Abstract
Background
Several experiences in the literature report SBRT as an effective treatment option for medically inoperable early stage non-small cell lung cancer (NSCLC) and oligometastatic disease. The optimal fractionation schedules and total dose remain controversial. In this study, we evaluated the safety in terms of toxicity and efficacy of using of 8–10 fractions schedules with Helical Tomotherapy (HT) for primary and metastatic lung lesions.
Methods
Between March 2014 and May 2016, a total of 39 patients (median age 72 years, range 26–91) were treated with HT-SBRT for malignant lung lesions: 22 patients with early stage NSCLC, 17 with oligometastases. Patients received 8–10 fractions with lower daily dose for central and ultracentral lesions. Treatment-related toxicity was evaluated using CTCAE v 4.0 scale. Local control (LC), overall survival (OS) and toxicity rates were prospectively collected.
Results
Median duration of RT was 15 days (range 10–26 days) and no interruption occurred. With a median follow-up of 13 months (range 3–29), we reported one G2 pneumonitis (2.6%) and one G2 chest pain (2.6%); no ≥ G2 esophagitis was registered. Actuarial local control rate was 95.5% both at 12 and 24 months for early stage NSCLC and 92.9% both at 12 and 24 months for metastatic patients. OS rate was 94.4 and 92.3% at 1 year, and 94.4 and 83.9% at 2 years in primary and metastatic group, respectively.
Conclusions
The use of 8–10 fractions schedule HT-SBRT for lung malignancies results in high LC and OS rates with minimal toxicities reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the second most common malignancy, after non-melanocytic skin cancers, and it causes more deaths than any other malignancy worldwide [1].
Standard treatment includes surgery, RT and chemotherapy, used alone or in combination, depending on stage of disease and clinical conditions. Stereotactic Body Radiation Therapy (SBRT) has allowed an improvement of oncological outcomes with negligible toxicity, compared to conventional RT, specifically for medically inoperable early stage NSCLC patients [2,3,4,5,6]. The efficacy and safety of SBRT have also been documented in patients affected by oligometastatic disease, i.e., the presence of 1–5 lesions [7].
SBRT is typically delivered in a limited number of fractions over the course of 1–2 weeks [8]. Based on the toxicity data following SBRT for centrally located lesions [9], a strategy of risk-adapted dose prescription was proposed to minimize SBRT-related adverse events, depending on the localization of target volumes within the lungs and proximity to mediastinal organs at risk (OARs) [10, 11].
Recent developments in lung SBRT, such as intensity-modulated RT (IMRT) and image-guided RT (IGRT), have allowed a high accuracy of dose distribution to the target volumes and a more precise assessment of tumor volume changes during the course of treatment by means of imaging on-board, lowering high doses to nearby normal tissues [12, 13].
Helical tomotherapy (HT) is a platform that combines IMRT with in-built image guidance using megavoltage (MV) CT scanning.
We report a single-center experience of 8–10 fractions schedule HT-SBRT for primary and single metastatic lung lesion.
Materials and methods
This is a retrospective mono-institutional study that received the ethics approval from our institutional ethic committee; informed consent was acquired from all participants enrolled in this series.
Primary endpoint of the present study is the feasibility of HT-SBRT for primary and secondary lung malignancies; Local Control (LC), Disease-Free Survival (DFS) and Overall Survival (OS) are secondary endpoints. SBRT was indicated when the following criteria were satisfied: medically inoperable Early Stage NSCLC, the presence of a single lung metastasis for oligometastatic patients, tumor size ≤ 5 cm, Karnofsky perfomance status ≥ 70, a life expectancy of at least 6 months.
Early stage NSCLC patients were staged with bronchoscopy, enhanced computed tomography (CT) scan of the lung and upper abdomen with contrast-medium and 18-fluorodesossiglucose positron emission tomography (PET). The biopsy was avoided in the presence of severe comorbidities; in this scenario, the metabolic imaging was considered a surrogate of malignancy, according to the literature [14]. Before local treatment, oligometastatic patients were evaluated with CT scan and PET.
HT-SBRT procedures
A 2.5 mm slice thickness CT was performed with the patient in supine position with arms up above the head. Immobilization was obtained with the aid of a breast board and an abdominal pressure mould mask. The Clinical Target Volume (CTV), equal to the Gross Tumor Volume (GTV), was defined merging the treatment planning CT with a megavolt computed tomography (MVCT) scan [15,16,17].
MVCT is a free breathing slow CT able to give information relative to the full extent of target motion during respiratory movement, as recommended by AAPM Task Group 101 report [16]. Planning CT images were fused with pre-SBRT diagnostic studies when necessary to facilitate GTV contouring.
The planning target volume (PTV) was obtained by adding a 10 mm margin in cranio-caudal direction and a 5 mm margin in all other directions.
Target volumes and OARs were contoured on the Pinnacle Planning system. The CT datasets were then transferred to the Tomotherapy Treatment Planning system (HT, Accuray Inc. Sunnyvale, CA, USA), where IMRT plans were generated with inverse treatment planning. Tumor dose was prescribed to the 95% isodose encompassing PTV. Different dose schedules were used according to the tumor site (central or peripheral) and maximum diameter of lesion: 60–70 Gy in 8–10 fractions for peripheral lesions, 50–60 Gy in 10 fractions for central lesions, 40 Gy in 10 fractions for ultra-central lesions. Also patients’ frailty, tumor size and location (especially for lesions whose PTV touches or extends into the ribs/pleura, or unable to meet the 3 or 5 fractions schedules constraints) had an impact in fractionation selection process [18, 19], leading to schedules with BED10 > 100 Gy administered in 39% of cases, and schedules with BED10 < 100 Gy in 61%. Dose constraints for OARs were derived from peer-reviewed literature [8, 10, 20]: volume of the lung, excluding PTV, receiving 20 Gy (V20) and 5 Gy (V5) ≤ to 10 and 35%, respectively; mean lung dose (MLD) ≤ 9 Gy; Dmax ≤ 28 Gy on spinal cord; central airways, brachial plexus and esophagus Dmax were limited to 40 Gy. Conformity Index (CI) and Homogeneity Index (HI) were assessed using the following formulas [21]: CI = (TVPIV)2/(TV × PIV) [TVPIV is the target volume covered by prescription isodose volume; TV is the target volume; PIV is the prescription isodose volume], HI is the [(maximum dose − minimum dose)/prescription dose].
Study design and statistical analysis
LC, DFS, OS and toxicity rates were prospectively collected. Dosimetric findings were retrospectively evaluated. LC, DFS and OS at 12 and 24 months from the end of SBRT were calculated using Kaplan–Meier analysis. The log-rank test was used to compare results between patient subsets and among radiation schedule groups. Fisher’s exact test was used to examine differences in LC, DFS, OS and toxicities, according to patients’ characteristics. The Wilcoxon rank-sum test and the equality-of-medians test were applied to examine differences in continuous variables among patient groups. A two-sided value of 0.05 or less was considered to assess statistical significance. All statistical analyses were carried out using Stata/SE 14.1 (Stata, Corp LP, Texas, USA).
Toxicity and follow-up
Adverse events were assessed according to the CTCAE version 4.0. Concerning pulmonary toxicity, patients who were asymptomatic with radiologic changes were not considered to have toxicity.
Contrast-enhanced Chest CT was performed every 3 months from the end of SBRT for the first 2 years. PET scanning was requested in selected cases after CT scan to evaluate tumor response after SBRT.
According to RECIST v1.1 Criteria, complete response (CR) was defined as the disappearance of lesions on the CT scan; a reduction of 30% was considered partial remission (PR); any increase in size ≥ 20% not clearly due to fibrosis was reported as progression of disease (PD); stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum of diameters [22]. PERCIST criteria were used to evaluate the metabolic response [23].
Results
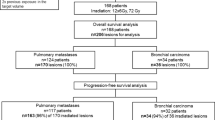
Between March 2014 and May 2016, a total of 39 consecutive patients (median age 72 years, range 26–91) were treated with HT-SBRT for malignant lung lesions: 22 patients were treated for early stage NSCLC, 17 for metastatic disease (Table 1).
In early stage NSCLC, pathological confirmation was obtained for 17 patients (77%). Chemo or biological therapy was administered prior to SBRT in 23 patients (59%). At the time of analysis, the median follow-up was 13 months (range 3–29 months). All patients completed SBRT without interruptions. The median overall treatment time was 15 days (range 10–26 days). SBRT was delivered in consecutive days in 19 patients (48.7%) and every other day in 20 patients (51.3%).
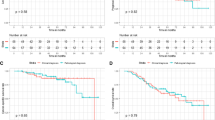
Actuarial local control rate was 95.5% both at 12 and 24 months for early stage NSCLC, and 92.9% both at 12 and 24 months for metastatic patients. Overall survival rate was 94.4 and 92.3% at 1 year, and 94.4 and 83.9% at 2 years in primary and metastatic lung cancer, respectively (Figs. 1, 2).
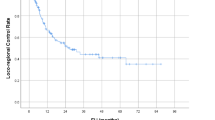
During follow-up, 10 (25.6%) patients developed distant progression, 5 occurred in NSCLC and 5 in metastatic group, leading to 1 year disease-free survival (DFS) rates of 78.9 and 67.9% for primitive lesions and metastases, respectively. 2 years-DFS rates were 52.6% for primitive and 60.4% for secondary lesions (Fig. 3). At the time of the analysis, CR was recorded in 11 (28.2%) lesions, PR in 8 (20.5%) and SD in 18 (46.2%) lesions. In-field irradiation failures were observed in 2 (5.1%) patients, one in the NSCLC group and one in the oligometastatic group.
Acute adverse events were registered as follows (Table 2): G2 chest pain in one (2.6%) patient with peripherally located tumor; no rib fractures were recorded. No patient developed ≥ G3 radiation-induced pneumonitis, reporting only one G2 pneumonitis (2.6%), successfully treated with steroids, in a patient who concomitantly underwent erlotinib after progression through platinum-based chemotherapy. The patient was treated with 10 daily fractions of 6 Gy equal to a BED10 to the tumor of 96 Gy10, reporting V20, V5 and MLD of 8.9, 44.8% and 8.2 Gy, respectively.
Concerning late adverse events, no patient developed any symptoms related to pulmonary fibrosis; a single case of G2 chest wall pain was reported. Regarding the three ultra-central lesions, no toxicity was observed.
A statistical relationship between tumor size, fractionation regimen, timing of fractions and LC or toxicities failed to be found.
Planning and dosimetric outcomes
The median GTV was 5.03 cc (range 0.87–47.27 cc) and the median PTV was 28.32 cc (range 7.32–114.21 cc). The median BED10 was 96 Gy (range 56–119 Gy). The median HI and median CI were 0.075 (range 0.043–0.136) and 0.84 (0.735–0.906), respectively. Median Total Lung V5, V10, V20 and Mean Lung Dose were 21.3% (range 6.5–45.5%), 13.23% (range 0.08–36%), 5.28% (range 0–19%), and 4.2 Gy (range 1.2–9.4 Gy), respectively (Table 3).
Discussion
In lung malignancies, both isocentric (LINAC-based) and non-isocentric (CyberKnife-based) SBRT techniques are routinely adopted in daily clinical practice. Linacs equipped with Flattening Filter Free delivery allow to reduce the treatment time and, probably, uncertainties related to organ motion during irradiation [24, 25]. Cyberknife system offers a precise tracking during breathing and adjustment to moving targets [26, 27] in order to compensate uncertainties related to the long treatment delivery time. HT could be largely criticized due to the relatively long treatment delivery in the absence of a lesion tracking system. Actually, on the basis of clinical evidence, this technology is safe for treating moving tumors considering that interplay of breathing and tomotherapy delivery motions did not affect significantly plan delivery accuracy [28].
To date, a BED10 ≥ 100 Gy remains a strong predictive factor of long-term LC [29]. Although in our series most patients received a BED10 inferior to 100 Gy, we reported an acceptable actuarial 2-years LC. This could be explained with experimental and clinical data supporting the role of the total dose as a more crucial factor comparing to BED10, based on the hypothesis that a moderate protracted schedule fractionation could improve re-oxygenation and, consequently, increase the tumor response [30, 31]. At 12 months, LC and OS rates were greater than 90% in both groups of patients treated with HT-SBRT in the present analysis. Apart from the present experience, other studies reported valuable results both in terms of LC and safety profile by means of HT-SBRT in lung malignancies (Table 4; [15, 18, 32,33,34,35,36,37,38]). Looking at HT-SBRT performances for stage I NSCLC, one of the larger data series [37] enrolled 79 patients treated with different doses schedules. Similar to our experience, 12-months LC was superior to 90%.
An Italian study [15], enrolling 56 patients with lung primary or secondary cancer, evaluated clinical outcomes of HT-SBRT in two different subgroups: ablative SBRT for 27 patients with T1–2 NSCLC and palliative SBRT for 29 patients with oligometastases. In their experience, the actuarial 2-years LC was 69.6% in case of primary tumor and 40% for the oligometastatic group. Regarding the setting of lung oligometastases, the choice of “the right patient” for local treatment remains largely debated. In the present series, we considered the “oligometastatic patient” in case of single lung metastasis in the absence of the primary tumor. At the time of the analysis, 24-months LC and OS were 92.9 and 83.9%, respectively, significantly superior compared to the experience by Marcenaro and colleagues [15] in which a subgroup of patients with multiple lesions was candidate to lung SBRT. These findings reinforce patient selection as a crucial factor in the decision-making process for lung oligometastatic disease.
Regarding the safety profile, the issue of the radiation-induced pneumonitis has been largely investigated during the last years. In fact, several authors [39,40,41] identified dosimetric parameters that might be useful as predictors of radiation pneumonitis, such as the mean lung dose, V20 and V5. Barriger and colleagues [41], among patients treated with SBRT for a total doses ranged between 42 and 60 Gy given in 8 fractions, reported Grade 2–4 pneumonitis in 4.3% of patients in case of mean lung dose ≤ 4 Gy compared to 17.6% for a mean lung dose > 4 Gy (p = 0.02); in their experience, a similar risk of moderate-severe pneumonitis was observed for V20 ≤ 4% (4.3% of patients) versus V20 > 4% (16.4% of patients, p = 0.03). Few authors highlighted the importance of low-dose radiation distribution in the development of lung toxicity with HT; Jo et al. [42] defined the value of V5 as crucial for the onset of symptomatic RP, recommending to keep its value inferior to 65%. Kim et al. [33] reported a significant dose–response relationship between RP and ipsilateral and contralateral V5. In the present study, HT allowed to respect available lung dose constraints in the majority of cases: mean lung dose < 9 Gy was respected in 97.4% of cases, V20 < 10% in 92.3%, V5 < 35% in 79.5%. The median values of total lung, V20 and V5 and mean lung dose were 5.28 and 21.3% and 4.2 Gy; median values of ipsilateral and contralateral V5 were 36.2 and 5.68%, respectively. In the present study population, only one patient in the metastatic group with slightly higher values of V20 and MLD underwent steroid treatment for respiratory symptoms due to a G2 RP; this patient was concomitantly treated with target agent anti-epidermal growth factor receptor (Erlotinib) after first-line platinum-based chemotherapy and, after a short course period of steroids treatment, continued Erlotinib. According to the data derived from few clinical trials, an increased toxicity is possible when targeted therapy is combined with SBRT [43].
It is also well recognized that lung lesions located in the so-called “No-Fly-Zone” are at particular risk of complications [12]. In this last clinical scenario, mediastinal OARs sparing, in addition to a more fractionated regimen, is crucial to minimize the risk of adverse events, especially in the challenging situation of ultra-central located lesions. As reported by Chi et al. in a dosimetric comparison with 2- and 8-arcs VMAT technique, HT allows sculpting the dose to the tumor while sparing nearby OARs offering a safe treatment option, despite the relatively higher low dose to the normal lung remains an issue of debate [18, 44]. In our series, the three ultra-central patients reported no significant toxicity.
In conclusion, the present study has several limitations such as: (1) the retrospective nature of the analysis; (2) the heterogeneity of the population (primary and metastatic lesions) here analyzed; (3) the limited follow-up. However, although the limitations abovementioned could affect the robustness of the present results, LC and OS rates here reported seem comparable to available clinical data, both for primary lung tumors as well as lung oligometastases after SBRT.
A longer follow-up and a wider sample size are advocated for more mature results both in terms of oncological outcomes and toxicity rates. Of course, in consideration of the above-mentioned limitations, keeping in mind the retrospective nature of the current analysis, long-term findings are warranted.
References
Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, Simo GV, Vansteenkiste J, Peters S, ESMO Guidelines Committee (2016) Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(suppl 5):v1–v27
Ricardi U, Frezza G, Filippi AR, Badellino S, Levis M, Navarria P, Salvi F, Marcenaro M, Trovò M, Guarneri A, Corvò R, Scorsetti M (2014) Stereotactic Ablative Radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung Cancer 84(3):248–253. https://doi.org/10.1016/j.lungcan.2014.02.015 (Epub 2014 Mar 13)
Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S (2010) Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 28(35):5153–5159. https://doi.org/10.1200/JCO.2010.30.0731 (Epub 2010 Nov 1)
Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, Bucci MK, McAleer MF, Mehran RJ, Roth JA, Komaki R (2008) Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 72(4):967–971. https://doi.org/10.1016/j.ijrobp.2008.08.001
Verstegen NE, Oosterhuis JWA, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, Mollema R, van Tets WF, Warner A, Joosten JJA, Amir MI, Haasbeek CJA, Smit EF, Slotman BJ, Senan S (2013) Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 24(6):1543–1548. https://doi.org/10.1093/annonc/mdt026 (first published online February 20, 2013)
Palma DA, Senan S (2012) Early-stage non-small cell lung cancer in elderly patients: should stereotactic radiation therapy be the standard of care? Int J Radiat Oncol Biol Phys 84(5):1058–1059. https://doi.org/10.1016/j.ijrobp.2012.07.2353
Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M (2012) Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 17(8):1100–1107. https://doi.org/10.1634/theoncologist.2012-0092 (Epub 2012 Jun 20; Review)
Guckenberger M, Andratschke N, Alheit H et al (2014) Definition of stereotactic body radiotherapy: Principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol 190(1):26–33. https://doi.org/10.1007/s00066-013-0450-y
Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J (2006) Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24(30):4833–4839
Li Q, Swanick CW, Allen PK, Gomez DR, Welsh JW, Liao Z, Balter PA, Chang JY (2014) Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: exploration of clinical indications. Radiother Oncol 112(2):256–261. https://doi.org/10.1016/j.radonc.2014.07.010
Bral S, Gevaert T, Linthout N, Versmessen H, Collen C, Engels B, Storme G (2011) Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 80(5):1343–1349
Mazzola R, Fiorentino A, Ricchetti F, Giaj Levra N, Fersino S, Di Paola G, Lo Casto A, Ruggieri R, Alongi F (2016) Cone-beam computed tomography in lung stereotactic ablative radiation therapy: predictive parameters of early response. Br J Radiol 20:20160146
Mazzola R, Fiorentino A, Di Paola G, Giaj Levra N, Ricchetti F, Fersino S, Tebano U, Pasetto S, Ruggieri R, Salgarello M, Alongi F (2017) Stereotactic ablative radiation therapy for lung oligometastases: predictive Parameters of early response by (18)FDG-PET/CT. J Thorac Oncol 12(3):547–555. https://doi.org/10.1016/j.jtho.2016.11.2234
Louie AV, Senan S, Patel P, Ferket BS, Lagerwaard FJ, Rodrigues GB, Salama JK, Kelsey C, Palma DA, Hunink MG (2014) When is a biopsy-proven diagnosis necessary before stereotactic ablative radiotherapy for lung cancer? A decision analysis. Chest 146(4):1021–1028. https://doi.org/10.1378/chest.13-2924
Marcenaro M, Vagge S, Belgioia L, Agnese D, Lamanna G, Mantero E, Gusinu M, Garelli S, Cavagnetto F, Agostinelli S, Corvò R (2013) Ablative or palliative stereotactic body radiotherapy with helical tomotherapy for primary or metastatic lung tumor. Anticancer Res 33(2):655–660
Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, Ramsey CR, Van Herk MB, Vedam SS, Wong JW, Yorke E (2006) The management of respiratory motion in radiation oncology report of AAPM Task Group 76a). Med Phys 33:3874–3900. https://doi.org/10.1118/1.2349696
Chang HJ, Ko HL, Lee CY, Wu RH, Yeh YW, Jiang JS, Kao SJ, Chi K (2012) Hypofractionated radiotherapy for primary or secondary oligometastatic lung cancer using Tomotherapy. Radiat Oncol 27(7):222. https://doi.org/10.1186/1748-717X-7-222
Arcangeli S, Agolli L, Portalone L, Migliorino MR, Lopergolo MG, Monaco A et al (2015) Patterns of CT lung injury and toxicity after stereotactic radiotherapy delivered with helical tomotherapy in early stage medically inoperable NSCLC. Br J Radiol 88:20140728
Franks KN, Jain P, Snee MP (2015) Stereotactic ablative body radiotherapy for lung cancer. Clin Oncol (R Coll Radiol) 27(5):280–289. https://doi.org/10.1016/j.clon.2015.01.006 (Epub 2015 Mar 4)
Navarria P, Ascolese AM, Tomatis S, Cozzi L, De Rose F, Mancosu P, Alongi F, Clerici E, Lobefalo F, Tozzi A, Reggiori G, Fogliata A, Scorsetti M (2014) Stereotactic body radiotherapy (sbrt) in lung oligometastatic patients: role of local treatments. Radiat Oncol 9(1):91. https://doi.org/10.1186/1748-717X-9-91
Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F, Ka Heng Chan M, Ernst I, Krieger T, Duma MN, Oechsner M, Ganswindt U, Heinz C, Alheit H, Blank H, Nestle U, Wiehle R, Kornhuber C, Ostheimer C, Petersen C, Pollul G, Baus W, Altenstein G, Beckers E, Jurianz K, Sterzing F, Kretschmer M, Seegenschmiedt H, Maass T, Droege S, Wolf U, Schoeffler J, Haverkamp U, Eich HT, Guckenberger M (2017) Planning benchmark study for SBRT of early stage NSCLC : results of the DEGRO Working Group Stereotactic Radiotherapy. Strahlenther Onkol 193(10):780–790. https://doi.org/10.1007/s00066-017-1151-8
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S. https://doi.org/10.2967/jnumed.108.057307 (Review)
Scorsetti M, Alongi F, Castiglioni S, Clivio A, Fogliata A, Lobefalo F, Mancosu P, Navarria P, Palumbo V, Pellegrini C, Pentimalli S (2011) Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiation oncology 6(1):113
Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozzi A, Iftode C, Reggiori G, Tomatis S, Infante M, Alloisio M (2013) Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiother Oncol 107(3):414–418
Lischalk JW, Woo SM, Kataria S, Aghdam N, Paydar I, Repka MC, Anderson ED, Collins BT (2016) Long-term outcomes of stereotactic body radiation therapy (SBRT) with fiducial tracking for inoperable stage I non-small cell lung cancer (NSCLC). J Radiat Oncol 5:379–387. https://doi.org/10.1007/s13566-016-0273-4
De Bari B, Filippi AR, Mazzola R, Bonomo P, Trovò M, Livi L, Alongi F (2015) Available evidence on re-irradiation with stereotactic ablative radiotherapy following high-dose previous thoracic radiotherapy for lung malignancies. Cancer Treat Rev 41(6):511–518. https://doi.org/10.1016/j.ctrv.2015.04.002 (Epub 2015 Apr 16. Review)
Sterpin E, Janssens G, Orban de Xivry J, Goossens S, Wanet M, Lee JA, Delor A, Bol V, Vynckier S, Gregoire V, Geets X (2012) Helical tomotherapy for SIB and hypo-fractionated treatments in lung carcinomas: a 4D Monte Carlo treatment planning study. Radiother Oncol 104(2):173–180. https://doi.org/10.1016/j.radonc.2012.06.005
Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Karasawa K, Hayakawa K, Niibe Y, Takai Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Kozuka T, Arimoto T, Hara R, Itami J, Araki T (2011) Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 81(5):1352–1358. https://doi.org/10.1016/j.ijrobp.2009.07.1751
Aoki M, Hatayama Y, Kawaguchi H, Hirose K, Sato M, Akimoto H, Fujioka I, Ono S, Tsushima E, Takai Y (2016) Clinical outcome of stereotactic body radiotherapy for primary and oligometastatic lung tumors: a single institutional study with almost uniform dose with different five treatment schedules. Radiat Oncol 20(11):5. https://doi.org/10.1186/s13014-016-0581-2
Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Miyakawa A, Murai T, Takaoka T, Hattori Y, Asai R (2015) Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol 10(6):960–964. https://doi.org/10.1097/JTO.0000000000000525
Hodge, Tome WA, Jaradat HA, Orton NP, Khuntia D, Traynor A, Weigel T, Mehta MP (2006) Feasibility report of image guided stereotactic body radiotherapy (IG-SBRT) with tomotherapy for early stage medically inoperable lung cancer using extreme hypofractionation. Acta Oncol 45(7):890–896 (NRO)
Kim JY, Kay CS, Kim YS, Jang JW, Bae SH, Choi JY, Yoon SK, Kim KJ (2009) Helical tomotherapy for simultaneous multitarget radiotherapy for pulmonary metastasis. Int J Radiat Oncol Biol Phys 75(3):703–710. https://doi.org/10.1016/j.ijrobp.2008.11.065 (Epub 2009 May 4)
Sole CV, Lopez Guerra JL, Matute R, Jaen J, Puebla F, Rivin E, Sanchez-Reyes A, Beltran C, Bourgier C, Calvo FA, Marsiglia H (2013) Stereotactic ablative radiotherapy delivered by image-guided helical tomotherapy for extracranial oligometastases. Clin Transl Oncol 15(6):484–491. https://doi.org/10.1007/s12094-012-0956-2 (Epub 2012 Nov 10)
Aibe N, Yamazaki H, Nakamura S, Tsubokura T, Kobayashi K, Kodani N, Nishimura T, Okabe H, Yamada K (2014) Outcome and toxicity of stereotactic body radiotherapy with helical tomotherapy for inoperable lung tumor: analysis of Grade 5 radiation pneumonitis. J Radiat Res. 55(3):575–582. https://doi.org/10.1093/jrr/rrt146 (Epub 2014 Jan 23)
Nagai A, Shibamoto Y, Yoshida M, Inoda K, Kikuchi Y (2014) Safety and efficacy of intensity-modulated stereotactic body radiotherapy using helical tomotherapy for lung cancer and lung metastasis. BioMed Res Int
Rosen LR, Fischer-Valuck BW, Katz SR, Durci M, Wu HT, Syh J, Patel B (2013) Helical image-guided stereotactic body radiotherapy (SBRT) for the treatment of early-stage lung cancer: a single-institution experience at the Willis-Knighton Cancer Center. Tumori 100(1):42–48
Casutt A, Bouchaab H, Beigelman-Aubry C, Bourhis J, Lovis A, Matzinger O (2015) Stereotactic body radiotherapy with helical tomotherapy for medically inoperable early stage primary and second-primary non-small-cell lung neoplasm: 1-year outcome and toxicity analysis. Br J Radiol 88(1049):20140687. https://doi.org/10.1259/bjr.20140687 (Epub 2015 Mar 4)
Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, Mueller G, Flentje M (2010) Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 97(1):65–70. https://doi.org/10.1016/j.radonc.2010.04.027 (Epub 2010 Jun 3)
Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Mantovani C, Fiandra C, Anglesio S, Ragona R (2009) Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol 48:571–577
Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, Johnstone PA, Fakiris AJ (2012) A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 82:457–462
Jo IY, Kay CS, Kim JY, Son SH, Kang YN, Jung JY, Kim KJ (2014) Significance of low-dose radiation distribution in development of radiation pneumonitis after helical-tomotherapy-based hypofractionated radiotherapy for pulmonary metastases. J Radiat Res 55(1):105–112. https://doi.org/10.1093/jrr/rrt080 (Epub 2013 Jun 11)
Wang Z, Zhu XX, Wu XH, Li B, Shen TZ, Kong QT, Li J, Liu ZB, Jiang WR, Wang Y, Hou B (2014) Gefitinib combined with stereotactic radiosurgery in previously treated patients with advanced non-small cell lung cancer. Am J Clin Oncol. 37(2):148–153. https://doi.org/10.1097/coc.0b013e31826e071b
Chi A, Ma P, Fu G et al (2013) Critical structure sparing in stereotactic ablative radiotherapy for central lung lesions: helical tomotherapy vs. volumetric modulated arc therapy. Chen C-T (ed) PLoS One 8(4):e59729. https://doi.org/10.1371/journal.pone.0059729
Acknowledgements
None of the authors involved in this study received financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Figlia, V., Mazzola, R., Cuccia, F. et al. Hypo-fractionated stereotactic radiation therapy for lung malignancies by means of helical tomotherapy: report of feasibility by a single-center experience. Radiol med 123, 406–414 (2018). https://doi.org/10.1007/s11547-018-0858-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0858-7