Abstract
Purpose
To determine the clinical effectiveness and long-term outcome of stent insertion for malignant superior vena cava (SVC) syndrome.
Materials and methods
From June 2010 to April 2016, 47 patients with malignant SVC syndrome were treated with stent insertion in our center. Data regarding the technical success, clinical success, and long-term outcome were collected and analyzed retrospectively.
Results
SVC stent insertion was successfully performed in all patients. A total of 65 stents were used. No procedure-related complication occurred in these patients. The mean SVC pressure gradient decreased from 17.8 mmHg before stent insertion to 7.6 mmHg after stent insertion (P < 0.001). Clinical success was 100%. During a mean follow-up period of 6 months (range 10 days–13 months), 25 patients underwent subsequent anti-cancer treatment. Six patients (12.8%) experienced re-obstruction of stent 1 to 189 days (median 76 days) after stent insertion. All patients died during the follow-up. The median stent patency time and survival were 339 and 167 days, respectively. The cumulative 3-, 6-, and 12-month stent patency rates were 93.4, 87.4, and 81.2%, respectively. The cumulative 3-, 6-, and 12-month survival rates were 83, 38.3, and 2.1%, respectively. The independent predictors of prolonging survival after stent insertion were lower tumor stage (P = 0.018) and subsequent anti-cancer treatment after stent insertion (P = 0.009).
Conclusion
Stent insertion is a simple, safe, and effective method for patients with malignant SVC syndrome. Subsequent anti-cancer treatment after stent insertion may increase the survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant superior vena cava (SVC) syndrome can be caused by lung cancer, esophageal cancer, or other mediastinal tumor due to the closely anatomic relationship [1–3]. Most patients with malignant SVC syndrome are usually poor candidates for surgical resection because of the advanced tumor stage [1–3]. In addition, patients with malignant SVC syndrome usually present with dyspnea, brain swelling, neck swelling, and upper limb swelling [4–6]. These symptoms also decrease the quality of life during their limited survival.
At present, stent insertion is usually used as the first-line treatment option in patients with SVC syndrome, because stent insertion dose not interfere the subsequent anti-cancer treatment and provide instant relief of symptoms [7–9]. However, the long-term outcome and predictor of influencing survival following stent insertion are still not clear. In the present study, we aimed to determine the clinical effectiveness and long-term outcome of stent insertion for malignant SVC syndrome.
Materials and methods
The retrospective study was approved by our Institutional Review Board. Informed consent for procedure and clinical data management was obtained.
Patients
From June 2010 to April 2016, 47 patients with malignant SVC syndrome were treated with stent insertion in our center. The baseline data of these 47 patients were demonstrated in Table 1. The inclusion criteria were as follows: (1) definite diagnosis of malignant SVC syndrome and (2) inoperable SVC syndrome. Data regarding the technical success, clinical success, and long-term outcome were collected and analyzed retrospectively. Baseline data included patients’ history, clinical presentation and imaging findings.
Diagnosis
SVC syndrome was diagnosed based on patients’ history, clinical presentation, and thoracic contrast enhanced computed tomography (CT) findings. The primary disease was diagnosed based on pathological results, which were obtained by bronchoscopy, percutaneous needle biopsy, esophageal endoscopy, or surgery. Tumor stage was evaluated based on the criteria of the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC).
Stents
All SVC stents were bare self-expanding metallic stents. SVC stents included Sinus-XL (OptiMed, Ettlingen, Germany), Zilver (Cook, Bloomington, Indiana, USA), Luminexx (Bard, Murray Hill, New Jersey, USA), and Smart (Cordis, Miami Lakes, Florida, USA) stents.
The distal and proximal margins of stent should be at least 10 mm longer than the distal and proximal margins of obstruction, respectively. The stent diameter should be 2 mm larger than the SVC diameter.
Treatment procedure
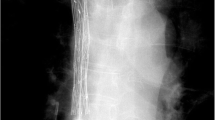
All procedures were performed by three interventional radiologists under fluoroscopic guidance (Fig. 1). SVC stent insertion was performed from the right femoral vein approach under local anesthesia (5 ml of 2% lidocaine). A 4 Fr VER catheter (Cordis) and a 0.035-inch guide wire (Terumo, Tokyo, Japan) were inserted to the proximal site of the SVC obstruction. Venography was performed to confirm the obstructed site. Then, a 0.035-inch stiff guide wire (Cook) was placed across the obstruction. The predilation was performed with a 10–14 mm-diameter balloon (Cook or Bard). Finally, the 7–10 Fr stent delivery system was advanced over the stiff guide wire, and the stent was deployed to cover the obstructed site. Venography was performed again to confirm the patency of SVC stent. The pressure gradient between the proximal and distal ends of the obstruction and stent was measured before and after stent insertion.
70-year-old man with malignant SVC syndrome and right internal jugular vein thrombosis underwent catheter-directed thrombolysis and SVC stent insertion. a SVC venography demonstrated the obstruction of SVC and bilateral BCVs and the right internal jugular vein thrombosis (arrow). The collateral circulations were established. b Thrombi were dissolved after thrombolysis. c Predilation was performed. d Stent was inserted and the venography demonstrated the patency of stent. The collateral circulations were disappeared
If the obstruction involved SVC and unilateral brachiocephalic vein (BCV), recanalization of SVC and the obstructed BCV was preformed. If the obstruction involved SVC and bilateral BCVs, unilateral stent insertion was performed and recanalization of SVC and right BCV was preferred. If the obstruction only involved SVC with sufficient tumor-free landing zone (more than 1 cm from the venous confluence), the stent was placed in SVC. If the proximal margin of the involved SVC was too close to the venous confluence (within 1 cm from the venous confluence), the stent was placed in SVC and right BCV.
After treatment, all patients were administered with subcutaneous low-molecular-weight heparin (5000 IU/12 h) for 3 days, followed by oral warfarin sodium. The international normalized ratio was maintained at 2–3.
Definitions and endpoints
Technical success of SVC stent insertion is defined as successful placement of the stent across the target lesion with the pressure gradient lower than 10 mmHg between the proximal and distal ends of the stent [8, 9]. Clinical success of SVC stent insertion is defined as a major improvement or the elimination of SVC syndrome after treatment [8, 9]. Survival time was evaluated after stent insertion. Stent patency time was defined as the period of time between stent insertion to stent dysfunction or patient death. Stent dysfunction was suspected when the patients experienced the recurrence of SVC syndrome. In the calculation of stent patency time, patients who died without stent dysfunction and living patients without stent dysfunction were regarded as censored data [10, 11]. In the previous studies, patient survival was shorter than the cumulative stent patency owing to loss of censored data [11].
The standard follow-up protocol included thoracic CT examination and telephone follow-up. Thoracic CT was performed 1 month and then every 2–3 months after stent insertion. Telephone follow-up was performed every 2 months for the duration of the follow-up period to confirm the patients’ general condition. Follow-up ended at the time of setting up of this study (December 2016) or patients’ death. Primary endpoint was survival time, and secondary endpoints included procedure-related complication and stent dysfunction.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were analyzed by t test. Numeric data were analyzed using the χ2 test or Fisher exact probability test. Patency and survival time were calculated using Kaplan–Meier curves. The predictors of survival were determined using univariate and multivariate Cox regression analyses. The covariates incorporated into the multivariate analysis were the variables with P < 0.1 on univariate analysis. A P value <0.05 was considered statistically significant.
Results
Technical and clinical success
SVC stent insertion was successfully performed in all patients. Two patients simultaneously had airway stenosis and underwent airway stent insertion before SVC stent insertion. A total of 65 stents (average 1.4 stents per case) were used. The diameter and length of stents varied from 12 to 18 mm and from 60 to 90 mm, respectively. Twenty-nine patients were placed with 1 stent and eighteen patients were placed with 2 stents. One patient had right internal jugular vein thrombosis and underwent catheter-directed thrombolysis (300,000 units of urokinase per day) for 6 days before stent insertion (Fig. 1). No procedure-related complication occurred in these patients. The mean SVC pressure gradient decreased from 17.8 mmHg before stent insertion to 7.6 mmHg after stent insertion (P < 0.001).
Clinical success was achieved in all patients. The symptoms of dyspnea, neck swelling, and upper limb swelling relieved instantly after stent insertion.
Follow-up
During a mean follow-up period of 6 months (range 10 days–13 months), 25 patients underwent subsequent anti-cancer treatment (chemotherapy: 11; radiotherapy: 7; chemo-radiotherapy: 7). Three patients experienced airway stenosis after SVC stent insertion and underwent airway stent insertion.
Six patients (12.8%) experienced re-obstruction of stent 1 to 189 days (median 76 days) after stent insertion (Table 2). The causes of re-obstruction included tumor growth (n = 5) and thrombosis development (n = 1). Among the 5 patients with tumor growth, two patients underwent second time stent insertion, one patient underwent chemotherapy, and 2 patients abandoned further treatment. The patient with thrombosis development underwent trans-opisthenar vein thrombolysis. The median stent patency time was 339 days (95% confidence interval [CI] 300–378). The cumulative 3-, 6-, and 12-month stent patency rates were 93.4, 87.4, and 81.2%, respectively (Fig. 2).
All patients died during the follow-up. The causes of death included tumor progression (n = 43) and respiratory failure (n = 4). The median survival was 167 days (95% CI 149–185). The cumulative 3-, 6-, and 12-month survival rates were 83, 38.3, and 2.1%, respectively (Fig. 3).
Based on univariate analysis, the predictors of prolonging survival after stent insertion were elder age, lower tumor stage, and subsequent anti-cancer treatment after stent insertion. Based on multivariate analysis, the independent predictors of prolonging survival after stent insertion were lower tumor stage (P = 0.018) and subsequent anti-cancer treatment after stent insertion (P = 0.009, Table 3).
Discussion
Approximately 4% patients with lung cancer simultaneously had SVC syndrome [12]. Chemotherapy and radiotherapy were usually considered as the traditional treatment for malignant SVC syndrome [13, 14]. However, these treatment options have a slow response [13, 14]. Although bypass surgery has been reported for palliative treatment of malignant SVC syndrome in selected patients, this type of surgery in terminally ill patients is difficult to justify and is rather invasive for a palliative procedure [15].
Stent insertion is a minimal invasive, simple, and effective method for patients with malignant SVC syndrome [7–9]. Stent insertion can provide an instant decrease of SVC pressure, resulting in rapid relief of symptoms [7–9]. In this present study, the symptoms of dyspnea, neck swelling, and upper limb swelling relieved in all patients after stent insertion. This clinical success rate is comparable to the previous studies of stent insertion for malignant SVC syndrome [7–9]. In addition, stent insertion dose not influence the subsequent anti-cancer treatment.
Approximately 10–60% SVC obstruction patients presented with SVC and bilateral BCVs obstruction [7–9]. Cordial et al. [16] reported a case about bilateral stent insertion for a patient with malignant SVC and bilateral BCVs obstruction. During the procedure, they first inserted a stent at SVC and right BCV, and then, they inserted the second stent via the first stent mesh hole. However, other studies proved that unilateral stent insertion can afford to the clinical success for patients with SVC and bilateral BCVs obstruction [7–9]. In this present study, 19 patients had SVC and bilateral BCVs obstruction, and all of them were successfully treated with unilateral stent insertion.
Although most patients with malignant SVC syndrome have a short life expectancy and the stent remained patent until death [7–9], recurrence of SVC syndrome after initially successful stent insertion still occurs in up to 41% patients [7, 8]. In this present study, six patients (12.8%) experienced re-obstruction of stent with the earliest 1 day after stent insertion. The cumulative 3-, 6-, and 12-month stent patency rates were 93.4, 87.4, and 81.2%, respectively. These rates are comparable to the previous study of stent insertion for malignant SVC syndrome [7]. Gwon et al. [8] reported in a single-center comparative cohort study that unilateral covered stent insertion was associated with higher cumulative stent patency time and lower stent occlusion rate than bare stent for treating SVC syndrome. However, that is a retrospective study and their results indicated that covered stent insertion did not improve patients’ survival time [8]. Therefore, we suggest the use of bare stent for patients with malignant SVC syndrome, because the cost of bare stent is lower.
In this present study, the median survival was 167 days, and the cumulative 3-, 6-, and 12-month survival rates were 83, 38.3, and 2.1%, respectively. These results are comparable to the previous studies [7–9]. Based on univariate and multivariate analyses, the independent predictors of prolonging survival after stent insertion were lower tumor stage (P = 0.018) and subsequent anti-cancer treatment after stent insertion (P = 0.009). In some studies about stent insertion for patients with malignant biliary, colorectal, or airway obstruction, the subsequent anti-cancer treatment after stent insertion can also prolong patients’ survival [17–19]. Therefore, if patients’ clinical condition permits, appropriate anti-cancer treatment should be performed.
This study has some limitations. First, this is a retrospective review, and therefore, the selection bias does exist. Further randomized controlled study should be performed. Second, the sample size is small and the patients only came from a single center; therefore, it is difficult to make definitive conclusions regarding this technique. Third, there is no control group. Therefore, we have no means of comparing this approach to other treatment options.
In conclusion, although further clinical trails are needed, this study demonstrated that stent insertion is a simple, safe, and effective palliative treatment for patients with malignant SVC syndrome. Subsequent anti-cancer treatment after stent insertion may prolong patients’ survival.
References
Wan JF, Bezjak A (2009) Superior vena cava syndrome. Emerg Med Clin North Am 27:243–255
Lepper PM, Ott SR, Hoppe H et al (2011) Superior vena cava syndrome in thoracic malignancies. Respir Care 56:653–666
Kapadia MR, de Hoyos AL, Blum MG (2009) Acute superior vena cava occlusion after stenting of tracheoesophageal fistula. Ann Thorac Surg 87:1260–1262
Morales JP, Sabharwal T, Man-Harun S et al (2007) Alleviation of severe compressive symptoms in a patient with advanced lung carcinoma using tracheal and superior vena cava stents. J Palliat Med 10:24–29
Fukuda M, Obase Y, Miyashita N et al (2006) Both bronchial and vascular stenting followed by chemoradiotherapy for locally advanced non-small cell lung cancer. Anticancer Res 26:565–567
Rabinstein AA, Wijdicks EF (2009) Fatal brain swelling due to superior vena cava syndrome. Neurocrit Care 10:91–92
Cho Y, Gwon DI, Ko GY et al (2014) Covered stent placement for the treatment of malignant superior vena cava syndrome: is unilateral covered stenting safe and effective? Korean J Radiol 15:87–94
Gwon DI, Ko GY, Kim JH et al (2013) Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology 266:979–987
Leung ST, Sung TH, Wan AY et al (2015) Endovascular stenting in the management of malignant superior vena cava obstruction: comparing safety, effectiveness, and outcomes between primary stenting and salvage stenting. Hong Kong Med J 21:426–434
Kitano M, Yamashita Y, Tanaka K et al (2013) Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol 108:1713–1722
Telford JJ, Carr-Locke DL, Baron TH et al (2010) A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc 72:907–914
Rowell NP, Gleeson FV (2002) Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 14:338–351
Dyet JF, Nicholson AA, Cook AM (1993) The use of the Wallstent endovascular prosthesis in the treatment of malignant obstruction of the superior vena cava. Clin Radiol 48:381–385
Urban T, Lebeau B, Chastang C et al (1993) Superior vena cava syndrome in small-cell lung cancer. Arch Intern Med 153:384–387
Picquet J, Blin V, Dussaussoy C et al (2009) Surgical reconstruction of the superior vena cava system: indications and results. Surgery 145:93–99
Cordial R, Moussavian MR, Corvalan J et al (2014) Percutaneous endovascular Y-stenting of a malignant superior vena cava and innominate vein obstruction. Vasc Endovascular Surg 48:77–79
Niu S, Cheng L, Qiao Y et al (2016) Combined stent insertion and high-intensity focused ultrasound ablation for patients with malignant obstructive jaundice. Surg Laparosc Endosc Percutan Tech 26:488–492
Xu YS, Fu YF, Du HT et al (2015) Palliative stent insertion for acute malignant colorectal obstruction: long-term patency and survival. Surg Laparosc Endosc Percutan Tech 25:500–504
Herth FJ, Peter S, Baty F et al (2010) Combined airway and oesophageal stenting in malignant airway–oesophageal fistulas: a prospective study. Eur Respir J 36:1370–1374
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Niu, S., Xu, YS., Cheng, L. et al. Stent insertion for malignant superior vena cava syndrome: effectiveness and long-term outcome. Radiol med 122, 633–638 (2017). https://doi.org/10.1007/s11547-017-0767-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0767-1