Abstract
Cardiomyopathies are a heterogeneous entity. The progress in the field of genetics has allowed over the years to determine its origin more and more often. The classification of these pathologies has changed over the years; it has been updated with new knowledge. Imaging allows to define the phenotypic characteristics of the different forms of cardiomyopathy. Cardiac magnetic resonance (CMR) allows a morphological evaluation of the associated (and sometimes pathognomonic) cardiac findings of any form of cardiomyopathy. The tissue characterization sequences also make magnetic resonance imaging unique in its ability to detect changes in myocardial tissue. This review aims to define the features that can be highlighted by CMR in hypertrophic and dilated forms and the possible differential diagnoses. In hypertrophic forms, CMR provides: precise evaluation of wall thickness in all segments, ventricular function and size and evaluation of possible presence of areas of fibrosis as well as changes in myocardial tissue (measurement of T1 mapping and extracellular volume values). In dilated forms, cardiac resonance is the gold standard in the assessment of ventricular volumes. CMR highlights also the potential alterations of the myocardial tissue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiomyopathy (CMP) is a disease that primarily affects the heart muscle with a heterogeneous clinical presentation and natural history [1, 2]. “Primary forms” of CMP must be distinguished from “Secondary forms,” in which the myocardial alteration is caused by a different pathology, such as a ventricular dilatation post-myocardial infarction associated with coronary artery disease (CAD). Although single forms are relatively rare, the overall prevalence of CMP reaches 3% [3]. In recent years, advances in genetics have made it possible to classify an increasing number of CMPs previously classified as being of “unknown origin” [4]. The recognition of familial CMP is important in creating a patient’s pathway, highlighting when screening of relatives is necessary, and distinguishing familial CMP from non-genetic forms of the disease (i.e., post-viral, autoimmune, immune-mediated sporadic CMPs, or from toxicity linked to endogenous or exogenous causes such as drugs and toxic agents). However, in the context of a complex diagnosis process, the recognition of a specific mutation can be associated with several different CMPs [5]. Since the World Health Organization (WHO) provided the first definition of CMP, there have been numerous classifications based on the morphology, origin (genetic and non-hereditary), and on a more complex functional morphology and classification scheme [1, 3]. A continual drive to acquire a more in-depth knowledge of the various phenotypes, and how these are linked to the etiology of CMP and its genetic basis, has led to imaging in CMPs playing an increasing role. When considering imaging, it is easier to follow a morphological classification that recognizes the following CMPs: the most frequent hypertrophic CMPs (HCM) associated with ventricular thickening; dilated CMPs (DCM), associated with an increase in ventricular volume; and the remaining CMPs associated with specific characteristics including arrhythmogenic right ventricular CMP (ARVC), restrictive CMP (RCM), and unclassified CMPs [3, 5, 6]. CMP may remain asymptomatic for a long time, but as the disease progresses, disorders relating to heart failure may appear, such as breathing difficulties (both under stress and at rest), swelling in the legs, ankles, and feet, coughing, fatigue, arrhythmias or palpitations, dizziness, and syncopation. These symptoms generally tend to worsen over time, regardless of the type of CMP [7]. Nearly 50% of patients dying suddenly in childhood or adolescence or undergoing cardiac transplantation are affected by CMPs [4]. The diagnosis of CMP is based on a medical examination in conjunction with taking an accurate family history [5, 8, 9]. The diagnostic pathway mainly utilizes echocardiogram, imaging methods, and, if possible, genetic tests. Potential complications of CMP include heart failure, thrombus formation, heart valve problems, and sudden death (SCD) [7, 10]. The most suitable treatment depends on the type of CMP diagnosed and the manifestation of the current disorder. However, the aim is to always reduce symptoms, prevent worsening of the disease, and reduce the risk of complications [4]. Imaging plays an essential role in the diagnosis, in the evaluation of the prognosis, and in the follow-up of a patient with CMP. Transthoracic echocardiography (TTE) still has a central role in diagnosis and in follow-up of patients with CMP [11]. TTE is a widespread low-cost technique that provides morphological and functional information. The use of 3D echo reduces some of the limitations of the standard two-dimensional technique, and with dedicated applications, it is possible to evaluate deformations of the ventricular wall. Furthermore, echocardiography provides for the evaluation of the diastolic function as well as an accurate assessment of the valve system. However, all these possibilities are sometimes limited by a suboptimal acoustic window and inter-observer variability. Finally, echocardiography as a method to characterize myocardial tissue is limited. Conversely, cardiac magnetic resonance (CMR) imaging is not only the gold standard for measuring volumes and cardiac mass, but also the only method that allows for tissue characterization [12, 13]. The “classic” sequences in late gadolinium enhancement (LGE) allow us to demonstrate the presence of areas of myocardial fibrosis. The most modern sequences, such as the mapping of T1 and T2, allow us to measure the values of myocardial T1 (native T1), the extravascular cellular volume (ECV) on post-contrast maps, and T2 values [1, 10, 14, 15]. In recent decades, cardiac CT (CCT) has established itself as a noninvasive imaging technique for the study of coronary arteries. However, technological advancements also make this method a valid tool for anatomical (e.g., measurement of volumes and ventricular thicknesses) and functional (e.g., using multi-phasic cine CT images global and regional kinetic) evaluation. Furthermore, with the most advanced scanning techniques it is possible to study dynamic perfusion and, with the use of dedicated software, to obtain iodine distribution maps [16,17,18,19,20,21,22].
In this review, we will describe the contribution of imaging in the diagnosis of the most common primitive forms of the disease: hypertrophic and dilated CMPs (Table 1). In particular, we will discuss the typical features of CMR and the potential for differential diagnoses.
Hypertrophic CMP
Hypertrophic CMP is the most common primary CMP. HCM occurs with primary ventricular thickening unrelated to valve diseases (e.g., aortic stenosis), hypertension, infiltrative diseases or the effects of training conditions on the myocardial muscle (e.g., athletes). Hypertrophy is linked with a non-dilated left ventricle with preserved or increased ejection fraction and is frequently associated with diastolic dysfunction and structural alterations such as the disarray of myocardial fibers and interstitial and focal fibrosis [3, 4]. HCM affects 1 in 500 individuals and is the most common heritable CMP. In 70% of cases, it has a familial origin. Earlier and more severe phenotype of HCM is associated with mutations in the β-myosin heavy chain gene, MYH7. Sarcomeric gene mutations are more likely to be found in younger patients who have a higher rate of SCD [10]. HCM has a high phenotypic heterogeneity [8, 23]. Most commonly, this is observed as an asymmetrical thickening of the septum. However, hypertrophy can also affect the apex of the left ventricle (LV), can be concentric, predominantly posterior, or also involve the right ventricle (RV) [24]. The risk of malignant arrhythmias is high, and this may be the first manifestation of the disease [7, 10]. The disarray of myocardial fibers shown in autopsy studies, and as shown by MRI, correlates with these arrhythmias. Atrial fibrillation is the most frequent arrhythmia in patients with HCM, and left atrium (LA) dilation predisposes patients to the risk of atrial fibrillation [12, 25]. CMR imaging provides information concerning the stratification and prediction of risk of atrial fibrillation (AF) and heart failure (HF) [2, 4, 26]. The presence of fibrosis visible with CMR on LGE images also represents a risk factor for patients with HCM. The presence and extent of fibrosis in LGE correlates with the risk of SCD/implantable cardioverter-defibrillator (ICD) [27].
CMR provides key information in relation to HCM pathology by making use of anatomical–functional sequences and tissue characterization sequences. Traditionally, cine sequences, steady-state free precession (SSFP), have been used to evaluate the volumes, thicknesses, and function of LV and RV in the cardiac planes (short axis, 2, 3, and 4 chambers), usually acquired with patient breath-hold or with new techniques in free breathing [14]. The volume of the left atrium must also always be calculated in these patients (using the biplane area–length method and indexed for body surface area) [12, 28].
In HCM, the thickness of the LV wall is increased in a predominantly asymmetrical manner (> 15 mm in one or more myocardial segments), typically involving the septum in 70% of patients [1, 2]. Different types of HCM are described according to the location in the heart of the thickened area of muscle [7, 8].
Asymmetrical septal hypertrophy
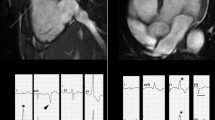
It is the most common presentation of the disease. The septum is thickened with or without signs of obstruction at the level of the outflow tract (Fig. 1). It is also possible to find posterior wall and isolated lateral wall hypertrophy. Reverse curvature of the interventricular septum (into the LV) is characteristic of overt HCM [29, 30]. If the septum thickening affects the basal segment can causes obstruction in the outflow tract (Fig. 2) [5, 31]. LV outflow tract obstruction is present at rest in about one-third of patients and can be provoked in another third. Mitral disease appears to be a key component in obstructive dynamics. Practically speaking, all HCM patients have an elongated anterior mitral flap, sometimes thickened with valve dysfunction [32,33,34]. When surgically correcting obstructive forms, it is often necessary to repair not only the septal thickening but also the mitral alteration [35].
A 55-year-old female: asymmetric hypertrophy with maximum thickness at level of basal and middle septum (21 mm) (white line) (a); lateral wall of LV with a thickness of 10 mm and hyper-trabeculated and spongy appearance (white arrowhead) (a); signs obstruction in the outflow tract (black arrow) (a, b). A 40-year-old male: thickening of septum at the basal segment (24 mm) (white line) with obstruction in the outflow tract and elongated aspect of the anterior mitral flap (white arrowhead) (c)
Apical hypertrophy
The thickened area is at the apex (1%) of the heart; it is also known as Yamaguchi syndrome. Apical hypertrophy usually reduces the volume of the ventricle (Fig. 3). In the Japanese population, apical HCM accounts for approximately 15% of cases, while in Caucasian populations, it accounts for up to 2% of cases [36].
Mid-ventricular hypertrophy obstructive cardiomyopathy and LV apical aneurysm
Mid-ventricular hypertrophic obstructive cardiomyopathy (HCM-MVO) is characterized by asymmetric left ventricular hypertrophy with mid-ventricular obstruction. HCM-MVO is a rare type of cardiomyopathy (incidence about 1%); several studies suggest that this subtype has worse outcomes than the common type of HCM and different genetic basis (Fig. 4) [37, 38].
This form may be present in patients who also have apical hypertrophy or apical aneurysm. The 2% of HCM patients have an apical aneurysm. In these cases, the ventricle wall can be thinned or hypokinetic. The presence of these findings increases the risk of sudden death, heart failure, and intraventricular thrombosis. It is important to demonstrate the possible presence of CMR on regional scaring and to exclude the presence of apical thrombosis (Fig. 5) [39, 40].
Symmetrical hypertrophy
The thickening affects the whole of the LV, reducing its volume. This is sometimes called “concentric” hypertrophy. In these cases, the CMR contributes to get differential diagnosis from other causes of symmetrical myocardial hypertrophy, including mild or moderate hypertrophy in hypertensive heart disease and aortic stenosis or athlete’s heart and other causes of myocardial hypertrophy (e.g., infiltrative diseases as cardiac amyloidosis or Fabry disease) (Fig. 6) [41].
Right hypertrophy
When we talk about hypertrophic CMP, we are usually referring to a thickening of the LV, even if we must not forget that the RV can also be involved (Fig. 7) [42]. Specific patterns of hypertrophy of the RV are recognized, which may lead to RV dysfunction. The wall of the RV may be affected by fibrosis, and a detailed description of the alterations affecting the RV should be part of the evaluation of a patient with HCM.
Additional morphological findings
When the hypertrophic phenotype is slight (mild phenotype), there are additional findings that may be present. That are typical of HCM such as anterior mitral valve leaflet elongation, myocardial crypts, para-septal muscle bundle, and abnormal apical trabeculation often found in both evident and mild hypertrophic forms (Fig. 8) [31]. These findings that are common in HCM patients are of great support in diagnosis.
The use of tissue characterization CMR sequences is fundamental in this pathology.
T2-weighted sequences and T2 maps
The role of the classic T2 fat suppression sequences (which highlight the presence of edema areas) is currently debated. The presence of focal hyperintensity areas in T2, possibly confirmed by T2 maps, can be important for arrhythmic risk stratification (Figs. 9, 10) [43].
A 48-year-old male with asymmetrical hypertrophy-involved anterior septum and anterior wall at the basal level of the LV (a); presence of edema as focal hyperintensity areas within the thickened region (white arrow) on T2 fat suppression sequence (b); enhancement of the hypertrophic segments of LV on LGE sequences 2-chamber (c) and short-axis (d) (white arrowhead)
A 32-year-old male. Diffuse hypertrophy asymmetric with a maximum thickness at the level of the septum of 28 mm (a) and diffuse hyperintensity areas in T2-weighted sequence (white arrowhead) (b); on LGE sequences 4-chamber intra-myocardial enhancement of septum and lateral wall (white arrow) (c); cine 4-chamber sequence shows the presence of a deep crypt in the lateral wall at the medium level (white arrow) (d)
T1 mapping
The T1 values measured in the native maps in hypertrophic CMPs are increased, as well as the expansion of the extracellular volume (ECV) obtained from the pre- and post-contrast maps. Native T1 values are significantly elevated in both non-hypertrophic and hypertrophic segments of HCM patients compared with controls [15].
Late gadolinium enhancement
LGE is understood to represent dense replacement fibrosis (Fig. 11). LGE sequences enable identification and quantification in vivo of myocardial fibrotic areas that have a prolonged retention of gadolinium compared with normal tissue. This is observed in approximately 60% of adult patients with HCM. However, the prevalence of LGE in children and adolescents with HCM is not well established [24, 27, 44]. LGE in HCM is typically non-ischemic, midwall with a patchy distribution located in the junctional area with the RV and in the hypertrophic segments. The extent and characteristics of LGE may have a prognostic role in HCM patients. The sequences for the evaluation of the LGE can also assist in the follow-up of patients to determine the evolution of the pathology [44, 45].
Role of cardiac CT
A noninvasive study of coronary arteries can be useful in these patients. The new CT applications also present an alternative to CMR in demonstrating perfusion alterations and the presence of fibrosis [21, 46, 47].
Differential diagnosis
It is important to be able to differentiate the forms of HCM from the paraphysiological and pathological conditions in which VS is hypertrophic. The key elements for diagnosis are the clinical and family history and the evaluation of EKG and imaging. In athletes, there may be an increase in heart mass with a thickening of the LV which, unlike the typical form of HCM, is symmetrical, rarely exceeds 14 mm, and regresses after a rest period (usually as early as 3 months) [48, 49]. In hypertrophy from hypertension or from aortic valve stenosis, the presence of these alterations guides the diagnosis. In infiltrative pathologies such as Fabry disease (FD) or cardiac amyloidosis (CA), CMR with tissue characterization sequences (e.g., mapping) can be decisive [50,51,52]. Native T1 and ECV on CMR imaging can allow for differentiation between patients with CA or HCM. Also, native T1 values at 1.5 and 3.0 T are significantly lower in patients with Fabry disease compared with those with HCM and provide independent and incremental diagnostic value beyond age, sex, and conventional imaging features [3, 53,54,55].
Dilated CMP
Dilated CMP (DCM) is defined as LV or biventricular dilation and systolic dysfunction in the absence of coronary artery disease, hypertension, valvular or congenital heart disease, and is sufficient to explain such a degree of systolic impairment [56]. It is the most frequent cause of HF and the leading indication for cardiac transplantation. Furthermore, it is associated with an increased risk of ventricular arrhythmias and/or SCD. The prevalence of DCM ranges from 1 in 25,00 to 1 in 250 people, mainly due to changes in diagnostic criteria and geographical variations [7, 23]. Advances in pharmacological and surgical treatment have significantly improved the prognosis of DCM with an estimated survival of up to 85% at 10 years free from heart transplantation [57]. LV reverse remodeling (LVRR), defined as an improvement in LV ejection fraction (LVEF) and a reduction in LV enlargement, is one of the main determinants of prognosis in DCM, and it is the key therapeutic goal. Cardiac adverse remodeling characteristics in DCM include LV dilation and wall thinning, LV dyssynchrony manifested by left bundle branch block, functional mitral regurgitation, myocardial fibrosis, remodeling of other cardiac chambers, and RV dysfunction.
Detailed characterization of these parameters has a pivotal role in the prognostic stratification of DCM patients and in improving clinical management [23, 58]. The concept that DCM likely represents not a single disease entity, but rather an end-stage manifestation of complex interactions between environmental insults and genetic predisposition is gaining acceptance [23, 58]. The term “idiopathic DCM” is often used in clinical practice when the exact cause remains unknown. Recent studies using genetic screening have suggested that up to 40% of DCM is inherited. Mutations in over 40 different genes, encoding cytoskeletal, sarcomeric, mitochondrial, desmosomal, nuclear membrane, and RNA-binding proteins, have been implicated in the pathogenesis of DCM. Mutations in the LMNA gene (encoding lamin A/C) cause up to 10% of DCM characterized by conduction system disease (atrioventricular block) and increased risk of life-threatening ventricular arrhythmias [59]. In some cases, a skeletal muscle involvement could be associated (limb-girdle or Emery–Dreifuss muscular dystrophy) [60]. Secondary causes of DCM include infectious agents (particularly viruses often producing myocarditis), toxins (including chronic excessive consumption of alcohol, chemotherapeutic agents, cocaine), autoimmune and systemic disorders (including sarcoidosis, hemosiderosis, vasculitis, connective tissue disorder), neuromuscular disorders (such as Duchenne/Becker muscular dystrophies), metabolic, endocrine (i.e., Cushing disease) and nutritional disorders (i.e., carnitine, selenium deficiencies), and the presence of persistent tachyarrhythmia and pregnancy (peripartum CMP) [7]. Since the term DCM refers to a spectrum of genetic and acquired disorders that manifest as dilated phenotype, identification of a specific underlying etiology is crucial so that targeted disease-specific therapy may be given to improve prognosis. It also enables clinicians to ascertain if screening of family members is indicated. Imaging techniques play a fundamental role in establishing the underlying pathological substrate leading to DCM. Echocardiography is the usual technique of frontline investigation and is an easily accessible tool for diagnosis and evaluation of DCM. It allows the estimation of LV volumes and LVEF and the exclusion of associated cardiac abnormalities, such as congenital and valvular heart disease. In addition, echocardiography is an important tool for prognostic stratification allowing the identification of many aspects of cardiac remodeling, such as functional mitral regurgitation [57].
Recent advances in technology, such as strain analysis and 3D echocardiography, have improved the diagnostic and prognostic capabilities of this technique. On the other hand, in recent years CMR has emerged as a fundamental tool for diagnosis, risk stratification, and management of DCM patients. CMR not only represents the gold standard for an accurate and reproducible assessment of ventricular volumes and function, but it is also the only technique that provides noninvasive tissue characterization. Using imaging techniques such as LGE and qualitative/quantitative parameters including T1 mapping, T2 mapping, and T2* mapping, tissue characterization is useful in the differential diagnosis of secondary causes of DCM and in the assessment of the probability of LVRR with a potential role in guiding individualized treatment strategies [54, 55, 61].
An accurate and reproducible cardiac evaluation always includes chamber size quantification, myocardial wall thicknesses, ventricular function and mass measurement with traditional cine sequences, steady-state free precession (SSFP), in short and long axis (2, 3, and 4 chamber) view. DCM is characterized by LV dilation, thin-walled, quite trabeculated, impaired contractility with diffuse hypo-akinesia, and reduced LVEF. At present, LVEF is considered one of the most important prognostic factors in DCM, and current guidelines recommend ICD in symptomatic heart failure with LVEF less than 35%. In addition, velocity-encoded CMR is a useful alternative to echocardiography for evaluating functional mitral regurgitation, another aspect of adverse myocardial remodeling.
The use of tissue characterization CMR sequences is fundamental in this pathology.
T2-weighted sequences and T2 maps
T2-weighted sequences with fat suppression highlight the presence of myocardial edema in inflammatory CMP or in acute myocardial infarction. T2-weighted short-tau inversion recovery (T2w-STIR) ECG-gated triple inversion recovery (IR) technique is recommended [1, 62]. The increase in T2 relaxation time is due to an increase in water content in myocardial tissues. Numerous studies have shown an increase in myocardial water content in patients with DCM [63]. T2 mapping sequences, due to quantitative analysis, improve the detection of a diffuse myocardial edema with higher reproducibility and clinical applicability [63]. Also, if a pathology such as acute or chronic myocarditis, Takotsubo syndrome, sarcoidosis, or acute myocardial infarction is suspected the T2 imaging is mandatory [54, 61].
T1 mapping
T1 mapping and extracellular volume calculation allow for the noninvasive detection of diffuse fibrosis. A recent meta-analysis demonstrated that DCM patients show significantly increased native myocardial T1 values and ECVs compared with controls. According to recent studies, ECV and native T1 are emerging as prognostic predictors of mortality independent of the presence of both LVEF and LGE [64]. Furthermore, an increased native T1 value seems to be present as an early imaging marker of adverse outcomes before the presence of LGE [7].
Late gadolinium enhancement
The pattern of LGE allows for the differential diagnosis between ischemic and non-ischemic DCM with good specificity. Myocardial infarction is characterized by subendocardial or transmural LGE in areas supplied by specific coronary arteries (Fig. 12). On the other hand, approximately 30% of DCM cases have a characteristic linear midwall pattern of LGE in a non-coronary distribution, predominantly within the interventricular septum (Fig. 13) [8, 24, 64]. Moreover, myocardial fibrosis is emerging as an important parameter of cardiac remodeling with prognostic and therapeutic implications. Currently, LVEF is the main factor for risk stratification in DCM. However, it is not a specific and sensitive marker for SCD and does not identify those patients who are more likely to respond to medical therapy. Randomized trials, such as the recent DANISH trial, have not shown that ICD implantation guided by LVEF alone is associated with improved clinical outcomes [27]. These findings have increased the interest on the potential role of CMR in the prognostic stratification of DCM helping to assess the risk for sudden cardiac death and the probability of LVRR (Fig. 14) [2, 7, 61]. According to the most recent studies, midwall fibrosis represents an independent predictor of mortality and morbidity beyond LVEF. Myocardial fibrosis is a pathophysiologic component of DCM and can be detected by CMR in two forms: as LGE, which corresponds to irreversible replacement fibrosis, and as T1 mapping alterations corresponding to diffuse interstitial fibrosis. Replacement myocardial fibrosis is associated with contractile impairment and is a potential substrate for ventricular arrhythmia. Assomul et al. [65] were the first to demonstrate a significant association between midwall fibrosis and all-cause mortality in DCM. This finding was subsequently confirmed in further studies, even after adjustment for potential confounders such as age, NYHA class, and LVEF. In a prospective study on the prognostic value of midwall LGE on a cohort of 472 consecutive patients with dilated CMP, comparison between DCM patients with and without LGE showed that the presence and extent of LGE was associated with an increased probability of death (26.8% vs 10.6%) and with an increased risk of arrhythmic event (29.6% vs 7%) [66]. In a study of lamin A/C mutation carriers having undergone CMR, typical midwall myocardial fibrosis in the LV septum was observed in 88% of LMNA mutation carriers, and this correlated significantly with the presence of conduction abnormalities. According to these findings, CMR is an accurate tool to recognize lamin A/C CMP with high risk of sudden cardiac death or severe heart failure at the early stage prior to LV remodeling [67]. The presence and extent of LGE identifies a cohort of patients who have a reduced likelihood of LVRR in response to medical therapy. Therefore, the presence of LGE may be used to guide ICD implantation. It may be reasonable to postpone ICD implantation in DCM patients without LGE because they have a high chance of LVRR and a low risk of SCD even when LVEF is < 35% [7].
Further studies are required to confirm the role of CMR in the risk stratification of DCM and to be able to assess if CMR-guided ICD implantation is able to improve survival in DCM. However, CMR parameters, such as LGE and T1 and T2 mapping, used as part of a multiparametric approach, promise to improve clinical outcomes and management in DCM [1, 2, 54].
Role of cardiac CT
In the diagnostic workup of systolic dysfunction, CCT is recommended in order to rule out ischemic heart disease as an alternative to invasive coronary angiography in patients with low pretest probability. Exclusion of significant coronary artery disease is the main role of CCT in patients with dilated phenotype. However, CCT is emerging as an alternative method to CMR for the detection of myocardial fibrosis in patients with contraindications to perform CMR [18, 47, 68, 69]. A recent study found an association between CMR and CCT on the presence of LGE in 82% of the DCM patients. However, in comparison with CMR, CCT is less sensitive in the detection of myocardial fibrosis because CMR has an inherent high tissue contrast. In addition, emerging data have suggested that CCT provides an accurate assessment of LVEF and LV volume, using retrospective ECG-gated spiral scanning. CCT may be useful in patients with suboptimal echocardiography and who are unable to undergo CMR due to contraindications, such as pacemakers and defibrillators [21].
Differential diagnosis
The identification of a reversible cause appears essential to promote targeted disease-specific treatment to induce LVRR and improve clinical outcome. An integrated approach between clinical and family history and the evaluation of ECGs and imaging is often necessary. The diagnosis of CMP related to cardiotoxins, such as alcohol, cocaine, amphetamines, or anabolic steroid, can be made in the presence of LV dilation and dysfunction in patients with history of substance abuse and no other known causes of myocardial disease (Fig. 15) [53, 56]. The onset of HF toward the end of pregnancy, or in the months after delivery, suggests peripartum CMP but in order to confirm the diagnosis alternative causes of CMP should be excluded. CMR with tissue characterization sequences aids the differential diagnosis of secondary causes of DCM, including inflammatory, autoimmune, neuromuscular diseases and toxic forms or several end-stage CMPs [62, 70]. T2-weighted sequences allow detection of myocardial edema in active myocarditis or sarcoidosis. In acute myocarditis, the pattern of LGE is usually sub-epicardial or patchy midwall. LGE positivity, edema on T2-weighted images, and hyperemia on early post-gadolinium T1-weighted sequences represent the so-called Lake Louise criteria [70, 71]. The presence of at least two Lake Louise criteria on CMR strengthens the diagnosis of clinically suspected myocarditis. In addition, T1 mapping and ECV calculation allow increasing CMR sensitivity in LGE negative patients with diffuse myocardial injury. T2 mapping can reduce artifacts and improve the detection of myocardial edema [1, 54, 62, 63]. CMR has a pivotal role in the detection of cardiac involvement in sarcoidosis usually identifying midwall LGE of the basal segments of the septum and lateral walls. CMR can identify early cardiac involvement in autoimmune CMP, such as systemic lupus erythematosus or rheumatoid arthritis, showing evidence of inflammation [56].
A 53-year-old male with dilated CMP related to abuse of anabolic steroid and ejection fraction of 15%; on 4-chamber cine sequence LV is markedly dilated (a); sub-epicardial and meso-cardial alteration in lateral wall (white arrowhead) (b) and linear midwall enhancement within the interventricular septum (white arrowhead) (c) on LGE; increased native T1 values at the altered segments (1063 ± 22 ms) (d)
Conclusions
The use of CMR in CMPs is of crucial importance today. The morphological evaluation done with the CMR helps with the diagnosis and often completes echocardiography data. CMR offers the ability to characterize tissues in order to add elements to the diagnosis. The presence of edema or fibrosis and the evaluation of their distribution often provide the differential diagnosis and are also important for risk stratification. Mapping sequences add significant quantitative data, with both T1 and T2 maps.
References
Patel AR, Kramer CM (2017) Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. JACC Cardiovasc Imaging 10:1180–1193. https://doi.org/10.1016/j.jcmg.2017.08.005
Baxi AJ, Restrepo CS, Vargas D et al (2016) Hypertrophic cardiomyopathy from A to Z: genetics, pathophysiology, imaging, and management. Radiographics 36:335–354. https://doi.org/10.1148/rg.2016150137
Kamal MU, Bin Riaz I, Janardhanan R (2016) Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: current state of the art. Cardiol J 23:250–263. https://doi.org/10.5603/CJ.a2016.0019
Geske JB, Ommen SR, Gersh BJ (2018) Hypertrophic cardiomyopathy: clinical update. JACC Hear Fail 6:364–375. https://doi.org/10.1016/j.jchf.2018.02.010
Neubauer S, Kolm P, Ho CY et al (2019) Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM registry. J Am Coll Cardiol 74:2333–2345. https://doi.org/10.1016/j.jacc.2019.08.1057
Masarone D, Kaski JP, Pacileo G et al (2018) Epidemiology and clinical aspects of genetic cardiomyopathies. Heart Fail Clin 14:119–128. https://doi.org/10.1016/j.hfc.2017.12.007
Kalisz K, Rajiah P, Roifman I et al (2018) Evolving concepts in dilated cardiomyopathy. J Am Coll Cardiol 19:364–375. https://doi.org/10.1161/CIRCULATIONAHA.117.032966
McKenna WJ, Maron BJ, Thiene G (2017) Classification, epidemiology, and global burden of cardiomyopathies. Circ Res 121:722–730. https://doi.org/10.1161/CIRCRESAHA.117.309711
O’Mahony C, Elliott P, McKenna W (2013) Sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythmia Electrophysiol 6:443–451. https://doi.org/10.1161/CIRCEP.111.962043
Ariga R, Tunnicliffe EM, Manohar SG et al (2019) Identification of myocardial disarray in patients with hypertrophic cardiomyopathy and ventricular arrhythmias. J Am Coll Cardiol 73:2493–2502. https://doi.org/10.1016/j.jacc.2019.02.065
Sivalokanathan S, Zghaib T, Greenland GV et al (2019) Hypertrophic cardiomyopathy patients with paroxysmal atrial fibrillation have a high burden of left atrial fibrosis by cardiac magnetic resonance imaging. JACC Clin Electrophysiol 5:364–375. https://doi.org/10.1016/j.jacep.2018.10.016
Minami Y, Haruki S, Yashiro B et al (2016) Enlarged left atrium and sudden death risk in hypertrophic cardiomyopathy patients with or without atrial fibrillation. J Cardiol 68:478–484. https://doi.org/10.1016/j.jjcc.2016.01.006
Minegishi S, Kato S, Takase-Minegishi K et al (2019) Native T1 time and extracellular volume fraction in differentiation of normal myocardium from non-ischemic dilated and hypertrophic cardiomyopathy myocardium: a systematic review and meta-analysis. IJC Hear Vasc 25:100422. https://doi.org/10.1016/j.ijcha.2019.100422
Roifman I, Gutierrez J, Wang E et al (2019) Evaluating a novel free-breathing accelerated cardiac MRI cine sequence in patients with cardiomyopathy. Magn Reson Imaging 61:260–266. https://doi.org/10.1016/j.mri.2019.06.008
Huang L, Ran L, Zhao P et al (2019) MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: tissue remodeling manifested prior to structure changes. Br J Radiol. https://doi.org/10.1259/bjr.20190634
Agostini A, Borgheresi A, Mari A et al (2019) Dual-energy CT: theoretical principles and clinical applications. Radiol Medica 124:1281–1295. https://doi.org/10.1007/s11547-019-01107-8
Schicchi N, Fogante M, Esposto Pirani P et al (2019) Third-generation dual-source dual-energy CT in pediatric congenital heart disease patients: state-of-the-art. Radiol Medica 124:1238–1252. https://doi.org/10.1007/s11547-019-01097-7
Scherer K, Hammel J, Sellerer T et al (2019) Dynamic quantitative iodine myocardial perfusion imaging with dual-layer CT using a porcine model. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-52458-1
Glockner JF (2018) Magnetic resonance imaging and computed tomography of cardiac masses and pseudomasses in the atrioventricular groove. Can Assoc Radiol J 69:78–91. https://doi.org/10.1016/j.carj.2017.12.004
Garcia-Figueiras R, Baleato González S, Padhani AR, Marhuenda A (2015) Advanced imaging of colorectal cancer: from anatomy to molecular imaging. World J Gastroenterol. https://doi.org/10.1007/s13244-016-0465-x
Ko SM, Hwang SH, Lee HJ (2019) Role of cardiac computed tomography in the diagnosis of left ventricular myocardial diseases. J Cardiovasc Imaging 27:73–92. https://doi.org/10.4250/jcvi.2019.27.e17
Di Cesare E, Patriarca L, Panebianco L et al (2018) Coronary computed tomography angiography in the evaluation of intermediate risk asymptomatic individuals. Radiol Medica 123:686–694. https://doi.org/10.1007/s11547-018-0898-z
Merlo M, Cannatà A, Gobbo M et al (2018) Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail 20:228–239. https://doi.org/10.1002/ejhf.1103
Soler R, Méndez C, Rodríguez E et al (2018) Phenotypes of hypertrophic cardiomyopathy. An illustrative review of MRI findings. Insights Imaging 9:1007–1020. https://doi.org/10.1007/s13244-018-0656-8
Khan MA, Yang EY, Zhan Y et al (2019) Association of left atrial volume index and all-cause mortality in patients referred for routine cardiovascular magnetic resonance: a multicenter study. J Cardiovasc Magn Reson 21:1–12. https://doi.org/10.1186/s12968-018-0517-0
Precone V, Krasi G, Guerri G et al (2019) Cardiomyopathies. Acta Biomed 90:32–43. https://doi.org/10.23750/abm.v90i10-s.8755
He D, Ye M, Zhang L, Jiang B (2018) Prognostic significance of late gadolinium enhancement on cardiac magnetic resonance in patients with hypertrophic cardiomyopathy. Hear Lung 47:122–126. https://doi.org/10.1016/j.hrtlng.2017.10.008
Maron MS, Rowin EJ, Maron BJ (2017) How to image hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 10:1–15. https://doi.org/10.1161/CIRCIMAGING.116.005372
Marian AJ, Braunwald E (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 121:749–770. https://doi.org/10.1161/CIRCRESAHA.117.311059
Reant P, Captur G, Mirabel M et al (2015) Abnormal septal convexity into the left ventricle occurs in subclinical hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 17:1–8. https://doi.org/10.1186/s12968-015-0160-y
Dulguerov F, Marcacci C, Alexandrescu C et al (2016) Hypertrophic obstructive cardiomyopathy: the mitral valve could be the key. Eur J Cardio-thoracic Surg 50:61–65. https://doi.org/10.1093/ejcts/ezv473
Nishimura RA, Seggewiss H, Schaff HV (2017) Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res 121:771–783. https://doi.org/10.1161/CIRCRESAHA.116.309348
Sherrid MV, Balaram S, Kim B et al (2016) Pathoanatomic findings and treatment during hypertrophic obstructive cardiomyopathy surgery: the role of mitral valve. Hear Lung Circ 28:477–485. https://doi.org/10.1016/j.hlc.2018.02.006
Pradella S, Grazzini G, Brandani M et al (2019) Cardiac magnetic resonance in patients with mitral valve prolapse: focus on late gadolinium enhancement and T1 mapping. Eur Radiol 29:1546–1554. https://doi.org/10.1007/s00330-018-5634-5
Spirito P, Iascone M, Ferrazzi P (2018) Mitral valve abnormalities in hypertrophic cardiomyopathy: a primary expression of the disease? Getting closer to the answer. Eur Heart J Cardiovasc Imaging 19:1107–1108. https://doi.org/10.1093/ehjci/jey112
Hughes RK, Knott KD, Malcolmson J et al (2020) Apical hypertrophic cardiomyopathy: the variant less known. J Am Heart Assoc 9:e015294. https://doi.org/10.1161/JAHA.119.015294
Cui L, Tse G, Zhao Z et al (2019) Mid-ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm: an important subtype of arrhythmogenic cardiomyopathy. Ann Noninvasive Electrocardiol 24:1–5. https://doi.org/10.1111/anec.12638
Inagaki N, Hayashi T, Takei Y et al (2018) Clinical and genetic backgrounds of hypertrophic cardiomyopathy with mid-ventricular obstruction. J Hum Genet 63:1273–1276. https://doi.org/10.1038/s10038-018-0509-9
Raza M, Chalfoun N, Chalfoun N, Wissam A et al (2018) Hypertrophic cardiomyopathy with a large apical ventricular aneurysm and mural thrombus. Glob Cardiol Sci Pract. https://doi.org/10.21542/gcsp.2018.9
Rowin EJ, Maron BJ, Haas TS et al (2017) Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 69:761–773. https://doi.org/10.1016/j.jacc.2016.11.063
Méndez C, Soler R, Rodríguez E et al (2018) Differential diagnosis of thickened myocardium: an illustrative MRI review. Insights Imaging 9:695–707. https://doi.org/10.1007/s13244-018-0655-9
Quarta G, Aquaro GD, Pedrotti P et al (2018) Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: the importance of clinical context. Eur Heart J Cardiovasc Imaging 19:601–610. https://doi.org/10.1093/ehjci/jex323
Hen Y, Takara A, Iguchi N et al (2018) High signal intensity on T2-weighted cardiovascular magnetic resonance imaging predicts life-threatening arrhythmic events in hypertrophic cardiomyopathy patients. Circ J 82:1062–1069. https://doi.org/10.1253/circj.CJ-17-1235
Becker MAJ, Cornel JH, van de Ven PM et al (2018) The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging 11:1274–1284. https://doi.org/10.1016/j.jcmg.2018.03.006
Maurizi N, Passantino S, Spaziani G et al (2018) Long-term outcomes of pediatric-onset hypertrophic cardiomyopathy and age-specific risk factors for lethal arrhythmic events. JAMA Cardiol 3:520–525. https://doi.org/10.1001/jamacardio.2018.0789
Ntsinjana HN, Hughes ML, Taylor AM (2011) The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson 13:51. https://doi.org/10.1186/1532-429X-13-51
Kalisz K, Rajiah P, Toth A et al (2017) Computed tomography of cardiomyopathies. Cardiovasc Diagn Ther 7:539–556. https://doi.org/10.21037/cdt.2017.09.07
Czimbalmos C, Csecs I, Toth A et al (2019) The demanding grey zone: sport indices by cardiac magnetic resonance imaging differentiate hypertrophic cardiomyopathy from athlete’s heart. PLoS ONE 14:1–14. https://doi.org/10.1371/journal.pone.0211624
Rodrigues JCL, Rohan S, Ghosh Dastidar A et al (2017) Hypertensive heart disease versus hypertrophic cardiomyopathy: multi-parametric cardiovascular magnetic resonance discriminators when end-diastolic wall thickness ≥ 15 mm. Eur Radiol 27:1125–1135. https://doi.org/10.1007/s00330-016-4468-2
Martinez-Naharro A, Treibel TA, Abdel-Gadir A et al (2017) Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 70:466–477. https://doi.org/10.1016/j.jacc.2017.05.053
Sado DM, Flett SA, Banypersad SM et al (2012) Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 98:1436–1441. https://doi.org/10.1136/heartjnl-2012-302346
Pandey T, Jambhekar K, Shaikh R et al (2013) Utility of the inversion scout sequence (TI scout) in diagnosing myocardial amyloid infiltration. Int J Cardiovasc Imaging 29:103–112. https://doi.org/10.1007/s10554-012-0042-4
Bozkurt B, Colvin M, Cook J et al (2016) Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. https://doi.org/10.1161/CIR.0000000000000455
Luetkens JA, Homsi R, Sprinkart AM et al (2016) Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17:154–161. https://doi.org/10.1093/ehjci/jev246
Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER et al (2015) Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 17:29. https://doi.org/10.1186/s12968-015-0111-7
Japp AG, Gulati A, Cook SA et al (2016) The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol 67:2996–3010. https://doi.org/10.1016/j.jacc.2016.03.590
Porcari A, De Angelis G, Romani S et al (2019) Expert Review of Cardiovascular Therapy Current diagnostic strategies for dilated cardiomyopathy: a comparison of imaging techniques. Expert Rev Cardiovasc Ther 17:53–63. https://doi.org/10.1080/14779072.2019.1550719
Schultheiss HP, Fairweather DL, Caforio ALP et al (2019) Dilated cardiomyopathy. Nat Rev Dis Prim. https://doi.org/10.1038/s41572-019-0084-1
Captur G, Arbustini E, Bonne G et al (2018) Lamin and the heart. Heart 104:468–479. https://doi.org/10.1136/heartjnl-2017-312338
Schiau C, Șerban S, Dudea SM, Manole S (2019) Cardiovascular magnetic resonance: contribution to the exploration of cardiomyopathies. Med Pharm Rep 92:326–336. https://doi.org/10.15386/mpr-1343
Spieker M, Katsianos E, Gastl M et al (2016) Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution. Eur J Radiol 56:1–19. https://doi.org/10.2967/jnumed.114.142729.The
Lota AS, Gatehouse PD, Mohiaddin RH (2017) T2 mapping and T2* imaging in heart failure. Heart Fail Rev 22:431–440. https://doi.org/10.1007/s10741-017-9616-5
Nishii T, Kono AK, Shigeru M et al (2014) Cardiovascular magnetic resonance T2 mapping can detect myocardial edema in idiopathic dilated cardiomyopathy. Int J Cardiovasc Imaging 30:65–72. https://doi.org/10.1007/s10554-014-0414-z
Brown PF, Miller C, Di Marco A, Schmitt M (2019) Towards cardiac MRI based risk stratification in idiopathic dilated cardiomyopathy. Heart 105:270–275. https://doi.org/10.1136/heartjnl-2018-313767
Assomull RG, Prasad SK, Lyne J et al (2006) Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 48:1977–1985. https://doi.org/10.1016/j.jacc.2006.07.049
Gulati A, Jabbour A, Ismail TF et al (2013) Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309:896–908. https://doi.org/10.1001/jama.2013.1363
Holmström M, Kivistö S, Heliö T et al (2011) Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. J Cardiovasc Magn Reson 13:30. https://doi.org/10.1186/1532-429X-13-30
Cerny V, Kuchynka P, Marek J et al (2017) Utility of cardiac CT for evaluating delayed contrast enhancement in dilated cardiomyopathy. Herz 42:776–780. https://doi.org/10.1007/s00059-016-4515-4
Schicchi N, Fogante M, Esposto Pirani P et al (2019) Third-generation dual-source dual-energy CT in pediatric congenital heart disease patients: state-of-the-art. Radiol Medica 124:1238–1252. https://doi.org/10.1007/s11547-019-01097-7
Ferreira VM, Schulz-Menger J, Holmvang G et al (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 72:3158–3176. https://doi.org/10.1016/j.jacc.2018.09.072
Amano Y, Tachi M, Tani H et al (2012) T2-weighted cardiac magnetic resonance imaging of edema in myocardial diseases. Sci World J 2012:1–7. https://doi.org/10.1100/2012/194069
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Although the nature of our study is a review regarding the material used (images), all procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients who perform imaging studies sign a written consent. All the cases (images) included in the work are obtained anonymously from our archive.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pradella, S., Grazzini, G., De Amicis, C. et al. Cardiac magnetic resonance in hypertrophic and dilated cardiomyopathies. Radiol med 125, 1056–1071 (2020). https://doi.org/10.1007/s11547-020-01276-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01276-x