Abstract
Rheumatoid arthritis (RA) is a chronic disease characterized mainly by joint inflammation that can lead to permanent structural damage. An accurate assessment of joint inflammation is of outmost importance for disease management. Over the past years, musculoskeletal ultrasound (MSUS) has proved to be a sensitive tool not only in detecting joint and periarticular inflammation, i.e., synovitis, tenosynovitis, and bursitis, but also in evaluating structural damages such as erosion and tendon damage. In addition to clinical and laboratory evaluation, MSUS has been successfully used to early diagnose RA. In RA patients, B-mode (BM) and Doppler techniques characterize better than clinical assessment joint inflammation (either synovial hypertrophy or effusion, either active or inactive synovitis) and reflect accurately disease inflammatory activity. Doppler-detected synovitis has been shown to be predictive for structural damage, even in patients in clinical remission. Furthermore, MSUS has proved successful in monitoring response to treatment and is also a valuable tool for guiding MSUS injections.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic, autoimmune disease affecting from 0.5 to 1 % of population. It is characterized primarily by joint inflammation that affects both large and small joints. Besides intra-articular structures, inflammation can also involve periarticular structures. The natural course of the disease can lead to progressive joint destruction and physical disability [1, 2]. Over the past years, the approach to RA management has changed considerably. Early diagnosis and rapid achievement of remission became the main goals with “treat-to-target” strategy in focus. In 2010, new classification criteria which excluded radiographic evidence of joint erosion meant that the patient could be diagnosed more quickly and easily [3]. Therapies such as conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and newer therapies, such as biologic DMARDs (bDMARDs), have proven to be effective in inducing and maintaining remission in RA patients. Therefore, disease prognosis became brighter as such therapies have proven efficacy in the reduction of structural damage, physical disability as well as many other comorbidities, particularly cardiovascular [4].
Over the last decade, an increased attention has been paid to the development of standardized clinical measurements of disease activity. The development of composite indexes and the “treat-to-target” strategy represent an important step forward in a tight control of the disease [5, 6]. Despite this, disease progression has been observed in some patients who achieved clinical remission status. The development of imaging modalities such as magnetic resonance imaging (MRI) and musculoskeletal ultrasound (MSUS) have enabled more accurate assessment of RA patients compared to clinical assessment [7–16]. In particular, MSUS has proven to be a reliable, noninvasive, practical, and accessible tool for assessing all peripheral joint and periarticular structures involved in RA patients in clinical practice.
In recent years, an increasing number of publications dealing with MSUS have shown that this technique can be an accurate, reliable, and sensitive-to-change tool in clinical practice. In RA, it has been used for both diagnosis purposes—including differential diagnosis—and for monitoring disease activity. B-mode (BM) examination provides important information regarding morphological aspects of intra- and periarticular structures and Doppler mode, either color Doppler (CD) or power Doppler (PD) examination, enables detection of low-speed blood flow, as it can be found in newly formed vessels in inflamed synovial tissue. Both BM and Doppler techniques are valid tools in assessing joint inflammatory diseases.
An important issue regarding MSUS is its reliability, as ultrasound (US) is considered a highly operator-depending technique. Its accuracy depends on both acquisition and interpretation of US images. This operator-dependent nature of MSUS has promoted the need for a uniform evaluation of US-detected pathologies. Therefore, to optimize MSUS as a diagnostic and monitoring tool, universal guidelines would be needed for pathology evaluation. However, progress has been made to address this point. In 2005, the Outcome Measures in Rheumatology (OMERACT) MSUS group defined the main US findings in inflammatory arthritis , i.e., synovitis, including synovial fluid/effusion (SE) and synovial hypertrophy (SH), tenosynovitis, bone erosions, and enthesopathy [17].Various scoring systems have also been developed and validated for these pathologies. Several studies that have investigated the intra- and interobserver reliability for US RA pathologies have shown good to excellent results [7, 10, 13, 18–22] .
The importance of MSUS in RA has recently been recognized by the European League against Rheumatism (EULAR), including this tool in its recommendations on the use of imaging in RA [23].
Ultrasound Findings
This section describes the most important US findings in RA patients. The evidence-based validation process of MSUS of these abnormalities is reviewed in this section .
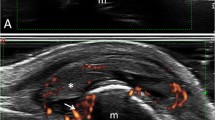
Synovitis is one of the most important features in RA. The term “synovitis” is used to indicate the presence of SE and/or SH (Fig. 3.1a). According to the OMERACT definitions published in 2005, SE was defined as an abnormal hypoechoic or anechoic (relative to subdermal fat, but sometimes may be isoechoic or hyperechoic) intra-articular material that is displaceable and compressible, but does not exhibit Doppler signal, and SH as an abnormal hypoechoic (relative to subdermal fat, but sometimes may be isoechoic or hyperechoic) intra-articular tissue that is non-displaceable and poorly compressible and which may exhibit Doppler signal [17]. PD and CD are used to visualize vascularization of inflamed synovium (Figs. 3.1b and 3.2). Choosing a Doppler modality for rheumatologic practice depends on the equipment sensitivity. Differences between CD and PD are significantly reduced with newer US equipment.
While knee arthroscopy, a frequent procedure in clinical practice, has permitted a relatively easier histopathological assessment of inflamed joints, MSUS, both BM and Doppler, were reported to be accurate in detecting joint synovitis in comparison to arthroscopy and histology, respectively [7, 24]. Earlier studies reported good correlation between histological and B-mode ultrasound (BMUS) findings in knee joint synovitis. Furthermore, in patients with knee joint involvement in different diseases, BMUS has shown a high sensitivity, specificity, and accuracy (Fig. 3.3) for detecting synovitis [7, 24]. In RA inflamed joints, there was a similar good correlation between histologic and Doppler inflammatory changes in different joints [25–27]. When comparing histopathology with BMUS, power Doppler ultrasound (PDUS), and MRI, the highest correlation was found for PDUS and histopathology [28]. Although false-negative results were found for Doppler techniques when compared to histology [27, 29], the presence of a positive Doppler signal in the synovium was an indicator of active synovial inflammation.
When using others imaging techniques as comparator, MSUS, both BM and Doppler, showed considerable sensitivity and specificity. In a number of studies, moderate to good correlations were found between US-detected synovitis (either BM or Doppler) and MRI-detected synovitis in hand finger joints [12, 30]. Using MRI as reference, Szkudlarek et al. found a good to excellent sensitivity and specificity of US, both BM and PD, for the detection of synovitis at metatarsophalangeal (MTP) and metacarpophalangeal (MCP) joints [31, 32]. Similarly, Scheel et al. reported a good agreement between US and MRI in the detection of BM synovitis at MCP and proximal interphalangeal (PIP) joints [33]. In concordance with these data, Terslev et al. depicted a high significant association between Doppler US indices of inflammation and post-contrast MRI scores at wrist and hand joints [34]; whereas Fukae et al. found a good correlation between the measurements of Doppler synovitis and the enhancement rate of MRI in MCP and PIP joints [30]. Recently, Kawashiri et al. observed a moderate to good correlation between US-detected synovitis (in both BM and PD) and MRI-detected osteitis [35] (Fig. 3.4) .
Good correlation was also reported in other studies investigating the link between MSUS-detected synovitis and inflammation identified through clinical examination and laboratory analysis. Naredo et al. found a moderate to good correlation between swelling joints count and MSUS-detected synovitis for both BM and PD. MSUS-detected synovitis was also found to better correlate with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) than clinically detected synovitis [10]. In the study by Scire et al. on patients with early RA who started csDMARDs treatment, both clinical and MSUS-detected synovitis which were significantly correlated with CRP in patients with active disease, while, in patients who achieved the clinical remission status, only PD correlated with CRP [14]. Kawashiri et al. found a significant moderate to good correlation between PDUS assessment of 12 joints with serum vascular endothelial growth factor (VEGF), matrix metalloprotease (MMP)-3, and metallopeptidase inhibitor (TIMP)-1 [36] .
Studies that have investigated intra- and interobserver reliability in a variety of joints for MSUS-detected synovitis, for both BM and Doppler showed moderate to excellent results [7, 10, 13, 30, 37–39]. A systematic review assessing the reliability of MSUS-detected synovitis in RA showed that US, particularly PD mode, was reliable in still-image interpretation when assessed by experienced ultrasonographers, while image acquisition was less reliable. Among all joints, the knee was the most reliable joint even in terms of image acquisition [40]. Mandel et al. compared the reliability of 11 different US scoring systems for synovitis including different combinations of joint counts [42, 28, 20, 16, 12, 10, 8, 7] and found that MSUS, both BM and PD, showed a better reliability than clinical assessment in evaluating synovitis. No differences in the reliability were observed between these scoring systems [41].
The sensitivity to change of US-detected synovitis has been investigated in several published studies. Regardless of how many joints were evaluated, a decrease of BM and Doppler variables has been shown in patients treated with bDMARDs [15, 42–46] or csDMARDs [47, 48]. In a randomized control trial, Taylor et al. studied patients with early RA treated with methotrexate (MTX) and infliximab (IFX) versus MTX and placebo using US and conventional radiography (CR) for the evaluation of MCP joints. After 18 weeks of treatment, patients under IFX therapy presented significant reduction in synovial thickness and joint vascularity measured as the number of CD pixels in a defined region of interest [49]. Responsiveness of US-detected synovitis has also been shown after steroid treatment, either intra-articular or systemic [50–54] .
Several scoring systems have been developed to assess synovitis, namely SH, SE, and inflammatory activity by Doppler US. Among them, semiquantitative scores have been the most used scores in clinical practice [18, 46]. In most published studies of MSUS in RA, the semiquantitative score for BM synovitis consisted of the following: grade 0—absence of synovial thickening, grade 1—mild synovial thickening, grade 2—moderate synovial thickening, grade 3—marked synovial thickening [18]. Similarly, in most published studies, the semiquantitative score for Doppler synovitis consisted of the following: grade 0—no flow in the synovium, grade 1—up to three single spots signals or up to two confluent spots or one confluent spot plus up to two single spots, grade 2—vessel signals in less than half of the area of the synovium, grade 3—vessel signals in more than half of the area of the synovium [18, 46]. Quantitative measurements of Doppler signals are obtained using a color recognition function that counts the number of total and color pixels within a region of interest. The number of color pixels is then expressed in relation to the total number of pixels as a fraction [44, 51, 55]. Terslev et al. found a good agreement between quantitative and semiquantitative scores for Doppler synovitis [56] .
While small joints can be more easily assessed by clinical examination, synovitis evaluation of larger joints mostly shoulders and hips, represents a challenge in daily clinical practice. In a recent study, Sakellariou et al. found inflammatory changes at glenohumeral (GH) joint in 14 % of RA studied patients [57]. Figure 3.5 shows an example of posterior GH recess showing BM synovitis with Doppler signal in an RA patient (Fig. 3.5). Even if it is not so frequently evaluated, hip involvement is not uncommon in RA patients. In an Italian cohort of RA patients, BMUS detected hip synovitis in 24 % of patients [58]. Figure 3.6 shows an example of the anterior recess of the hip showing BM synovitis. However, Doppler activity is not detected so frequently in these joints, due to the lower sensitivity of Doppler techniques for deep areas .
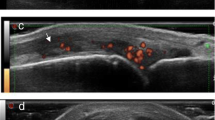
Tenosynovitis is another important feature in RA patients. US-detected tenosynovitis is defined as hypoechoic or anechoic thickened tissue with or without fluid within the tendon sheath, which is seen in two perpendicular planes (Fig. 3.7) and which may exhibit Doppler signal [17]. Tenosynovitis on Doppler mode is defined as the presence of peri-tendinous Doppler signal within the synovial sheath (Fig. 3.8), seen in two perpendicular planes, excluding normal nutrient vessels in mesotenon or vinculae, only if the tendon shows peri-tendinous synovial sheath widening on BM [37].
Compared to MRI, MSUS has shown to be accurate in detection of tenosynovitis. MSUS has also shown a high specificity, but a fair to moderate sensitivity for detecting tenosynovitis [21]. A number of studies have compared US and MRI evaluation of tenosynovitis. In the study by Hoving et al., MSUS detected more tendon effusion than MRI at wrist and hand tendons [59]. Scheel et al. found a good agreement between US and MRI for tenosynovitis at ankle flexors and peroneus tendons while for ankle extensor tendons they reported a lower agreement [11].
Regarding the intra-observer consistency of US-assessed tenosynovitis, various studies have shown good to excellent reliability [19–21] and moderate to excellent interobserver reliability [19, 20, 22, 47]. Hammer et al. studied the sensitivity to change of US-detected tenosynovitis in RA patients initiating adalimumab (ADA) treatment. They assessed flexor and extensor tendons of bilateral wrist and ankle and observed a significant reduction of tenosynovitis after 12 months for all studied tendons. The MSUS assessment of a reduced number of tendons (i.e., extensor carpi ulnaris (ECU), tibialis posterior (TP), and flexor digitorum longus) was as sensitive to change as the assessment of all studied tendons [60] .
For tenosynovitis, the most studied tendons have been the hand and ankle tendons. However, in RA not all tendons are affected in the same way. At hand and finger level, the most frequently involved tendons are the ECU and the flexor tendons of the second, third, and fourth fingers [61]. In an MRI study of RA patients with hindfoot pain, the most frequently involved tendons were TP and peroneal tendons and the least common involved tendons were tibialis anterior (TA) and the extensor tendons [62]. Figure 3.9 shows an example of BM tenosynovitis of TP tendon in an RA patient.
Recently, the OMERACT MSUS group developed a four-grade semiquantitative scoring system for BM and Doppler tenosynovitis which showed a good intra- and interobserver reliability [19]. This score is as follows: grade 0—normal, grade 1—minimal, grade 2—moderate, and grade 3—severe. Doppler tenosynovitis was scored as following: grade 0—no Doppler signal, grade 1—minimal, grade 2—moderate, and grade 3—severe pathological peri-tendinous Doppler signal within the synovial sheath [19] .
Bone erosions are defined, according to OMERACT, as intra-articular discontinuity of the bone surface that is visible in two perpendicular planes (Fig. 3.10) [17].
Over the past decades, CR has been the primary choice in assessing bone erosions. However, early in the disease course, CR cannot always detect bone changes. MSUS was shown to be more sensitive than CR in detecting bone erosions at finger and toe joint level [12, 31, 63–65]. This was supported by various studies which revealed a high agreement between US and MRI in detecting bone erosion at hand and foot finger joints [12, 31]. At the hand finger joint level, the agreement was higher in joints with good accessibility for US, like second and fifth MCP [12]. Finzel et al. reported a good correlation between the severity of erosions detected by US and by micro-computed tomography (micro-CT) [66]. Furthermore, using MRI and CT as reference, US has shown a high specificity with a moderate sensitivity in detecting bone erosions [12, 67]. However, sensitivity improved considerably, without losing specificity, when only US-accessible areas, i.e., radial second MCP, ulnar fifth MCP, and all dorsal/palmar aspects, were included [67]. In another study, Døhn et al. compared US- and MRI-detected erosions with CT-detected erosions at MCP joints in RA patients without CR-detected erosions. With CT as reference, US and MRI resulted in high specificity in detecting bone erosions even in normal radiographic MCP joints [68]. On the other hand, when compared to CT, false-positive results for US-detected erosions could be noted, especially in small joints. This is mostly due to the misinterpretation of normal vascular bone channels and normal grooves of the metacarpal heads that can mimic bone erosions [66] .
On another front, MSUS has shown a good reliability for detecting bone erosions. Several studies revealed good to excellent intra- and interobserver reliability for both small and large joints [18, 39, 63, 69]. For small joints, the highest agreement was found at second MCP joint [18].
In published studies on RA patients, the most commonly used score for bone erosions has been semiquantitative scores. There are a number of different semiquantitative scores for bone erosion used in these studies. Szkudlarek et al. proposed a semiquantitative scoring from 0 to 3 (0—regular bone surface, 1—irregularity of the bone surface without formation of a defect seen in two planes, 2—formation of a defect in the surface of the bone seen in two planes, 3—bone defect creating extensive bone destruction) system that demonstrated a good intra-observer agreement [18]. Another semiquantitative scoring system was proposed by Wakefiel et al. based on the size of erosions (small erosion ≤ 2 mm, moderate erosion 2–4 mm, and large erosion ≥ 4 mm), that also showed good intra- and interobserver agreement [63] .
The most frequent site for MSUS-detected erosions in RA patients at hand level are the second MCP and fifth MCP joints, and at foot level are the first MTP and fifth MTP joints, while the fewest erosions are detected at fourth MCP joint [63, 64, 70, 71]. An explanation for these findings could be the acoustic window for US beams at this level. The majority of erosions were detected at the metacarpal heads and on the radial or ulnar sites of the joints, while lesser erosions were detected at the phalangeal bases and on the dorsal and volar aspect of the joints [63]. In contrast, at wrist level, US evaluation of erosions is difficult due to the irregularities of the bone margins, the presence of several nutrition channels, and poor US-window for structure visualization [72]. Humeral head erosions can be seen in a significant number of healthy people [73]. Thus, clinical conclusion cannot be drawn from US-detected erosion at this level, especially for small erosions.
Tendon damage is a common finding in long-standing RA patients as repeated or persistent tendon inflammation can lead to structural damage and tendon rupture. Compared to MRI, US is seemingly more sensitive in detecting partial finger extensor tendon tear [74]. However, until recently, there was no commonly agreed definition or scoring system for tendon damage. In 2013, the OMERACT MSUS group defined tendon damage on BM as internal and/or peripheral focal tendon defect (i.e., absence of fibers) in the region enclosed by tendon sheath (Fig. 3.11), seen in two perpendicular planes [75] . For tendon damage, a three-grade semiquantitative scoring system has recently been developed (grade 0—normal, grade 1—partial, and grade 3—complete rupture). This scoring system resulted in good interobserver agreement and excellent intra-observer agreement [75]. Good to excellent intra- and interobserver agreement was found in various studies for tendon damage at hand and ankle tendons [20, 22].
Bursitis Inflammation of periarticular soft tissue, including synovial bursae, is a major cause of pain in RA patients. Accurate diagnosis of such pathologies is of utmost importance for adequate management of these patients. The most studied bursal sites are at the shoulder and foot level. Figure 3.12 shows an example of subacromial subdeltoid (SASD) bursitis in an RA patient. In a study carried out by Bruyn et al., they reported a good overall agreement between US and MRI in detecting SASD bursitis. In the same study, intra- and interobserver reliability for BM and PD ranged from poor to moderate [76]. At forefoot bursitis, the most frequently involved site was the 4/5 inter-metatarsal space [77] .
An important cause of knee pain is the presence of Baker’s cyst. Figure 3.13 shows an example of Baker cyst showing SH with Doppler signal and SE in an RA patient. Diagnosis is essential as treatment is different from other knee pathologies. In a number of studies, almost a half of RA patients assessed had US-detected Baker’s cyst [8, 78, 79] of which less than a half were detected by clinical examination [78].
Enthesopathy was defined as abnormal hypoechoic (loss of fibrillar architecture) and/or thickened tendon at its bony attachment seen in two perpendicular planes that may exhibit Doppler signal and/or bony changes including enthesophytes, erosions, or irregularity [17]. Although entheseal abnormalities in RA patients are insufficiently studied, it seems that these are more frequent than has been previously estimated. Genc et al. compared tendon and entheseal US abnormalities of lower and upper limb in RA patients with spondyloarthropathy (SpA) and healthy controls. They found that there were no significant differences between RA and SpA patients in terms of tendon and entheseal involvement, whereas RA patients presented more tendon and entheseal pathologies than healthy controls. The most affected entheseal sites in RA and SpA patients were the distal and proximal patellar tendon and Achilles tendon. No differences from the control group were found in the involvement of plantar aponeurosis [80] .
Rheumatoid nodules are more frequently found at pressure sites, usually associated with more severe disease. At US examinations, they appear oval shaped, with well-defined hypoechoic formation, generally homogenous, and in the majority of cases they are usually found close to the bone surface. They can present a central very hypoechoic, well-defined area. Compared to gout tophi, rheumatoid nodules show less frequent posterior acoustic shadowing and less erosion at adjacent bone level [81] .
Clinical Applications
Besides demonstrating to be a valid, reliable, and sensitive-to-change tool in inflammatory arthritis , MSUS has also been shown to be more sensitive than clinical assessment in detecting joint inflammation in RA patients. Irrespective of the number of joints studied, disease activity, or duration, MSUS has detected inflammation in significantly more joints than clinical assessment [7–16, 39].
Currently, the main role of US assessment in RA includes diagnosis, monitoring disease activity and treatment response as well as guiding intra-articular procedures.
Diagnosis
Although several joint abnormalities can be detected by MSUS, none of them are pathognomonic for RA. However, a number of studies have shown that MSUS can be used for diagnostic purposes in addition to clinical evaluation [82, 83], especially in seronegative patients [84]. Noteworthy US findings were not interpreted out of clinical context. For the diagnostic purposes, the majority of studies have investigated the added value of US-detected abnormalities at small joint level (i.e., MCP, PIP, MTP wrist and ankle joints). Early US-detected abnormalities at this level were mostly synovitis and tenosynovitis, although erosion detection was not uncommon. Results of these studies paved the way for EULAR recommendations regarding the use of US when diagnostic doubts arise, as this would improve the certainty of an RA diagnosis above clinical criteria alone [23].
For BM synovitis, there is no consensus regarding the relevance of grade 1 of synovitis, especially in small joints. BM synovitis grade 1 can be detected in a significant percentage of healthy people, and at least for diagnosis purposes its use is debatable [85]. Figure 3.14 shows an example of grade 1 BM synovitis in healthy people. Although the presence of intra-articular Doppler signals is associated more frequently with pathology, it can be detected also in healthy people [86]. This is possible mainly due to the improvement of machines sensitivity which allows the detection of normal vessels. Thus, the sensitivity of the machine must be considered and settings adjusted accordingly. The presence of grade 1 synovitis, especially in one isolated joint, without other inflammatory changes should be carefully considered as diagnosis value. Noteworthy, all the above remarks are valid when assessing patients without any anti-inflammatory treatment, as this can mask the BM synovitis and Doppler activity. Also synovitis, erosions can also be seen in healthy people [31, 68, 87].
The minimal threshold enough to diagnose active inflammatory arthritis remains a matter of controversy. This may include the minimal degree of synovitis, number of joints with synovitis, degree of erosions, or a combination of any three that are necessary to make an RA diagnosis. Millet et al. suggested a minimum two joints showing grade 2 or 3 for BM-detected synovitis or two cases of bone erosion [87]. Other studies added tendon evaluation to US assessment of inflammatory arthritis to make an RA diagnosis. These US findings—together with clinical and laboratory findings—increase the probability of an inflammatory arthritis diagnosis. In the study by Freeston et al., US evaluation of wrists and MCP joints and flexor tendons was added to clinical examination in patients with very early inflammatory arthritis. In seronegative patients with positive CRP, swollen joints and erosion on CR, the presence of a grade 3 BM synovitis, at least a grade 1of PD synovitis, or at least one erosion increases the probability of inflammatory arthritis from 30 to 94 % [84]. In a study of early, untreated oligoarthritis, following US assessment, about one third of patients fulfilled polyarthritis classification criteria owing to the presence of subclinical synovitis [9]. According to a study by Scire et al., a PD score of two or more was highly specific for the diagnosis of RA [88]. Thus, the tendon evaluation can add valuable information about inflammatory activity. Furthermore, it is important to remember that a number of the tendons’ synovial sheaths can communicate to the synovial joint (e.g., biceps tendon sheath, foot first finger flexor tendon).
In early, untreated RA patients, finger flexor tenosynovitis was observed more frequently than peri-extensor tenosynovitis (Fig. 3.15), and the most frequently involved tendons were the tendons from second and third fingers [21]. In an MRI study, hand flexor tenosynovitis was a strong predictor for RA in early, unspecified arthritis or suspected RA [89].
The detection of erosions is also useful in RA diagnosis. Although erosions can be detected in several rheumatic diseases, some areas can be considered as target for RA. US assessment of the styloid process of the ulnar, the radial part of second MCP joint, and the ulnar part of fifth MCP joint can provide important information for RA diagnosis. Zayat et al. investigated the specificity and sensitivity of US-detected erosion in RA compared to different musculoskeletal diseases (i.e., psoriatic arthritis (PsA) , osteoarthritis (OA), and gout) and healthy controls. Although RA patients presented more US-detected erosions than other groups, the differences were not significant. When RA-target sites only were included, i.e., second and third MCP, fifth MTP, and distal ulna, the sensitivity improved but was still not specific for RA. However, the presence of large erosions covering between one- to two-thirds of the surface of one quadrant in any of RA-target sites was highly specific for RA. Furthermore, the presence of any erosion at the level of fifth MTP was both specific and sensitive for RA [90].
New classification criteria for RA have been developed by American College of Rheumatology (ACR) and EULAR and published in 2010. A number of recent studies have shown the value of adding US in making the diagnosis of RA [82, 91]. Furthermore, based on US finding, patients were more accurately classified as requiring MTX treatment [82].
In addition to its diagnostic role, the US-detected inflammation can also be used to predict the progression of undifferentiated inflammatory arthritis to RA. Salaffi et al. found that the strongest independent predictor factor for developing RA in early, undifferentiated arthritis was PD positivity. Moreover, the positivity of PD in more than three joints increased significantly the risk of progression to RA [92]. Furthermore, van de Stadt et al. found that the presence of both BM and PD synovitis increases the risk for the development of arthritis in patients with arthralgia, without arthritis at clinical examination and positive anti-citrullinated protein antibodies (ACPA) and/or immunoglobulin M-rheumatoid factor (IgM-RF) [93]. In another work, Navalho et al. studied the association between MRI-detected synovitis and tenosynovitis at hand level with progression to RA. They found that ECU tenosynovitis, finger flexor tenosynovitis of the second finger, and radio-carpal synovitis were significantly associated with progression to RA [94].
In other rheumatic diseases, joint inflammatory activity can also be detected. Synovitis, tenosynovitis, erosions, and Doppler signals were reported in a variety of inflammatory and noninflammatory diseases, e.g., PsA, OA. Some US findings can help differentiate RA from other inflammatory diseases. For example, peritenon inflammation of finger extensor tendons is highly characteristic of PsA [95], while Doppler activity at the enthesis level is characteristic in SpA patients [96].
However, it should be highlighted that RA patients may also experience other rheumatic diseases that have different treatments and prognosis. Joints included in clinical scores (e.g., shoulder, knee) are often affected by degenerative processes and clinical differentiation of these pathologies can be challenging. Figure 3.16 shows an example of degenerative changes of the knee joint in an RA patient with knee pain, whereas, Fig. 3.17 shows an example of full-thickness tear of the supraspinatus tendon in an RA patient with shoulder pain. MSUS is useful in identifying pathologic changes related to degenerative musculoskeletal disorders (e.g., OA) or regional pain syndromes in RA patients, thus helping in differentiating these pathologies from active disease. Figure 3.18 shows an example of BM synovitis with Doppler signal of a PIP joint in an OA patient.
Furthermore, in patients already diagnosed with RA, MSUS can help to differentiate active disease from chronic structural damage. Although there is no consensus in the definition of US-active synovitis, the presence of Doppler signal is considered as a sign of active inflammation. At tendon level, MSUS can help in differentiating active tendon inflammation from chronic tendon damage. Although clinical examination can identify complete tendon tear, partial tear remains undiagnosed. Thus, tendon damage although secondary to persistent tendon inflammation, does not represent active disease; thereby does not require changes in DMARDs treatment.
Monitoring Disease Activity
In several studies, MSUS has shown a good sensitivity to change [15, 42–48]. When compared to MRI, MSUS showed a high sensitivity in detecting both synovitis and tenosynovitis [21, 59, 64]. MSUS was also reported to be more sensitive than CR and as sensitive as MRI, in detecting bone erosion [69]. In addition, earlier studies revealed that MSUS was more sensitive than clinical assessment in detecting joint inflammation [31, 47, 79]. All the evidence coming from the studies points to MSUS as a useful and valuable tool in monitoring RA patients. Taking all of this into consideration, EULAR recommendations for the use of imaging in RA endorsed the use of US for more accurate assessment of inflammation [23].
As far as monitoring disease activity, a comprehensive US assessment of all accessible peripheral joints would be time-consuming and not necessarily practical in daily clinical practice. However, until now, there is no consensus on how many and which joints should be assessed. Several reduced joint counts that have been studied are discussed below. Scheel et al. suggested that US evaluation of second to fourth PIP and MCP joints with a semiquantitative score is sufficient for diagnosis and follow-up in RA patients [33]. A study carried out by Naredo et al. depicted high correlation of a reduced 12-joint count, i.e., bilateral elbow, wrist, second and third MCP and PIP, knee and ankle, with a comprehensive 44-joint count. Moreover, this reduced 12-joint count was shown to represent accurately the response to biologic therapy in RA patients [46]. In another study, Backhaus et al. developed a reduced US joint count which included assessment of only seven small joints, i.e., wrist, second and third MCP and PIP, second and fifth MTP of the clinically dominant side, combining soft tissue changes such as synovitis, tenosynovitis, paratenonitis with erosive bone lesions. This US seven-joint count showed to be a sensitive tool in monitoring patients with inflammatory arthritis (i.e., RA and PsA) in daily clinical practice [97]. This score was found to be sensitive to change in a cohort of patients with RA treated with csDMARDs or bDMARDs [98]. According to the study by Perricone et al., a reduced 6-joint count, i.e., bilateral wrist, second MCP, and knee, correlated excellently with the 12-joint count and was also shown to be sensitive to change in RA patients treated with etanercept (ETA) [99].
On the other hand, other studies focused on Doppler US as the cornerstone for monitoring the disease activity. In the study done by Ellegaard et al., Doppler US quantitative assessment of synovitis of the most symptomatic wrist showed fair to moderate correlation with disease activity index (DAS) for 28 joints (DAS28), swollen joint count, CRP, and ESR in RA patients starting anti-tumor necrosis factor (TNF) therapy. In conclusion, the authors suggested that CDUS examination of only one affected joint could be sufficient in assessing disease activity [100]. In another study, Damjanov et al. calculated the disease activity using US score (US DAS) by replacing tender joint count with PD semiquantitative score for synovitis for 22 joints, including wrists, MCP, and MTP joints, and swollen joint count with BM semiquantitative score for synovitis for 28 joints, i.e., MCP joints, PIP joints, wrists, elbows, shoulders, and knees. With MRI as reference, US DAS was more reliable than DAS28 in assessing both disease activity and further joint damage [101]. Another US score for large joints was developed by Hartung W et al. Sonography of large joints in rheumatology (SOLAR) score was used to assess the grade of inflammation in the shoulder, elbow, hip, and knee joints in RA patients. They calculated a score for each joint by summing the BM/PD scores recorded for each joint’s recesses. After 12 months of treatment, all parameters showed significant improvement, except PDUS scores for shoulder and hip. They concluded that the SOLAR score is a feasible tool for evaluating large joints in patients with RA [102]. Lastly, Hammer BH and Kvien T compared previously described reduced joint counts (i.e., 7, 12, 28, and 44 joints [14]) with comprehensive 78 joints, 36 tendons, and 2 bursae count in RA patients starting bDMARDs. They observed high correlations between the comprehensive 78-joint count and all of the reduced joint count, for both BM and PD variables [103].
However, a difficult question remains unanswered, which score to be used? In an attempt to address this question, Mandel et al. compared the reliability and discriminant capacity of 11 different US scoring systems, including different combinations of 42, 28, 20, 16, 12, 10, 8, 7 joints counts. They found no significant differences between these systems, suggesting that simplified joint counts may perform at least as well as more comprehensive scores [41]. On another front, in standard daily clinical practice, the significance of BM synovitis grade 1, is another challenge which needs to be addressed especially in long-standing disease where fibrotic, irreversible changes can also be found. Witt et al. investigated the clinical relevance of grade 1 synovitis in the wrist, MCP, PIP, and MTP joints, comparing early onset and established RA patients with healthy controls. Considering the frequency of BMUS synovitis grade 1, at MCP joints there were no significant differences between the three groups; neither were significant differences found between established RA patients and healthy controls at PIP and MTP joint level. In contrast, at wrist level, they found significant more synovitis in patients with early onset and established RA disease compared to healthy controls. After 6 months of treatment, significant more joints with initial synovitis grade 1 remained unchanged compared to initial synovitis grade 2 and 3. On the other hand, as expected, the majority of joints with synovitis grade 1 were neither painful nor swollen on clinical examination nor exhibited PD activity [104]. However, in this study, the issue of predictive value in relation to structural damage was not assessed.
Attempts to define US-active synovitis have been deliberated, taking into consideration the evidence just mentioned. As structural damage has been associated mostly, in the majority of studies, with the presence of Doppler signal [105, 106], synovitis was considered as active if Doppler signal was detected [107, 108]. Other authors considered synovitis active if SH is greater than two together with the presence of Doppler activity [109]. At the patient level, a cumulative BM or PD score for defining active disease has not yet been developed. However, care must be taken with patients on anti-inflammatory medication such as nonsteroidal anti-inflammatory drugs (NSAIDs), steroidal anti-inflammatory drugs, as these may mask the BM and Doppler activity. Zayat et al. found a significant increase in BM and Doppler parameters after stopping treatment with NSAIDs for five drug half-life times [110]. Yet, pharmacokinetics of subcutaneous anti-TNF drugs showed no effect on US findings [111].
With respect to bone erosions, an important issue to be addressed was whether US could be used to monitor the erosion course. In RA patients, US-detected inflammation has shown predictive value in relation to structural damage, measured either by CR or MRI. This was documented by the results of earlier studies which revealed an increase of US-detected erosions at hand finger lever in RA patients, both at short- and long-term follow-up [47, 59, 112]. In most of the studies, the detection of vascularization in synovial proliferation by Doppler techniques has been shown to be the strongest predictor for further structural damage [48, 49, 105, 106, 113, 114].
Taylor et al. evaluated the predictive value of US-detected synovitis in a randomized controlled trial of early RA patients treated with IFX versus placebo. After 54 weeks, in the placebo group, there was a significant correlation between both baseline synovial thickness and vascularity and progression in total radiographic score, whereas in the IFX group, the negative correlation was insignificant [49]. According to a study by Naredo et al., US assessment of joint inflammation in early RA patients found that time-integrated values of Doppler variables were the strongest predictors for disease activity during subsequent visits [48]. Dougados et al. found that in active RA patients, radiographic progression was observed more frequently if BM or PD-detected baseline synovitis was present and persistent following 4 months of treatment [115]. In another study, Bøyesen et al. compared the predictive value of clinical and laboratory variables, BMUS and MRI of dominant wrist and CR of wrists and hands in early RA. After a 1-year follow-up period, only BMUS and MRI bone marrow edema (BME) were found to be independent predictors for MRI erosive progression. Moreover, BMUS inflammation, MRI synovitis, and BME performed slightly better than clinical and laboratory variables in identifying early RA patients at risk of developing MRI erosions [113]. This was in concordance with the results of another study carried out by Lillegraven et al. who investigated the predictive value of hand inflammatory US findings in patients with early RA. Baseline ECU tenosynovitis was found to be an independent factor for MRI progression at 1-year follow-up and for radiographic progression at 3-year follow-up [114].
Another interesting issue that remains relatively unknown is the predictive value of baseline US-detected inflammatory activity in relation to treatment response. In the study by Ellegaard et al., the presence of Doppler activity at baseline showed to be predictive for treatment persistence at 1 year in patients staring anti-TNF treatment, while clinical and laboratory variables showed no significant association [116]. This has been endorsed by the EULAR recommendations for the use of imaging in RA, as EULAR sanctioned the use of imaging techniques to predict response to treatment [23].
Furthermore, the role of US in identifying subclinical inflammation represents a further expansion of this tool’s use in standard clinical practice. Several studies have demonstrated the presence of MSUS-detected synovitis in patients in clinical remission. This subclinical synovitis has been detected in RA patients irrespective of the treatment received, whether synthetic or biologic DMARD [14, 16, 21, 38, 105, 106, 117–121]. Figures 3.19 and 3.20 show examples of BM synovitis in an RA patient in clinical remission. Interestingly, earlier studies revealed that regardless of what remission criteria were used (DAS28 or ACR remission criteria) there were no significant differences in the prevalence of US-detected synovitis [38]. In the study carried out by Kawashiri et al., more than a half of RA patients in clinical remission according to simplified disease activity index (SDAI) and without any tender or swollen joint at clinical assessment, presented BM synovitis and/or Doppler activity [122]. However, patients were deemed to be in clinical remission by their attending rheumatologist. As expected, the patients who did not fulfill ACR or DAS28 remission criteria, were more likely to have a significant higher number of joints with US-detected SH. Moreover, even if clinical assessment did not detect tender and swollen joints, BMSH and PD synovitis were still detected in a high number of patients [38]. Adding the disease duration to the equation was assessed in the study performed by Peluso et al. The study revealed that the patients with early onset RA who achieved remission had lower PD synovitis score and were more likely to present no synovitis on imaging compared to patients with long-standing RA in remission [117]. Saleem et al. compared RA patients in clinical remission who received bDMARD as first-line therapy with those with delayed treatment. They found a significantly lower BM synovitis scores in the first group, but similar PD scores [118]. The findings of these studies may have an important role in providing an explanation for radiologic progressive joint damage found in patients with prolonged clinical remission according to ACR criteria [123]. An MRI study in patients with early RA suggested a direct link between synovial inflammation and structural damage as no MRI-detected new erosions were seen in any joint without synovitis. The authors of this study concluded that synovitis appears to be the primary abnormality and the likelihood of bone erosions is related to the level of synovitis [124].
Predictive value of subclinical synovitis in relation to radiographic structural damage and disease relapse/flare had been investigated in several studies. The great majority of studies found associations between Doppler variables, not BM synovitis or structural damage, and/or disease relapse/flare. An explanation of these results can be that BM SH may reflect active disease, especially in early RA, which is reversible with treatment, but also may represent a chronically thickened, fibrotic, and irreversible synovial tissue in later stages of disease. In contrast, Doppler signals reflect increased vascularity which is associated with active inflammation. The impact of the persistence of enhanced vascularity assessed by PD in RA patients who achieved clinical remission has been investigated in several other studies. Outcome of these studies revealed that PDUS predicts radiographic progression and disease relapse or flare in RA patients in clinical remission [14, 105, 106, 119]. In patients treated with either csDMARDs or bDMARDs, the baseline variables for Doppler activity were associated with radiographic progression [105] and disease relapse [106]. In the study carried out by Scire et al., the persistence of PD signal in a single joint had proved to be the main predictor for short-term relapse in RA patients in clinical remission with an odds ratio (OR) of 12.8 [14]. In concordance with these findings, the study carried out by Saleem et al. revealed that the presence of PD signal was found to be the strongest independent predictor for disease flare with an OR of 4.08 [119].
Recently, Fukae et al. studied the association between quantitative measurements of Doppler signal at hand finger level and radiographic progression at 52 weeks in patients with long-term clinical low disease activity (DAS28 < 3.2). They found that structural damage occurred more frequent in joint with synovial hypervascularity and progression of structural damage occurred irrespective to the level of cumulative synovial vascularity. These results show that joints can demonstrate radiographic progression in the presence of any Doppler activity, even if sustained low disease activity is achieved [125]. Considering that the presence of PDUS in RA patients in clinical remission may be predictive of radiographic progression and disease flare or relapse [14, 105, 118], EULAR recommended the use of US for assessing persistent inflammation even in patients in clinical remission [23].
A reduced joint count for detecting subclinical synovitis in patients in clinical remission was proposed by Naredo et al. Evaluation of bilateral wrist, second to fifth MCP joints, ankle, and second to fifth MTP joints showed a high correlation with a comprehensive 44-joint count for both BM and PD [16]. The same high correlation was found also for previous described 12-joint counts [16]. However, further studies are needed to investigate the maximal acceptable synovitis at joint level that will not produce structural damage and therefore will not require more aggressive treatment. Besides, studies are needed to established cutoff values at patient level for which a BM synovitis can be considered relevant in relation to disease activity and progression. Ongoing studies try to find if using US as a target for remission in RA patients can improve outcomes.
Guided Intra-Articular Procedures
Joint puncture for fluid aspiration purposes or intra-articular injection of different drugs represents routine procedures for rheumatologists. US guidance has a number of advantages against blinded injections. Firstly, US allows for better diagnosis and better characterization of the pattern of joint inflammation, whether it be SH or SE. Secondly, US allows direct visualization of the needle within the joint structure facilitating fluid aspiration. Therefore US-guided procedures significantly improve the accuracy of intra-articular injections [126–129]. Moreover, US-guided punctures significantly reduce patient discomforts and shorten procedure (Fig. 3.21). However, in a study by Cunnington et al., no differences were found regarding improvement in clinical outcomes between US-guided and blinded corticosteroid injection [129].
Conclusions
In conclusion, MSUS is a valid, reliable, and sensitive-to-change tool in RA. Moreover, MSUS has demonstrated to be more sensitive than clinical assessment in detecting joint inflammation. The main clinical applications of MSUS in RA include diagnosis, monitoring disease activity and treatment response, and guiding intra-articular procedures. For RA diagnosis, MSUS has been successfully used in addition to clinical evaluation. For monitoring disease activity, several scoring systems including a reduced number of joints have been developed, with similar performances. US-detected joint inflammation has been observed in patients in clinical remission. Doppler-detected synovitis has showed predictive value in relation to radiographic damage and disease flare/relapse in both active and remission disease. The US guidance of procedures improves the accuracy of intra-articular injections.
Abbreviations
- ACPA:
-
Anti-citrullinated protein antibodies
- ACR:
-
American College of Rheumatology
- ADA:
-
Adalimumab
- b:
-
Biologic
- BM:
-
B-mode
- BME:
-
Bone marrow edema
- CD:
-
Color Doppler
- CR:
-
Conventional radiography
- CRP:
-
C-reactive protein
- cs:
-
Conventional synthetic
- CT:
-
Computed tomography
- DAS:
-
Disease activity index
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ECU:
-
Extensor carpi ulnaris
- ESR:
-
Erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- GH:
-
Glenohumeral
- IFX:
-
infliximab
- IgM-RF:
-
immunoglobulin M-rheumatoid factor
- MCP:
-
Metacarpophalangeal
- MMP:
-
Matrix metalloprotease
- MRI:
-
Magnetic resonance imaging
- MS:
-
Musculoskeletal
- MTP:
-
Metatarsophalangeal
- MTX:
-
Methotrexate
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- OMERACT:
-
Outcome measures in Rheumatology
- OR:
-
Odds ratio
- PD:
-
Power Doppler
- PIP:
-
Proximal interphalangeal
- PsA:
-
Psoriatic arthritis
- RA:
-
Rheumatoid arthritis
- SASD:
-
Subacromial subdeltoid
- SDAI:
-
Simplified disease activity index
- SE:
-
Synovial fluid/effusion
- SH:
-
Synovial hypertrophy
- SOLAR:
-
Sonography of large joints in rheumatology
- SpA:
-
Spondyloarthropathy
- TA:
-
Tibialis anterior
- TIMP:
-
Metallopeptidase inhibitor
- TNF:
-
Tumor necrosis factor
- TP:
-
Tibialis posterior
- US:
-
Ultrasound
- VEGF:
-
Vascular endothelial growth factor
References
Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27(8):864–72.
Scott DL, Symmons DP, Coulton BL, Popert AJ. Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 1987;1:1108–11.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res. 2011;63(4):522–9.
Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7.
Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014;73(3):516–28.
Karim Z, Wakefield RJ, Quinn M, Conaghan PG, Brown AK, Veale DJ, et al. Validation and reproducibility of ultrasonography in the detection of synovitis in the knee: a comparison with arthroscopy and clinical examination. Arthritis Rheum. 2004;50(2):387–94.
Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30(5):966–71.
Wakefield RJ, Green MJ, Marzo-Ortega H, Conaghan PG, Gibbon WW, McGonagle D, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63(4):382–5.
Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64(3):375–81.
Scheel AK, Schmidt WA, Hermann KG, Bruyn GA, D’Agostino MA, Grassi W, et al. Interobserver reliability of rheumatologists performing musculoskeletal ultrasonography: results from a EULAR ‟Train the trainers” course. Ann Rheum Dis. 2005;64(7):1043–9.
Szkudlarek M, Klarlund M, Narvestad E, Court-Payen M, Strandberg C, Jensen KE, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther. 2006;8(2):R52.
Salaffi F, Filippucci E, Carotti M, Naredo E, Meenagh G, Ciapetti A, et al. Inter-observer agreement of standard joint counts in early rheumatoid arthritis: a comparison with grey scale ultrasonography–a preliminary study. Rheumatology 2008;47(1):54–8.
Scire CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology 2009;48(9):1092–7.
Hammer HB, Sveinsson M, Kongtorp AK, Kvien TK. A 78-joints ultrasonographic assessment is associated with clinical assessments and is highly responsive to improvement in a longitudinal study of patients with rheumatoid arthritis starting adalimumab treatment. Ann Rheum Dis. 2010;69(7):1349–51.
Naredo E, Valor L, De la Torre I, Martinez-Barrio J, Hinojosa M, Aramburu F, et al. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: how many and which joints should be assessed? Arthritis Care Res. 2013;65(4):512–7.
Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485–7.
Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Ostergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48(4):955–62.
Naredo E, D’Agostino MA, Wakefield RJ, Moller I, Balint PV, Filippucci E, et al. Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis. 2013;72(8):1328–34.
Bruyn GA, Moller I, Garrido J, Bong D, dʼAgostino MA, Iagnocco A, et al. Reliability testing of tendon disease using two different scanning methods in patients with rheumatoid arthritis. Rheumatology 2012;51(9):1655–61.
Wakefield RJ, O’Connor PJ, Conaghan PG, McGonagle D, Hensor EM, Gibbon WW, et al. Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imaging. Arthritis Rheum. 2007;57(7):1158–64.
Micu MC, Serra S, Fodor D, Crespo M, Naredo E. Inter-observer reliability of ultrasound detection of tendon abnormalities at the wrist and ankle in patients with rheumatoid arthritis. Rheumatology 2011;50(6):1120–4.
Colebatch AN, Edwards CJ, Ostergaard M, van der Heijde D, Balint PV, DʼAgostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804–14.
Fiocco U, Cozzi L, Rigon C, Chieco-Bianchi F, Baldovin M, Cassisi GA, et al. Arthroscopic synovectomy in rheumatoid and psoriatic knee joint synovitis: long-term outcome. Br J Rheumatol. 1996;35(5):463–70.
Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44(2):331–8.
Walther M, Harms H, Krenn V, Radke S, Kirschner S, Gohlke F. Synovial tissue of the hip at power Doppler US: correlation between vascularity and power Doppler US signal. Radiology 2002;225(1):225–31.
Andersen M, Ellegaard K, Hebsgaard JB, Christensen R, Torp-Pedersen S, Kvist PH, et al. Ultrasound colour Doppler is associated with synovial pathology in biopsies from hand joints in rheumatoid arthritis patients: a cross-sectional study. Ann Rheum Dis. 2014;73(4):678–83.
Takase K, Ohno S, Takeno M, Hama M, Kirino Y, Ihata A, et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin Exp Rheumatol. 2012;30(1):85–92.
Koski JM, Saarakkala S, Helle M, Hakulinen U, Heikkinen JO, Hermunen H. Power Doppler ultrasonography and synovitis: correlating ultrasound imaging with histopathological findings and evaluating the performance of ultrasound equipments. Ann Rheum Dis. 2006;65(12):1590–5.
Fukae J, Kon Y, Henmi M, Sakamoto F, Narita A, Shimizu M, et al. Change of synovial vascularity in a single finger joint assessed by power Doppler sonography correlated with radiographic change in rheumatoid arthritis: comparative study of a novel quantitative score with a semiquantitative score. Arthritis Care Res. 2010;62(5):657–63.
Szkudlarek M, Narvestad E, Klarlund M, Court-Payen M, Thomsen HS, Ostergaard M. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum. 2004;50(7):2103–12.
Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44(9):2018–23.
Scheel AK, Hermann KG, Kahler E, Pasewaldt D, Fritz J, Hamm B, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2005;52(3):733–43.
Terslev L, Torp-Pedersen S, Savnik A, von der Recke P, Qvistgaard E, Danneskiold-Samsoe B, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum. 2003;48(9):2434–41.
Kawashiri SY, Suzuki T, Nakashima Y, Horai Y, Okada A, Nishino A, et al. Synovial inflammation assessed by ultrasonography correlates with MRI-proven osteitis in patients with rheumatoid arthritis. Rheumatology 2014;53(8):1452–6.
Kawashiri SY, Kawakami A, Iwamoto N, Fujikawa K, Satoh K, Tamai M, et al. The power Doppler ultrasonography score from 24 synovial sites or 6 simplified synovial sites, including the metacarpophalangeal joints, reflects the clinical disease activity and level of serum biomarkers in patients with rheumatoid arthritis. Rheumatology 2011;50(5):962–5.
Naredo E, Moller I, Moragues C, de Agustin JJ, Scheel AK, Grassi W, et al. Interobserver reliability in musculoskeletal ultrasonography: results from a †Teach the Teachers” rheumatologist course. Ann Rheum Dis. 2006;65(1):14–9.
Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–73.
Filer A, de Pablo P, Allen G, Nightingale P, Jordan A, Jobanputra P, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis. 2011;70(3):500–7.
Cheung PP, Dougados M, Gossec L. Reliability of ultrasonography to detect synovitis in rheumatoid arthritis: a systematic literature review of 35 studies (1,415 patients). Arthritis Care Res. 2010;62(3):323–34.
Mandl P, Balint PV, Brault Y, Backhaus M, DʼAgostino MA, Grassi W, et al. Metrologic properties of ultrasound versus clinical evaluation of synovitis in rheumatoid arthritis: results of a multicenter, randomized study. Arthritis Rheum. 2012;64(4):1272–82.
Hau M, Kneitz C, Tony HP, Keberle M, Jahns R, Jenett M. High resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alpha receptor (etanercept). Ann Rheum Dis. 2002;61(1):55–8.
Ribbens C, Andre B, Marcelis S, Kaye O, Mathy L, Bonnet V, et al. Rheumatoid hand joint synovitis: gray-scale and power Doppler US quantifications following anti-tumor necrosis factor-alpha treatment: pilot study. Radiology 2003;229(2):562–9.
Terslev L, Torp-Pedersen S, Qvistgaard E, Kristoffersen H, Rogind H, Danneskiold-Samsoe B, et al. Effects of treatment with etanercept (Enbrel, TNRF:Fc) on rheumatoid arthritis evaluated by Doppler ultrasonography. Ann Rheum Dis. 2003;62(2):178–81.
Filippucci E, Iagnocco A, Salaffi F, Cerioni A, Valesini G, Grassi W. Power Doppler sonography monitoring of synovial perfusion at the wrist joints in patients with rheumatoid arthritis treated with adalimumab. Ann Rheum Dis. 2006;65(11):1433–7.
Naredo E, Moller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58(8):2248–56.
Scheel AK, Hermann KG, Ohrndorf S, Werner C, Schirmer C, Detert J, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis. 2006;65(5):595–600.
Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57(1):116–24.
Taylor PC, Steuer A, Gruber J, Cosgrove DO, Blomley MJ, Marsters PA, et al. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum. 2004;50(4):1107–16.
Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response–preliminary observations. Radiology 1996;198(2):582–4.
Terslev L, Torp-Pedersen S, Qvistgaard E, Danneskiold-Samsoe B, Bliddal H. Estimation of inflammation by Doppler ultrasound: quantitative changes after intra-articular treatment in rheumatoid arthritis. Ann Rheum Dis. 2003;62(11):1049–53.
Filippucci E, Farina A, Carotti M, Salaffi F, Grassi W. Grey scale and power Doppler sonographic changes induced by intra-articular steroid injection treatment. Ann Rheum Dis. 2004;63(6):740–3.
Stone M, Bergin D, Whelan B, Maher M, Murray J, McCarthy C. Power Doppler ultrasound assessment of rheumatoid hand synovitis. J Rheumatol. 2001;28(9):1979–82.
Teh J, Stevens K, Williamson L, Leung J, McNally EG. Power Doppler ultrasound of rheumatoid synovitis: quantification of therapeutic response. Br J Radiol. 2003;76(912):875–9.
Qvistgaard E, Rogind H, Torp-Pedersen S, Terslev L, Danneskiold-Samsoe B, Bliddal H. Quantitative ultrasonography in rheumatoid arthritis: evaluation of inflammation by Doppler technique. Ann Rheum Dis. 2001;60(7):690–3.
Terslev L, Ellegaard K, Christensen R, Szkudlarek M, Schmidt WA, Jensen PS, et al. Head-to-head comparison of quantitative and semi-quantitative ultrasound scoring systems for rheumatoid arthritis: reliability, agreement and construct validity. Rheumatology 2012;51(11):2034–8.
Sakellariou G, Iagnocco A, Filippucci E, Ceccarelli F, Di Geso L, Carli L, et al. Ultrasound imaging for the rheumatologist XLVIII. Ultrasound of the shoulders of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(6):837–42.
Di Geso L, Filippucci E, Riente L, Sakellariou G, Delle Sedie A, Meenagh G, et al. Ultrasound imaging for the rheumatologist XL. Sonographic assessment of the hip in rheumatoid arthritis patients. Clin Exp Rheumatol. 2012;30(4):464–8.
Hoving JL, Buchbinder R, Hall S, Lawler G, Coombs P, McNealy S, et al. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis. J Rheumatol. 2004;31(4):663–75.
Hammer HB, Kvien TK. Ultrasonography shows significant improvement in wrist and ankle tenosynovitis in rheumatoid arthritis patients treated with adalimumab. Scand J Rheumatol. 2011;40(3):178–82.
Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. H and tendon involvement in rheumatoid arthritis: an ultrasound study. Semin Arthritis Rheum. 2012;41(6):752–60.
Wakefield RJ, Freeston JE, O’Connor P, Reay N, Budgen A, Hensor EM, et al. The optimal assessment of the rheumatoid arthritis hindfoot: a comparative study of clinical examination, ultrasound and high field MRI. Ann Rheum Dis. 2008;67(12):1678–82.
Wakefield RJ, Gibbon WW, Conaghan PG, OʼConnor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43(12):2762–70.
Schmidt WA, Schicke B, Ostendorf B, Scherer A, Krause A, Walther M. Low-field MRI versus ultrasound: which is more sensitive in detecting inflammation and bone damage in MCP and MTP joints in mild or moderate rheumatoid arthritis? Clin Exp Rheumatol. 2013;31(1):91–6.
Weidekamm C, Koller M, Weber M, Kainberger F. Diagnostic value of high-resolution B-mode and doppler sonography for imaging of hand and finger joints in rheumatoid arthritis. Arthritis Rheum. 2003;48(2):325–33.
Finzel S, Ohrndorf S, Englbrecht M, Stach C, Messerschmidt J, Schett G, et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 2011;63(5):1231–6.
Dohn UM, Terslev L, Szkudlarek M, Hansen MS, Hetland ML, Hansen A, et al. Detection, scoring and volume assessment of bone erosions by ultrasonography in rheumatoid arthritis: comparison with CT. Ann Rheum Dis. 2013;72(4):530–4.
Dohn UM, Ejbjerg BJ, Court-Payen M, Hasselquist M, Narvestad E, Szkudlarek M, et al. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8(4):R110.
Baillet A, Gaujoux-Viala C, Mouterde G, Pham T, Tebib J, Saraux A, et al. Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology 2011;50(6):1137–47.
Riente L, Delle Sedie A, Scire CA, Filippucci E, Meenagh G, Iagnocco A, et al. Ultrasound imaging for the rheumatologist. XXXI. Sonographic assessment of the foot in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29(1):1–5.
Grassi W, Filippucci E, Farina A, Salaffi F, Cervini C. Ultrasonography in the evaluation of bone erosions. Ann Rheum Dis. 2001;60(2):98–103.
Salaffi F, Carotti M, Ciapetti A, Ariani A, Gasparini S, Grassi W. Validity of a computer-assisted manual segmentation software to quantify wrist erosion volume using computed tomography scans in rheumatoid arthritis. BMC Musculoskelet Disord. 2013;14:265.
Schmidt WA, Schmidt H, Schicke B, Gromnica-Ihle E. Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis. 2004;63(8):988–94.
Swen WA, Jacobs JW, Hubach PC, Klasens JH, Algra PR, Bijlsma JW. Comparison of sonography and magnetic resonance imaging for the diagnosis of partial tears of finger extensor tendons in rheumatoid arthritis. Rheumatology 2000;39(1):55–62.
Bruyn GA, Hanova P, Iagnocco A, d’Agostino MA, Moller I, Terslev L, et al. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focussing on the diagnostic reliability. Ann Rheum Dis. 2013;73:1929–34
Bruyn GA, Naredo E, Moller I, Moragues C, Garrido J, de Bock GH, et al. Reliability of ultrasonography in detecting shoulder disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68(3):357–61.
Bowen CJ, Culliford D, Dewbury K, Sampson M, Burridge J, Hooper L, et al. The clinical importance of ultrasound detectable forefoot bursae in rheumatoid arthritis. Rheumatology 2010;49(1):191–2.
Andonopoulos AP, Yarmenitis S, Sfountouris H, Siamplis D, Zervas C, Bounas A. Baker’s cyst in rheumatoid arthritis: an ultrasonographic study with a high resolution technique. Clin Exp Rheumatol. 1995;13(5):633–6.
Riente L, Delle Sedie A, Filippucci E, Scire CA, Iagnocco A, Gutierrez M, et al. Ultrasound Imaging for the rheumatologist XXVII. Sonographic assessment of the knee in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2010;28(3):300–3.
Genc H, Cakit BD, Tuncbilek I, Erdem HR. Ultrasonographic evaluation of tendons and enthesal sites in rheumatoid arthritis: comparison with ankylosing spondylitis and healthy subjects. Clin Rheumatol. 2005;24(3):272–7.
Nalbant S, Corominas H, Hsu B, Chen LX, Schumacher HR, Kitumnuaypong T. Ultrasonography for assessment of subcutaneous nodules. J Rheumatol. 2003;30(6):1191–5.
Nakagomi D, Ikeda K, Okubo A, Iwamoto T, Sanayama Y, Takahashi K, et al. Ultrasound can improve the accuracy of the 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis to predict the requirement for methotrexate treatment. Arthritis Rheum. 2013;65(4):890–8.
Agrawal S, Bhagat SS, Dasgupta B. Improvement in diagnosis and management of musculoskeletal conditions with one-stop clinic-based ultrasonography. Mod Rheumatol. 2009;19(1):53–6.
Freeston JE, Wakefield RJ, Conaghan PG, Hensor EM, Stewart SP, Emery P. A diagnostic algorithm for persistence of very early inflammatory arthritis: the utility of power Doppler ultrasound when added to conventional assessment tools. Ann Rheum Dis. 2010;69(2):417–9.
Ten Cate DF, Luime JJ, Swen N, Gerards AH, De Jager MH, Basoski NM, et al. Role of ultrasonography in diagnosing early rheumatoid arthritis and remission of rheumatoid arthritis–a systematic review of the literature. Arthritis Res Ther. 2013;15(1):R4.
Terslev L, Torp-Pedersen S, Bang N, Koenig MJ, Nielsen MB, Bliddal H. Doppler ultrasound findings in healthy wrists and finger joints before and after use of two different contrast agents. Ann Rheum Dis. 2005;64(6):824–7.
Millot F, Clavel G, Etchepare F, Gandjbakhch F, Grados F, Saraux A, et al. Musculoskeletal ultrasonography in healthy subjects and ultrasound criteria for early arthritis (the ESPOIR cohort). J Rheumatol. 2011;38(4):613–20.
Scire CA, Iagnocco A, Meenagh G, Riente L, Filippucci E, Delle Sedie A, et al. Ultrasound imaging for the rheumatologist XXXIII. Sonographic assessment of the foot in early arthritis patients. Clin Exp Rheumatol. 2011;29(3):465–9.
Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, et al. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology 2009;48(8):887–91.
Zayat AS, Ellegaard K, Conaghan PG, Terslev L, Hensor EM, Freeston JE, Emery P, Wakefield RJ. The specificity of ultrasound-detected bone erosions for rheumatoid arthritis. Ann Rheum Dis. 2014 Apr 30 [Epub ahead of print].
Kawashiri SY, Suzuki T, Okada A, Yamasaki S, Tamai M, Nakamura H, et al. Musculoskeletal ultrasonography assists the diagnostic performance of the 2010 classification criteria for rheumatoid arthritis. Mod Rheumatol. 2013;23(1):36–43.
Salaffi F, Ciapetti A, Gasparini S, Carotti M, Filippucci E, Grassi W. A clinical prediction rule combining routine assessment and power Doppler ultrasonography for predicting progression to rheumatoid arthritis from early-onset undifferentiated arthritis. Clin Exp Rheumatol. 2010;28(5):686–94.
van de Stadt LA, Bos WH, Meursinge Reynders M, Wieringa H, Turkstra F, van der Laken CJ, et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther. 2010;12(3):R98.
Navalho M, Resende C, Rodrigues AM, Ramos F, Gaspar A, Pereira da Silva JA, et al. Bilateral MR imaging of the hand and wrist in early and very early inflammatory arthritis: tenosynovitis is associated with progression to rheumatoid arthritis. Radiology 2012;264(3):823–33.
Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W. Differential diagnosis between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70(6):1111–4.
D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48(2):523–33.
Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum. 2009;61(9):1194–201.
Backhaus TM, Ohrndorf S, Kellner H, Strunk J, Hartung W, Sattler H, et al. The US7 score is sensitive to change in a large cohort of patients with rheumatoid arthritis over 12 months of therapy. Ann Rheum Dis. 2013;72(7):1163–9.
Perricone C, Ceccarelli F, Modesti M, Vavala C, Di Franco M, Valesini G, et al. The 6-joint ultrasonographic assessment: a valid, sensitive-to-change and feasible method for evaluating joint inflammation in RA. Rheumatology 2012;51(5):866–73.
Ellegaard K, Torp-Pedersen S, Terslev L, Danneskiold-Samsoe B, Henriksen M, Bliddal H. Ultrasound colour Doppler measurements in a single joint as measure of disease activity in patients with rheumatoid arthritis–assessment of concurrent validity. Rheumatology 2009;48(3):254–7.
Damjanov N, Radunovic G, Prodanovic S, Vukovic V, Milic V, Simic Pasalic K, et al. Construct validity and reliability of ultrasound disease activity score in assessing joint inflammation in RA: comparison with DAS-28. Rheumatology 2012;51(1):120–8.
Hartung W, Kellner H, Strunk J, Sattler H, Schmidt WA, Ehrenstein B, et al. Development and evaluation of a novel ultrasound score for large joints in rheumatoid arthritis: one year of experience in daily clinical practice. Arthritis Care Res. 2012;64(5):675–82.
Hammer HB, Kvien TK. Comparisons of 7- to 78-joint ultrasonography scores: all different joint combinations show equal response to adalimumab treatment in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R78.
Witt M, Mueller F, Nigg A, Reindl C, Leipe J, Proft F, et al. Relevance of grade 1 Gy-scale ultrasound findings in wrists and small joints to the assessment of subclinical synovitis in rheumatoid arthritis. Arthritis Rheum. 2013;65(7):1694–701.
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958–67.
Foltz V, Gandjbakhch F, Etchepare F, Rosenberg C, Tanguy ML, Rozenberg S, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012;64(1):67–76.
Szkudlarek M, Wakefield RJ, Backhaus M, Terslev L. The discriminatory capacity of ultrasound in rheumatoid arthritis: active vs inactive, early vs advanced, and more. Rheumatology 2012;51(Suppl 7):vii6–9.
Cheung PP, Gossec L, Ruyssen-Witrand A, Le Bourlout C, Mezieres M, Dougados M. The relationship of patient-reported joints with active synovitis detected by power Doppler ultrasonography in rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(4):490–7.
Ramirez J, Ruiz-Esquide V, Pomes I, Celis R, Cuervo A, Hernandez MV, et al. Patients with rheumatoid arthritis in clinical remission and ultrasound-defined active synovitis exhibit higher disease activity and increased serum levels of angiogenic biomarkers. Arthritis Res Ther. 2014;16(1):R5.
Zayat AS, Conaghan PG, Sharif M, Freeston JE, Wenham C, Hensor EM, et al. Do non-steroidal anti-inflammatory drugs have a significant effect on detection and grading of ultrasound-detected synovitis in patients with rheumatoid arthritis? Results from a randomised study. Ann Rheum Dis. 2011;70(10):1746–51.
Naredo E, Hinojosa M, Valor L, Hernandez-Florez D, Mata-Martinez C, Serrano-Benavente B, et al. Does ultrasound-scored synovitis depend on the pharmacokinetics of subcutaneous anti-TNF agents in patients with rheumatoid arthritis? Rheumatology. 2014;53:2088–94
Backhaus M, Burmester GR, Sandrock D, Loreck D, Hess D, Scholz A, et al. Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis. 2002;61(10):895–904.
Boyesen P, Haavardsholm EA, van der Heijde D, Ostergaard M, Hammer HB, Sesseng S, et al. Prediction of MRI erosive progression: a comparison of modern imaging modalities in early rheumatoid arthritis patients. Ann Rheum Dis. 2011;70(1):176–9.
Lillegraven S, Boyesen P, Hammer HB, Ostergaard M, Uhlig T, Sesseng S, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2049–50.
Dougados M, Devauchelle-Pensec V, Ferlet JF, Jousse-Joulin S, D’Agostino MA, Backhaus M, et al. The ability of synovitis to predict structural damage in rheumatoid arthritis: a comparative study between clinical examination and ultrasound. Ann Rheum Dis. 2013;72(5):665–71.
Ellegaard K, Christensen R, Torp-Pedersen S, Terslev L, Holm CC, Konig MJ, et al. Ultrasound Doppler measurements predict success of treatment with anti-TNF- & alpha; drug in patients with rheumatoid arthritis: a prospective cohort study. Rheumatology 2011;50(3):506–12.
Peluso G, Michelutti A, Bosello S, Gremese E, Tolusso B, Ferraccioli G. Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann Rheum Dis. 2011;70(1):172–5.
Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Wakefield R, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis. 2011;70(5):792–8.
Saleem B, Brown AK, Quinn M, Karim Z, Hensor EM, Conaghan P, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012;71(8):1316–21.
Balsa A, de Miguel E, Castillo C, Peiteado D, Martin-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology 2010;49(4):683–90.
Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Karim Z, et al. Disease remission state in patients treated with the combination of tumor necrosis factor blockade and methotrexate or with disease-modifying antirheumatic drugs: a clinical and imaging comparative study. Arthritis Rheum. 2009;60(7):1915–22.
Kawashiri SY, Suzuki T, Nakashima Y, Horai Y, Okada A, Iwamoto N, et al. Ultrasonographic examination of rheumatoid arthritis patients who are free of physical synovitis: power Doppler subclinical synovitis is associated with bone erosion. Rheumatology 2014;53(3):562–9.
Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50(1):36–42.
Conaghan PG, O’Connor P, McGonagle D, Astin P, Wakefield RJ, Gibbon WW, et al. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003;48(1):64–71.
Fukae J, Isobe M, Kitano A, Henmi M, Sakamoto F, Narita A, et al. Structural deterioration of finger joints with ultrasonographic synovitis in rheumatoid arthritis patients with clinical low disease activity. Rheumatology 2014;53(9):1608–12.
Raza K, Lee CY, Pilling D, Heaton S, Situnayake RD, Carruthers DM, et al. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology 2003;42(8):976–9.
Naredo E, Cabero F, Beneyto P, Cruz A, Mondejar B, Uson J, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308–14.
Sibbitt WL, Jr., Peisajovich A, Michael AA, Park KS, Sibbitt RR, B and PA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892–902.
Cunnington J, Marshall N, Hide G, Bracewell C, Isaacs J, Platt P, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joint of patients with inflammatory arthritis. Arthritis Rheum. 2010;62(7):1862–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Naredo, E., Montoro, M., Janţă, I. (2015). Rheumatoid Arthritis. In: El Miedany, Y. (eds) Musculoskeletal Ultrasonography in Rheumatic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-15723-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-15723-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15722-1
Online ISBN: 978-3-319-15723-8
eBook Packages: MedicineMedicine (R0)