12–15 μm, broadly clavate, surrounded by similar shaped pseudoparaphyses, 24–32 × 13–21 μm. Lamellae edge sterile. Cheilocystidia numerous, (27)35–80 × 10–35 μm, extremely variable in shape, mostly broadly lageniform or utriform with rounded apex, more rarely clavate, subcylindrical or irregular-shaped, hyaline, thin-walled. Pleurocystidia absent. Pileipellis hymeniform, composed of 25–75 × 7–20 μm, broadly clavate, piriform or spheropedunculate elements, thin-walled, hyaline or with yellowish intracellular pigment. Pileocystidia absent. Stipitipellis a cutis, containing fascicles of caulocystidia, 27–70 × 11–23 μm, highly varying in shape: utriform, subcylindrical with subcapitate apex, barrel-shaped, clavate, broadly lageniform, sometimes with lateral projections, hyaline, thin-walled. Clamp-connections absent.
Habitat
Solitary or in small groups on soil, litter, straw, decayed wood or dung, in different types of forest, grassland, ruderal sites and pastures.
Material examined
RUSSIA. European part – Vologda Region, Kirillovsky District, National Park “Russky Sever”, meadow, on litter, 16 Sept. 2004, col. and det. O. Kirillova (LE 235346); Leningrad Region, Priozersky District, Borisovsky peninsula, vicinity of Otradnoye, pasture, 29 Sept. 1994, col. A. Kovalenko, O. Sutyrina, det. E. Malysheva (LE 202345); Moscow Region, Prioksko-Terrasny Nature Reserve, broadleaf forest, on decayed wood, 20 Jun. 1990, col. and det. G. Levitskaya (LE 216994); Samara Region, Krasnoglinsky District, vicinity of Pribrezhny, mixed forest, on soil, 8 Jun. 2001, col. and det. E. Malysheva (LE 303562); Samara Region, Zhigulevsky Nature Reserve, vicinity Bakhilova Polyana village, broadleaf forest, on decayed wood, 10 Jul. 2003, col. and det. E. Malysheva (LE 227424); Republic of Adygeya, Maykop District, National Park “Bolshoi Tkhach”, hillside of Bolshoi Tkhach mountain (h = 2000 m.a.s.l.), subalpine meadow, on soil, 13 Aug. 2004, col. and det. A. Kiyashko (LE 265066). Urals – Sverdlovsk Region, vicinity of Bolshiye Galashki village, lawn, on soil, col. and det. L. Marina (LE 258041). Western Siberia – Altai Territory, Altaisky Nature Reserve, Teletskoye Lake, Chiri River, birch forest, on cow dung, 14 Aug. 1985, col. and det. A. Kovalenko (LE 11335).
Notes
In general, the studied specimens of this subclade demonstrate considerable variation in morphological characteristics in terms of basidiocarp size, colouration, and substrate preferences, as well as basidiospore dimension, which explains the difference in species names given on morphological identification (B. vitellinus, B. variicolor, B. titubans, B. lacteus). However, the main features of this subclade are brightly coloured and fragile basidiocarps with a distinct yellow tint in combination with large, thick-walled basidiospores. One collection (LE 202345) is an exception, and is characterised by pure white basidiocarps, but with large, thick-walled basidiospores.
Most of the Russian collections studied belong to this subclade. Sequences of three collections retrieved from GenBank and labeled as B. vitellinus and B. elegans are also included in the group (Fig. 1).
Subclade II
Four of our collections belonging to this highly supported (BS = 100 %, PP = 1) subclade (Fig. 2) differ from the first phylogenetic group (subclade I) primarily in basidiocarp colouration and basidiospore size. All of the studied specimens have faded basidiocarps with whitish, pale yellowish or beige pileus (2A2-3, 4C3-4), without bright yellow tones, and white or pale lemon-yellow (1A2-3) stipe. Basidiospores are slightly larger (13.5–18(21) × (8)9–10.5(11) μm, Q = 1.46–1.89, Qav. = 1.65, n = 35) but identically shaped.
In the field these specimens can be confused with B. lacteus. However, based on combination of macro- and micromorphological characters the group does not correspond to the B. lacteus concept accepted by a majority of authors (Arnolds 2005), in which the main diagnostic features recognized are small basidiocarps together with small basidiospores (10–13(14) × (5.5)6–7.5 μm) and cheilocystidia not exceeding 40 μm long and 20 μm wide.
Habitat
Solitary or in small groups on soil, litter or humus, in different types of habitat (grassland, pastures and forest).
Material examined
RUSSIA. European part – Rostov Region, Sholokhovsky District, vicinity of Veshenskaya, pine forest, on litter, 21 Jul. 2003, col. Yu. Rebriev, det. E. Malysheva (LE 287256); Stavropol Territory, on pile of humus, 5 Oct. 2010, col. and det. T. Svetasheva (LE 289428); Stavropol Territory, Grachevsky District, vicinity of Lisichki village, pasture, on soil among grass, 23 Sept. 2009, col. T. Svetasheva, det. T. Svetasheva, E. Malysheva (LE 303575, LE 303577).
The sequences of two collections retrieved from GenBank and labeled as B. vitellinus (MSC 378484) and B. lacteus (MSC 378485) are also included in the subclade (Fig. 1).
Subclade III
Fig. 6
One Russian collection belonging to this phylogenetic group is characterised by muted colouration of basidiocarps, not containing vivid yellow tones, but having greyish yellow, yolk-yellow (3B8, 4B8), or olive-yellow (3C4-6) pileus, wrinkled or venous at center. Basidiospores are non-significantly smaller than in previous groups, (12.5)13–15(16) × (7.5)8–9.5 μm, and most of cheilo- and caulocystidia have subglobose shape.
The sequences of two collections retrieved from GenBank and one collection from Sweden (LE 214359) labeled as B. variicolor are also included in the subclade (Fig. 1).
Based on morphology, we have not yet found a clear taxonomic criterion, apart from pileus colouration, to separate this collection from other groups inside the B. titubans clade.
Habitat
In small groups, on sawdust or horse dung.
Material examined
RUSSIA. Far East – Primorye Territory, vicinity of Terney, on sawdust, 1 Sept. 2012, col. and det. E. Malysheva (LE 303556).
Subclade IV
One collection (LE 303576), occupying a sister position to subclade III (Figs. 1 and 2), differs from its members by the presence of a long insertion (21 nucleotides) in 146–166 positions of the nrITS sequence. However, we have no convincing morphological evidence of its independent status within the whole B. titubans group other than significantly smaller basidiospores, with dimensions not overlapping those in other studied collections (11–12.5(13) × 6–7.5 μm).
Material examined
RUSSIA. European part – Samara Region, vicinity of Zadel’noye village, floodplain of Volga River, deciduous forest (Populus, Ulmus), on soil, 25 Jun. 2011, col. and det. E. Malysheva (LE 303576).
We can conclude that B. titubans is clearly a complex of different morphotypes, also supported phylogenetically, but the question of whether the members of this complex represent independent taxa will require further research.
Bolbitius lacteus J.E. Lange, Fl. agar. dan. 5, Appendix: II, 1940.
Fig. 8
Pileus 15 mm broad, conico-convex when young, then plano-convex becoming applanate, with small low umbo; soon deliquescent; surface slightly viscid, not hygrophanous, whitish (1A1, 1A2), yellowish or cream at center (2A3, 4A3); margin sulcate-striate up to ½ of radius or to center. Lamellae free, crowded, thin, soon deliquescent, pale yellow (3A3) at first, then hazel (6E8), ochre-brown or orange-brown (5C8, 6C8), with flocculose whitish edge. Stipe 45 × 1.5 mm, cylindrical, fistulose, fragile, white or cream, entirely pruinose-flocculose. Context thin, fragile, white. Smell and taste not distinctive.
Basidiospores 10.5–11.5(12) × (6)6.5–8 μm, Q = 1.35–1.66(1.74), Qav. = 1.49, n = 30, broadly ellipsoid or ovoid-oblong, slightly flattened, sometimes subphaseoliform in side view, yellow-brown in KOH, thick-walled, with wide (1.5–2.5 μm) central or slightly eccentric germ pore. Basidia 4-spored, 18–27 × 10–15 μm, broadly clavate, surrounded by similar-shaped pseudoparaphyses. Lamellae edge sterile. Cheilocystidia numerous, 25–40 × 8–14 μm, clavate, subcylindrical, broadly lageniform with rounded apex or utriform, rarely irregular-shaped, hyaline, thin-walled. Pleurocystidia absent. Pileipellis hymeniform, composed of 27–50 × 8–20 μm, broadly clavate or subcylindrical elements, thin-walled, hyaline. Pileocystidia absent. Stipitipellis a cutis, containing fascicles of caulocystidia, 20–60 × 8–12 μm, varying in shape: utriform, subcylindrical clavate or broadly lageniform, sometimes with lateral projections, hyaline, thin-walled. Clamp-connections absent.
Habitat
Solitary, on soil in forest. It is a rare species and known from Russia on the basis of only one collection.
Material examined
RUSSIA. Far East – Primorye Territory, Sikhote-Alinsky Nature Reserve, Kunaleika field station, Khanova brook, mixed forest, on soil, 29 Aug. 2013, col. A. Kovalenko, det. E. Malysheva (LE 303559).
Notes
Bolbitius lacteus is characterised by small white or very pale-coloured basidiocarps and small basidiospores. Morphologically, it is similar to B. reticulatus var. pluteoides (M.M. Moser) Arnolds, when the latter is accepted, but differs from it in its larger basidiospores with a wide germ pore.
B. lacteus is distinguished from the pale-coloured phenotypic variant of B. titubans by smaller basidiospores and significantly smaller cheilocystidia.
Molecularly, this species is closer to B. titubans than to the B. reticulatus clade, and is well separated from both on the resulting phylogenetic tree of the combined nrITS-nrLSU dataset (Fig. 1).
Bolbitius coprophilus (Peck) Hongo, Mem. Fac. Educ. Shiga Univ. Nat. Sci. 9: 82, 1959.
Pileus 15–50 mm broad and 10–20 mm high, oblong-ovoid at first, then campanulate or conico-convex becoming applanate, usually umbonate with broad low umbo or slightly depressed at center; surface viscid, more rarely dry, glabrous, weakly hygrophanous, whitish, pale pink (10A2-3), at the center finally becoming greyish pink (9B2-3), ochre-brown or olive-brown with pinkish tint, with pale-coloured whitish or pinkish margin; up to ½ radius striate. Lamellae free, crowded, thin, firstly whitish, pale pink, soon becoming ochre, ochre-brown or rusty brown (4E8, 5E8), with concolorous edge, deliquescent. Stipe 60–130 × 3–7 mm, cylindrical or uniformly broadened towards slightly bulbous base (up to 10 mm wide), fistulose, fragile, white to pale pink (9-10A2-3), turning pale ochraceous or sandy (4B3-4), entirely pruinose. Context thin, fragile, white to pinkish. Smell and taste not distinctive.
Basidiospores (12.5)13–16.5(18.5) × 8–11(12.5) μm, Q = (1.18)1.31–1.86(2.03), Qav. = 1.57, n = 40, highly variable in size and shape, predominantly broadly ellipsoid, ovoid-oblong, sometimes weakly angular or lentiform, slightly flattened or phaseoliform in side view, golden brown to rusty brown in KOH, thick-walled, with wide (up to 2.5 μm) central or slightly eccentric germ pore. Basidia 4-spored, 24–27 × 13–17.5 μm, broadly clavate, surrounded by pseudoparaphyses similar-shaped and difficult to find. Lamellae edge sterile. Cheilocystidia numerous, 35–80 × 20–40(50) μm, highly variable in shape, mostly broadly lageniform with short and wide neck, utriform with rounded apex, broadly clavate, spheropedunculate or subcylindrical, hyaline, thin-walled. Pleurocystidia absent. Pileipellis hymeniform, composed of 16–62 × 8–20 μm, broadly clavate or piriform elements, thin-walled, hyaline or with pale pink intracellular pigment. Pileocystidia absent. Stipitipellis a cutis, containing fascicles of caulocystidia, 25–62(80) × 13.5–33 μm, varying considerably in shape from subcylindrical and clavate to utriform, broadly lageniform, spherical and irregular shaped with lateral projections, hyaline, thin-walled. Clamp-connections absent.
Habitat
Usually scattered or gregarious on compost, dung or straw in ruderal sites and pastures. It is not rare, probably a thermophilous species.
Material examined
RUSSIA. European part – Penza Region, Shemysheisky District, on rotten straw, 24 Jul. 1989, col. A. Ivanov, det. A. Hausknecht, E. Malysheva (LE 18905, 18599); Rostov Region, Orlovsky District, vicinity of Manych, on dung, 17 May 2008, col. Yu. Rebriev, det. T. Svetasheva (LE 287244). Eastern Siberia – Irkutsk Region, vicinity of Kob’ village, near a farm, on dung, 28 Jul. 1984, col. and det. V. Astapenko (LE 11317).
Notes
Bolbitius coprophilus is characterised by pale-colored basidiocarps with a distinct pinkish tinge and large thick-walled spores, and inhabits rich organic substrates, e.g. dung or compost. It can be separated from the other species of the genus by this particular combination of features. However, it is hardly distinguishable from two species, B. incarnatus Hongo and B. demangei (Quél.) Sacc. & D. Sacc., based on morphological data alone. B. incarnatus, originally described from Japan, with pink-coloured pileus and large spores (up to 16 μm long: Hongo 1958), may represent the same taxon as B. coprophilus in light of new morphological and molecular data, but we had no opportunity to examine B. incarnatus collections in the framework of this study. B. demangei differs from B. coprophilus in the darker overall colouration of its pileus, with the presence of a violaceous tint (Arnolds 2005; Hausknecht and Contu 2006), and obviously smaller spores (8.5–14 × 6–8 μm), whereas the nrITS sequence designated as B. demangei (JF907771) available in the public database (GenBank) appeared to be identical with the sequences of our collections (with 98–99 % similarity), determined as B. coprophilus. The natural geographical distribution of B. demangei is apparently understudied because the vast majority of records were made inside greenhouses or in ruderal places indicating their adventive origin (Hausknecht and Contu 2006). Therefore, the question of the identity of both species raised by M. Enderle et al. (1985) is still open and requires further investigation with additional material.
Bolbitius reticulatus (Pers.) Rick., Blätterpilze 1: 68, 1915.
Pileus 8–20 mm broad and 5–8 mm high, hemispherical when young, then broadly conical to convex becoming applanate, usually umbonate with small low umbo; surface glutinous to viscid, glabrous, often wrinkled at center, not or weakly hygrophanous, highly variable in colouration – from whitish, white-pink or pinkish cream to beige (8A2, B2), ochre, often with vinaceous tint (10D8, E8), grey or grey-brown (6D3-4), with dark grey, brownish violet or almost black (7F3-4, 11E8) center; up to ½ radius striate. Lamellae free, crowded, thin, pale yellow-pink or yellow-brown (5A4, 5C8) at first, then hazel, orange-brown or rusty brown (6D8, 6E8), with flocculose concolorous edge. Stipe 15–35 × 1–3 mm, cylindrical or uniformly broadened towards base but without distinct bulb, fistulose, fragile, white to pale yellowish (1A3-4), entirely pruinose to flocculose. Context thin, fragile, whitish or greyish. Smell and taste not distinctive.
Basidiospores (8)10–13 × (5)5.5–6(7) μm, Q = 1.59–2.27, Qav. = 1.99, n = 30, narrowly to broadly ellipsoid or ovoid-oblong, slightly amygdaliform or phaseoliform in side view, yellow-brown or golden brown in KOH, thin- or slightly thick-walled, with small to medium-sized central germ pore (up to 1–1.5 μm wide). Basidia mostly 4-spored, 16–22 × 8–11 μm, broadly clavate, surrounded by similar-shaped pseudoparaphyses, often difficult to notice. Lamellae edge sterile. Cheilocystidia numerous, 23–45 × (5.5)8–18 μm, predominantly broadly lageniform with short and wide neck, utriform with rounded apex, more rarely clavate, subcylindrical or irregular-shaped, hyaline, thin- or slightly thick-walled. Pleurocystidia absent. Pileipellis hymeniform, composed of 16–45 × 10–35 μm, broadly clavate, piriform or globose elements, thin-walled, hyaline or with greyish intracellular pigment. Pileocystidioid elements rather numerous, represented by apical cylindrical, narrowly lageniform or narrowly clavate cells, 18–35 × 5.5–8 μm, hyaline or weakly pigmented, thin-walled, at the top of erect hyphae towering above the layer of globose elements. Stipitipellis a cutis, containing clusters of caulocystidia, 20–45 × 8–17.5 μm, varying in shape like cheilocystidia, broadly lageniform, utriform, barrel-shaped, clavate or irregular with lateral projections, hyaline, thin- to slightly thick-walled. Clamp-connections absent.
Habitat
Solitary or in small groups on decayed wood, more rarely on litter or soil, in different types of forest. In Russia it is a rather common species, widely distributed throughout the whole territory.
Material examined
RUSSIA. European part – Leningrad Region, Boksitogorsky District, vicinity of Somino, Dolgoye swamp, spruce forest, on decayed coniferous wood, 26 Aug. 1999, col. and det. O. Morozova (LE 215469); Moscow Region, Odintsovsky District, Zvenigorod biological station, grassy site, on buried wood, 2 Jul. 2012, col. and det. E. Voronina (LE 253961); Samara Region, Zhigulevsky Nature Reserve, vicinity of Bakhilova Polyana village, broadleaf forest, on wood, 4 Jul. 2005, col. and det. E. Malysheva (LE 234342); the same place, aspen forest, on litter, 12 Jul. 2003, col. and det. E. Malysheva (LE 227536). Far East – Primorye Territory, Sikhote-Alinsky Nature Reserve, Venera field station, pine forest, on branch of Acer, 29 Aug. 2012, col. and det. E. Malysheva (LE 303561); the same place, vicinity of Kabany field station, spruce forest, on decayed wood, 26 Aug. 2011, col. and det. E. Malysheva (LE 303560).
Notes
Bolbitius reticulatus is a morphologically variable species that includes several phenotypic variants differing in the size of basidiocarps, colouration, pileus surface structure and spore size. Because of these significant morphological differences, some varieties and forms have been recognized within B. reticulatus (Arnolds 2005). In our study, however, the collections with small, pale-coloured basidiocarps, which should be referred to as var. pluteoides, formed a single monophyletic clade together with collections having medium-sized basidiocarps and a smooth pileus (considered by some authors as f. aleuriatus) and collections representing typical morphological variants of B. reticulatus f. reticulatus on the phylogenetic tree (Fig. 1). Accordingly, we use a wide species concept for B. reticulatus, without subdividing it into traditionally accepted varieties and forms.
Bolbitius bisporus E.F. Malysheva, sp. nov.
Fig. 11
MycoBank No. MB 812349
Diagnosis
The main distinctive characters are small basidiocarps with dark greyish violet pileus, 2-spored basidia, and large, thick-walled basidiospores (av. 13–17 × 6.5–8.5 μm) with medium-sized germ pore.
Pileus 15 mm broad, convex when young, then plano-convex becoming applanate, without umbo; surface slightly viscid or dry, glabrous, wrinkled at center, weakly hygrophanous, dark greyish violet (17E2-3), with pale-coloured grey-brown (5D2) margin; margin translucently striate. Lamellae free, crowded, thin, pale ochre-brown to orange-brown (6C6-8), with concolorous edge. Stipe 25 × 1.5–2.5 mm, uniformly broadened towards base but without distinct bulb, fistulose, fragile, white to pale ochre-yellow (3A2-3), entirely pruinose. Context thin, fragile, whitish. Smell and taste not distinctive.
Basidiospores (11)13.5–17(18.5) × 6–8.5(9) μm, Q = 1.66–2.10(2.31), Qav. = 1.97, n = 30, highly variable in size and shape, from narrowly to broadly ellipsoid, ellipsoid-oblong, some lentiform, slightly flattened or amygdaliform in side view, yellow-brown in KOH, slightly thick-walled, with medium-sized (1–1.7 μm wide) central or slightly eccentric germ pore. Basidia 1- or 2-spored, 18–26 × 8–11 μm, broadly clavate, surrounded by similar-shaped or subglobose pseudoparaphyses, 13–18 × 8–11 μm. Lamellae edge sterile. Cheilocystidia numerous, 25–40 × 7–14 μm, mostly broadly lageniform with wide neck (up to 8 μm) and rounded apex, subcylindrical, clavate or utriform, hyaline, thin-walled. Pleurocystidia absent. Pileipellis hymeniform, composed of 15–40 × 10–32 μm, broadly clavate or subglobose and globose elements, in chains, thin-walled, hyaline. Pileocystidioid elements rather numerous, represented by apical cylindrical, narrowly lageniform or narrowly clavate cells, 20–30 × 5.5–9.5 μm, hyaline or weakly pigmented with intracellular greyish pigment, thin-walled, at the top of erect hyphae towering above the layer of globose elements. Stipitipellis a cutis, containing fascicles of caulocystidia, 16–60 × 8–20 μm, as variable as cheilocystidia, utriform, broadly lageniform, subcylindrical, clavate, sometimes irregular-shaped, hyaline, slightly thick-walled. Clamp-connections absent.
Habitat
Solitary on litter in lowland coniferous-broadleaf forest. It is known only from the type locality.
Material examined
RUSSIA. Primorye Territory, Sikhote-Alinsky Nature Reserve, Kunaleika field station, Khanova brook, mixed forest, on litter, 29 Aug. 2013, col. E. Malysheva (HOLOTYPE, LE 303558).
Etymology
The name refers to the number of spores per basidium.
Notes
Both ML and BI analyses indicated, with strong support values, that the closest relative taxon of B. bisporus was B. reticulatus. Our new species differs from B. reticulatus mainly in its 2-spored basidia and large, thick-walled spores with medium-sized germ pore.
The nrITS sequence of our specimen matches the sequence from GenBank labeled B. aleuriatus (JF907770), which is distributed in Italy. The sequences differ by only three nucleotides, which shows that the species is quite widespread, but additional data are needed to clarify its true geographical distribution.
Bolbitius pallidus E.F. Malysheva & T. Svetasheva, sp. nov.
Fig. 12
MycoBank No. MB 812350
Diagnosis
Distinguished from B. reticulatus by the lack of vinaceous tint in pileus colouration, slightly longer and broader basidiospores, and supcapitate cheilo- and caulocystidia.
Pileus 10–20 mm broad and 5–7 mm high, hemispherical when young, then broadly conical to convex becoming applanate, usually umbonate with small low umbo; surface weakly viscid to dry, glabrous, not or weakly hygrophanous, very pale, whitish, cream or yellowish cream at center (3A3, 4A3-4); margin translucently striate up to ½ radius. Lamellae free or almost free, crowded, thin, pale yellowish at first (2A3), then ochre or hazel (6E8), with flocculose whitish edge. Stipe 35–50 × 1.5–3.5 mm, cylindrical or uniformly broadened towards base but without distinct bulb, fistulose, fragile, pale lemon-yellow (1A4, 2A6), entirely pruinose to flocculose. Context thin, fragile, whitish. Smell and taste not distinctive.
Basidiospores (9.5)10.5–13.5(14.5) × 5–7(8) μm, Q = (1.67)1.74–2.08(2.20), Qav. = 1.93, n = 40, narrowly to broadly ellipsoid, ellipsoid-oblong or ovoid-oblong, slightly amygdaliform or phaseoliform in side view, yellow-brown or golden brown in KOH, thin- or slightly thick-walled, with small central germ pore (up to 1–1.5 μm wide). Basidia mostly 4-spored, but 2-spored also present, 18–24 × 8–11 μm, broadly clavate, surrounded by similar-shaped pseudoparaphyses, often difficult to find. Lamellae edge sterile. Cheilocystidia numerous, 25–55 × 8–20 μm, mostly utriform, with inflated and distinctly subcapitate apex, broadly lageniform, clavate or subcylindrical, hyaline or with yellowish intracellular pigment, thin-walled. Pleurocystidia absent. Pileipellis hymeniform, composed of densely packed, 12–40 × 9–30 μm, broadly clavate, globose or spheropedunculate elements in chains, thin-walled, hyaline or with yellowish intracellular pigment. Pileocystidioid elements scattered as apical subcylindrical, lageniform or narrowly clavate cells, 20–30 × 8–13 μm, hyaline or weakly pigmented, thin-walled, terminated hyphae rising above the layer of globose elements. Stipitipellis a cutis, containing clusters of caulocystidia, 25–68 × 8–20 μm, varying in shape, predominantly subcylindrical with distinct subcapitate apex, broadly lageniform, utriform or irregular with thick lateral projections, hyaline, slightly thick-walled. Clamp-connections absent.
Habitat
Solitary or in small groups on decayed wood or litter, in broadleaf or mixed forests. There are only two records, both in the Samara Region.
Material examined
RUSSIA. European part – Samara Region, Zhigulevsky Nature Reserve, vicinity of Bakhilova Polyana village, broadleaf forest, on decayed deciduous wood, 3 Jul. 2005, col. E. Malysheva (HOLOTYPE, LE 234343); Samara Region, vicinity of Pribrezhny, mixed forest, on decayed wood in litter, 22 Jun. 2005, col. E. Malysheva (LE 303557).
Etymology
The name is given to this taxon because of the very pale colouration of its pileus.
Notes
Bolbitius pallidus is characterised by small basidiocarps, with a very pale and smooth pileus, and utriform or subcylindrical cheilo- and caulocystidia, with distinct subcapitate apex.
The subcapitate cystidia is not a typical character in the genus Bolbitius, which helps to distinguish the species from the other taxa. The most morphologically similar species is B. reticulatus, with which B. pallidus shares the most characters, differing only in cystidia shape and spore size (they are longer and broader in B. pallidus) and in the absolute lack of vinaceous tint in its pileus colouration. In the resulting phylogenetic tree of the combined nrITS-nrLSU dataset (Fig. 1), the sequences of the two studied specimens of B. pallidus form one monophyletic clade with moderate support (BS = 69 %) separated from B. reticulatus. The genetic distance between the sequences of these two species amounts to 5–7 %, allowing them to be considered as two different taxa.
References
Arnolds E (2005) Bolbitius Fr. In: Noordeloos ME, Kuyper TW, Vellinga EC (eds) Flora Agaricina Neerlandica, vol 6. CRC Press, Boca Raton, pp 112–119
Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T (2009) TrimAl, a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi:10.1093/bioinformatics/btp348
Enderle M, Kajan E, Krieglsteiner GJ (1985) Studien in der Gattung Bolbitius Fries. Mitt Arbeitsgem Pilzk Niederrhein 3:5–34
Fries EM (1838) Epicrisis Systematis Mycologici, seu Synopsis Hymenomycetum. Typographia Academica, Uppsala, 1836
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Hausknecht A, Contu M (2006) Bolbitius demangei in Italien. Österr Z Pilzk 1:7–10
Hongo T (1958) Notes on Japanese larger fungi (12). J Jpn Bot 33:41–48
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298. doi:10.1093/bib/bbn013
Kornerup A, Wanscher JH (1978) Methuen Handbook of Colour, 3rd edn. Eyre Methuen, London
Matheny PB, Curtis JM, Hofstetter V et al (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98(6):982–995
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY et al (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23:357–400
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Ronquist F, Huelsenbeck JP (2003) MrBayes version 3.0: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. doi:10.1093/sysbio/sys029
Singer R (1951) The Agaricales (Mushrooms) in modern taxonomy. Lilloa 22:1–832
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Tóth A, Hausknecht A, Krisai-Greilhuber I, Papp T, Vágvölgyi C, Nagy LG (2013) Iteratively refined guide trees help improving alignment and phylogenetic inference in the mushroom family Bolbitiaceae. PLoS ONE 8:e56143. doi:10.1371/journal.pone.0056143
White TJ, Bruns T, Lee S, Taylor JW (1990) AmpliWcation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: A Guide to Methods and Applications. Academic Press, New York, pp 315–322
Watling R (1982) British Fungus Flora. Agarics and Boleti 3. Bolbitiaceae: Agrocybe, Bolbitius and Conocybe. HMSO, Edinburgh
Acknowledgments
We are grateful to Dr. Yu. Rebriev (Institute of Arid Zones at the Southern Scientific Center, Russian Academy of Science, Rostov-on-Don, Russia) for kindly providing some photographs. This study was funded by the Russian Foundation for Basic Research (project no.13-04-00838). The morphological data analysis was carried out within the framework of the institutional research project (no. 01201255602) of the Komarov Botanical Institute of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Zhu-Liang Yang
Rights and permissions
About this article
Cite this article
Malysheva, E.F., Malysheva, V.F. & Svetasheva, T.Y. Molecular phylogeny and taxonomic revision of the genus Bolbitius (Bolbitiaceae, Agaricales) in Russia. Mycol Progress 14, 64 (2015). https://doi.org/10.1007/s11557-015-1087-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-015-1087-2