Abstract
Objective
To evaluate the correlation between aortic root calcification (ARC) markers and coronary artery calcification (CAC) derived from coronary artery calcium scoring (CACS) and their ability to predict obstructive coronary artery disease (CAD).
Methods
We retrospectively analyzed 189 patients (47% male, age 60.3 ± 11.1 years) with an intermediate probability of CAD who underwent clinically indicated CACS and coronary CT angiography (CCTA). ARC markers [aortic root calcium score (ARCS) and volume (ARCV)] were calculated and compared to CAC markers: coronary artery calcium score (CACS), volume (CACV), and mass (CACM). CCTA datasets were visually evaluated for significant CAD (stenosis ≥ 50%) and the ability of ARC markers to predict obstructive CAD was assessed.
Results
ARCS (mean 67.7 ± 189.5) and ARCV (mean 67.3 ± 184.7) showed significant differences between patients with and without CAC (109.4 ± 238.6 vs 9.42 ± 31.4, p < 0.0001; 108.5 ± 232.4 vs 9.9 ± 30.5, p < 0.0001). A strong correlation was found for ARCS and ARCV with CACS, CACM, and CACV (all p < 0.0001). In a multivariate analysis, ARCS (OR 1.09, p = 0.033) and ARCV (OR 1.12, p = 0.046) were independent markers for CAC. Using a receiver-operating characteristics analysis, the AUC to detect severe CAC was 0.71 (p < 0.0001) and 0.71 (p < 0.0001) for ARCS and ARCV, respectively. ARCS (0.67, p < 0.0001) and ARCV (0.68, p < 0.0001) showed discriminatory power for predicting obstructive CAD, yielding sensitivities 61 and 78% and specificities of 62 and 80%, respectively.
Conclusion
ARC markers are associated with and independently predict the presence of CAC and obstructive CAD. Further testing is required in patients with severe ARC and significant CAD in order to reliably obtain these markers from thoracic-CT or X-ray for proper risk classification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery calcification (CAC) can be assessed during routine coronary computed tomographic angiography (CCTA) using non-enhanced calcium scans. Typically, CAC is calculated as the Agatston calcium score [1]. Recently, a significant amount of evidence has highlighted the prognostic value of coronary artery calcium and its importance as an independent marker of obstructive coronary artery disease (CAD) and cardiac risk stratification for assessing mortality and morbidity in patients with atherosclerosis [2–4].
Recent studies have shown that calcification of the aortic and mitral valve, aortic arch, and thoracic aorta shares risk factors with CAD, and is strongly associated with increased cardiovascular risk and coronary plaque burden [5–8]. For patients undergoing transcatheter aortic valve replacement, CT imaging of the aortic root is used for preoperative procedural planning [9–11].
Few studies have specifically evaluated the role of aortic root calcification (ARC) as a predictor of CAC and obstructive CAD. Previous studies evaluating the association between aortic calcification, CAC, and CAD also included mitral annular or mitral valve calcification, aortic valve calcification, or patients with known CAD [12, 13]. The relationship between ARC markers quantified as calcium and volume score, and CAC assessed with calcium, volume, and mass scores has not been evaluated in a population with an intermediate pre-test probability for CAD. Furthermore, the ability of ARC markers to predict obstructive CAD has not been sufficiently investigated to date.
Thus, we sought to evaluate the association between ARC markers and the presence of CAC and to investigate their discriminatory power for predicting obstructive CAD as defined by coronary CT angiography.
Materials and methods
Study population
The research study protocols were approved by the institutional review board with a waiver of informed consent due to the retrospective nature of this investigation. The study was performed in compliance with HIPAA practices.
We retrospectively analyzed data of patients with intermediate pre-test probability of CAD who underwent clinically indicated non-enhanced coronary calcium scans and CCTA assessing the possibility of CAD between June 2012 and May 2014. Indications for cardiac CT included abnormal exercise or nuclear stress test results, chest pain, and/or routine follow-up. The patient’s Framingham risk score was calculated to reflect clinical risk for cardiovascular events. Patients were excluded if they had a history of known CAD, heart, or valve surgery (valve replacement/repair, coronary artery bypass grafting, or aortic surgery), aortic disease, or cardiac valve disease, including valve calcification. Furthermore, CCTA datasets with non-diagnostic image quality were excluded. We identified 189 eligible patients who were enrolled in this study. Covariates and patient baseline characteristics were obtained from medical records.
CT acquisition parameters
All CCTA examinations were performed using either a first- or second-generation dual-source CT (DSCT) system (Somatom Definition or Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany). All patients underwent an initial non-contrast-enhanced calcium scoring scan (collimation, 32 × 1.2 mm; 120 kV tube voltage; tube current, 75 mA; 3 mm slice thickness with 1.5 mm increment). For the subsequent contrast-enhanced CCTA, scan parameters were as follows: a retrospectively ECG-gated protocol for the 1st generation DSCT scanner and a prospectively ECG-triggered sequential scan protocol with a padding window for the 2nd generation DSCT scanner; tube voltage of 100–120 kV, tube current of 320–412 mA, temporal resolution of 83 or 75 ms, and 2 × 32 × 0.6 mm or 2 × 64 × 0.6 mm collimation with z-flying focal spot. Contrast enhancement was achieved by injecting 50–80 mL of iopromide (Ultravist 370 mg I/mL, Bayer, Wayne, NJ) at 4–6 mL/s followed by a 30 mL saline bolus chaser. Beta blockers and nitroglycerin were used at the discretion of the attending physician. Weighted filtered back projection image reconstruction was performed in the cardiac phase with the least motion using the following parameters: section thickness of 0.75 mm, reconstruction increment of 0.5 mm, and a smooth convolution kernel (B26f).
Analysis of CCTA data and CAC markers
All datasets were transferred to a post-processing workstation (syngo.via VA30, Siemens, Forchheim, Germany) for further analysis performed with dedicated software (syngo.via VB10 Calcium Scoring, Siemens). Transverse sections and automatically generated curved multiplanar reformations were available for assessment. Using CCTA acquisitions, coronary artery stenosis was visually determined as either non-significant (<50% luminal narrowing) or significant (≥50% luminal narrowing) [14]. An area >1 mm2 with an attenuation threshold >130 Hounsfield units (HU) was considered to be calcified plaque [15]. The extent of CAC was quantified semi-automatically on non-contrast scans with the following markers: calcium mass (CACM), volume (CACV), and calcium score (CACS). Calcium scores according to the Agatston method were used to grade CAC: 1 = minimal (0–10), 2 = mild (10–100), 3 = moderate (100–400), or 4 = severe (>400) [14].
Analysis of ARC markers
ARC evaluation was performed with the same software and acquisition protocol used for the CAC analysis. The aortic root was defined as the region of the ascending aorta between the aortic annulus and the sinotubular junction, including the aortic sinuses. Calcifications of aortic valve leaflets were not included in the ARC evaluation. ARC markers were quantified as aortic root calcification score (ARCS) and volume (ARCV). Consistent with CAC, any area >1 mm2 showing an attenuation >130 Hounsfield units (HU) was considered to be a calcified lesion. The degree of ARC was graded according to the CAC measurement.
All examinations were evaluated independently by two observers, each with more than 3 years of experience in cardiovascular imaging. The observers were blinded to patient characteristics as well as the imaging report. All discordant cases were resolved by a consensus reading.
Statistical analysis
MedCalc (MedCalc Software, version 15, Ostend, Belgium) was used for all statistical analyses. Continuous variables were presented as mean ± standard deviation or as median with interquartile range when not normally distributed. Student t test and Mann–Whitney U test were used for parametric or non-parametric data. Normal distribution was assessed using the Kolmogorov–Smirnov test. In addition, Pearson’s correlation coefficient was used to analyze the correlation between ARC markers and CAC markers. A multivariate logistic regression analysis was performed to calculate odds ratios (ORs) with a corresponding 95% confidence interval (CI) to detect independent markers of CAC. Variables that were significant in a univariate analysis were entered into this multivariate logistical regression model. Receiver-operating characteristics (ROC) curve analysis was used to evaluate ARC markers' ability to detect CAC and obstructive CAD. In order to determine discriminatory power, an area under the ROC curve (AUC) was measured according to the DeLong method [16]. Sensitivity and specificity were derived from ROC curve analysis and presented with a 95% confidence interval. For the analysis of inter-rater agreement, Cohen ĸ analysis was used. k values greater than 0.81, 0.61–0.80, 0.41–0.60, 0.21–0.40, and less than 0.20 were defined as excellent, good, moderate, fair, and poor tests, respectively [17]. Statistical significance was assumed with a p value of ≤0.05.
Results
Study population
Of the 256 patients initially identified for the study, 67 were excluded due to previous percutaneous stent implantation (n = 34), coronary artery bypass grafting (n = 7), aortic valve replacement (n = 6), mitral valve repair/replacement (n = 8), and non-diagnostic image quality (n = 12). The remaining 189 patients (47% male, age 60.3 ± 11.1 years) were included. Patient demographics and baseline characteristics are presented in Tables 1 and 2.
CCTA analysis and association of ARC markers and CAC markers
Patients with ARC (n = 74) had a significantly higher presence of CAC compared to patients without ARC (n = 115) (CAC 78.4% vs 43.5%, p = 0.002). Furthermore, patients with ARC showed significantly more obstructive CAD (58.2%) compared to patients without ARC (20.5%, p = 0.016). Mean CACS was 208.9 ± 546.7, and patients with ARC (368.6 ± 708.4) displayed significantly higher scores when compared to patients without ARC (106.1 ± 379.9, p = 0.043). Additionally, CACM (mean 33.3 ± 96.3) and CACV (mean 171.8 ± 419.3) showed relevant differences between patients with and without ARC (56.7 ± 122.9 vs. 20.0 ± 74.7, p = 0.040 and 293.3 ± 530.2 vs. 92.2 ± 304.3, p = 0.041, respectively) (Table 1).
Mean ARCS was 67.7 ± 189.5 with significant differences between patients with CAC (109.4 ± 238.6) and patients without CAC (9.42 ± 31.4, p < 0.0001). Similar results regarding ARCV (mean 67.3 ± 184.7) showed significant differences between patients with CAC (108.5 ± 232.4) and patients lacking CAC (9.9 ± 30.5, p < 0.0001) (Table 2).
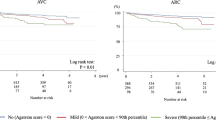
Patients with mild to moderate CAC demonstrated lower ARCS (mean 36.6 ± 109.1) and ARCV (mean 34.9 ± 93.2) compared to patients with severe CAC (251.7 ± 378.2 and 259.4 ± 382.4, p = 0.002 and p = 0.001) (Table 3). Excellent inter-rater agreement was found between the observers K = 0.94 (95%CI 0.89–0.97). Representative examples of ARC and CAC are shown in Figs. 1 and 2.
74-year-old female with severe calcification of the aortic root (a) and left anterior descending artery (b) is shown yielding an ARCV of 822 mm3, ARCS of 1014, and coronary Agatston score of 2011. The patient underwent invasive catheter angiography followed by coronary artery bypass grafting for myocardial revascularization
Pearson’s correlation coefficients for ARCS with CAC markers were as follows: CACS, r = 0.53 (p < 0.0001), CACM, r = 0.48 (p < 0.0001), and CACV, r = 0.52 (p < 0.0001). Correlation coefficients between ARCV and the CAC markers were as follows: CACS, r = 0.48 (p < 0.0001), CACM, r = 0.43 (p < 0.0001), and CACV, r = 0.48 (p < 0.0001). Likewise, ARCS and ARCV showed good correlation with CACS and CACV for individual coronary arteries. Correlation coefficients for ARCS with CACS for LM, LAD, LCX, and RCA were: r = 0.34, r = 0.47, r = 0.38, and r = 0.32 (all p < 0.0001), respectively. Coefficients for ARCV with CACV for LM, LAD, LCX, and RCA were: r = 0.53, r = 0.41, r = 0.37, and r = 0.31 (all p < 0.0001), respectively. ARCS and ARCV showed good correlation with the number of obstructive coronary vessels (≥50% luminal narrowing) as defined by CCTA, resulting in r = 0.72 (p < 0.0001) and r = 0.72 (p < 0.0001), respectively.
Using a multivariate regression analysis, age (OR 1.07, 95%CI 1.02–1.13, p = 0.005), male sex (OR 5.18, 95%CI 2.46–10.95, p = 0.023), ARCS (OR 1.09, 95%CI 0.98–1.21, p = 0.033), and ARCV (OR 1.12, 95%CI 0.96–1.28, p = 0.046) were all independent markers for the presence of CAC. In contrast, only age (OR 1.06, 95%CI 1.03–1.10, p = 0.008) was an independent marker for ARC, whereas CACS (OR 1.01, 95%CI 0.99–1.03, p = 0.203), CACV (OR 1.00, 95%CI 0.99–1.00, p = 0.859), and CACM (OR 0.95, 95%CI 0.89–1.03, p = 0.216) were not independent markers of ARC.
ROC curve analysis of the ARC markers showed discriminatory power of ARCS and ARCV to identify any amount of CAC (AUC 0.69, 95%CI 0.62–0.75, p < 0.0001 and AUC 0.70, 95%CI 0.61–0.76, p < 0.0001), including mild to moderate CAC (AUC 0.68, 95%CI 0.61–0.75, p < 0.0001 and 0.69, 95%CI 0.61–0.76, p < 0.0001) and severe CAC (AUC 0.71, 95%CI 0.59–0.83, p < 0.0001 and 0.72, 95%CI 0.59–0.85, p < 0.0001). Corresponding sensitivity and specificity to identify any CAC were 50 and 87% for ARCS and 52 and 88% for ARCV, respectively. Sensitivity and specificity to determine mild to moderate CAC were 71 and 67% for ARCS and 73 and 68%, for ARCV, respectively. Sensitivity and specificity in determining severe CAC were 67 and 71% for ARCS and 66 and 73% for ARCV, respectively.
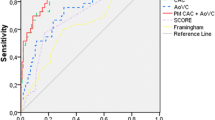
The discriminatory power of these markers to predict obstructive CAD, as defined by CCTA, yielded an AUC of 0.67 (95%CI 0.59–0.72, p < 0.0001) for ARCS and 0.68 (95%CI 0.59–0.73, p < 0.0001) for ARCV (Fig. 3). Therefore, the sensitivity and specificity for ARCS and ARCV to detect obstructive CAD were 61 and 78%, and 62 and 80%, respectively.
Discussion
Our results demonstrate a significant correlation between the presence of ARC and CAC. Patients with ARC show a significantly higher prevalence of CAC with higher calcium burden assessed with CACS, CACV, and CACM. The ARC markers, ARCS and ARCV, portend predictive value for obstructive CAD, resulting in AUC values of 0.67 (p < 0.0001) and 0.68 (p < 0.0001) and corresponding sensitivity and specificity of 61 and 78%, and 62 and 80%, respectively. These results are in accordance with previous studies, including CCTA and x-ray examinations, which showed a significant association between different aortic calcification markers, CAC, and CAD [8, 18, 19].
In reference to the association between ARC and CAC, our study displayed a significant correlation between the ARC markers and the CAC-related markers: CACS, CACV, and CACM. The validity of ARCS and ARCV measurements compared to CACS has been previously evaluated, and our results further confirm the conclusions and the prognostic value of these markers [18, 19]. Another important finding elucidated by our study was the significant correlation between ARCS and both the individual coronary artery vessels, as well as the number of coronaries with significant stenosis (≥50% luminal narrowing), which is similar to previously reported results [19].
Our findings through multivariate regression analysis show that age (OR 1.06, p = 0.008) is an independent marker for the presence of ARC, which is in accordance with previous studies. This ultimately demonstrates that age is an independent marker for the prevalence of ascending aorta calcification in both men and women aged 50–60 years, with a growing probability for patients older than 70 years [7, 13]. Stewart et al. found that aortic valve and root calcification are associated with a twofold increased risk for each 10 year increase in age [20]. A recent study by Jeon et al. demonstrated that besides age, hypertension and male sex were associated with significant ARC [13]. However, we did not confirm these specific findings in our study. Additionally, our results showed an excellent correlation between CACS and the presence of ARC (r = 0.53, p < 0.0001), which further supports the findings of two recent studies that also examined this relationship [18, 19]. Alternatively, CACV and CACM did not show significant correlation to detect ARC.
Previous studies showed significantly greater coronary calcification for men compared to women in correlation with ascending aorta calcifications [21, 22]. In addition, recent results derived from the MESA study (Multi-Ethnic Study of Atherosclerosis) determined that age, male sex, and aortic calcification were strong predictors for CAC [5, 23, 24]. Thus, the focus of this study supports these previous findings by demonstrating that age, male sex, as well as both markers of ARC are independent markers for CAC. Multivariate analysis for ARCS (OR 1.09, p = 0.033) and ARCV (OR 1.12, p = 0.046) further corroborates the diagnostic value of these parameters as additional risk markers for the presence of CAC.
In addition, we demonstrated significant discriminatory power of ARCS and ARCV to detect mild to moderate CAC (AUC 0.68, p < 0.0001 and 0.69, p < 0.0001) and severe CAC (AUC 0.71, p < 0.0001 and 0.72, p < 0.0001). More importantly, ARCS (AUC 0.67, p < 0.0001) and ARCV (AUC 0.68, p < 0.0001) revealed significant discriminatory power and emerged as predictors for obstructive CAD, ultimately providing incremental diagnostic value. Our results compare favorably to a study conducted by Adar et al. that demonstrated the significant discriminative power of aortic arch calcification (AUC 0.89, p < 0.0001) to predict severe CAC. However, these results did not investigate predictive value for obstructive CAD [8].
Several limitations in this study deserve mention. We present a retrospective, single-center study with a limited number of enrolled subjects. We only involved patients with intermediate pre-test probability of coronary artery disease, which may have caused a selection bias, influencing our results. Larger studies that encompass a more representative study population will be necessary to validate our data. Furthermore, we did not compare our results to the golden standard of invasive catheter angiography to confirm the severity of CAD.
In conclusion, this study demonstrates that ARC markers are strongly associated with and independently predict the presence of CAC and obstructive CAD. Additionally, further testing is required in patients with severe ARC and significant CAD in order to reliably obtain these markers from thoracic-CT or X-ray for proper risk classification.
Abbreviations
- ARC:
-
Aortic root calcification
- ARCS:
-
Aortic root calcification score
- ARCV:
-
Aortic root calcification volume
- BMI:
-
Body mass index
- CACS:
-
Coronary artery calcium score
- CACM:
-
Coronary artery calcium mass
- CACV:
-
Coronary artery calcium volume
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomographic angiography
- CI:
-
Confidence interval
- DSCT:
-
Dual-source CT
- LAD:
-
Left anterior descending
- LCX:
-
Left circumflex
- LM:
-
Left main
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- RCA:
-
Right coronary artery
- SD:
-
Standard deviation
References
Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS et al (2007) F. American College of Cardiology Foundation Clinical Expert Consensus Task, I. Society of Atherosclerosis, Prevention, T. Society of Cardiovascular Computed, ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 49(3):378–402
Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K (2014) Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 35(33):2232–2241
Bauer RW, Thilo C, Chiaramida SA, Vogl TJ, Costello P, Schoepf UJ (2009) Noncalcified atherosclerotic plaque burden at coronary CT angiography: a better predictor of ischemia at stress myocardial perfusion imaging than calcium score and stenosis severity, AJR. Am J Roentgenol 193(2):410–418
Thilo C, Gebregziabher M, Mayer FB, Zwerner PL, Costello P, Schoepf UJ (2010) Correlation of regional distribution and morphological pattern of calcification at CT coronary artery calcium scoring with non-calcified plaque formation and stenosis. Eur Radiol 20(4):855–861
Nasir K, Katz R, Al-Mallah M, Takasu J, Shavelle DM, Carr JJ, Kronmal R, Blumenthal RS, O’Brien K, Budoff MJ (2010) Relationship of aortic valve calcification with coronary artery calcium severity: the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr 4(1):41–46
Oberoi S, Schoepf UJ, Meyer M, Henzler T, Rowe GW, Costello P, Nance JW (2013) Progression of arterial stiffness and coronary atherosclerosis: longitudinal evaluation by cardiac CT. AJR Am J Roentgenol 200(4):798–804
Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, Budoff MJ (2008) Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 155(4):765–771
Adar A, Erkan H, Gokdeniz T, Karadeniz A, Cavusoglu IG, Onalan O (2015) Aortic arch calcification is strongly associated with coronary artery calcification. Vasa 44(2):106–114
Blanke P, Spira EM, Ionasec R, Meinel FG, Ebersberger U, Scheuering M, Canstein C, Flohr TG, Langer M, Schoepf UJ (2014) Semiautomated quantification of aortic annulus dimensions on cardiac CT for TAVR. JACC Cardiovasc Imaging 7(3):320–322
Blanke P, Schoepf UJ, Leipsic JA (2013) CT in transcatheter aortic valve replacement. Radiology 269(3):650–669
Escobedo C, Schoenhagen P (2013) Aortic root imaging in the era of transcatheter aortic valve implantation/transcatheter aortic valve replacement. Rev Esp Cardiol (Engl Ed) 66(11):839–841
Pressman GS, Crudu V, Parameswaran-Chandrika A, Romero-Corral A, Purushottam B, Figueredo VM (2011) Can total cardiac calcium predict the coronary calcium score? Int J Cardiol 146(2):202–206
Jeon DS, Atar S, Brasch AV, Luo H, Mirocha J, Naqvi TZ, Kraus R, Berman DS, Siegel RJ (2001) Association of mitral annulus calcification, aortic valve sclerosis and aortic root calcification with abnormal myocardial perfusion single photon emission tomography in subjects age ≤ 65 years old. J Am Coll Cardiol 38(7):1988–1993
Wu FZ, Wu MT (2015) SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 9(2):e3
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15(4):827–832
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33(2):363–374
Hu X, Frellesen C, Kerl JM, Bauer RW, Beeres M, Bodelle B, Lehnert T, Vogl TJ, Wichmann JL (2015) Association of aortic root calcification severity with the extent of coronary artery calcification assessed by calcium-scoring dual-source computed tomography. Eur J Radiol 84(10):1910–1914
Nafakhi H, Al-Nafakh HA, Al-Mosawi AA, Al Garaty F (2015) Correlations between aortic root calcification and coronary artery atherosclerotic markers assessed using multidetector computed tomography. Acad Radiol 22(3):357–362
Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM (1997) Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol 29(3):630–634
Liyanage L, Lee NJ, Cook T, Herrmann HC, Jagasia D, Litt H, Han Y (2016) The impact of gender on cardiovascular system calcification in very elderly patients with severe aortic stenosis. Int J Cardiovasc Imaging 32(1):173–179
Henein M, Hallgren P, Holmgren A, Sorensen K, Ibrahimi P, Kofoed KF, Larsen LH, Hassager C (2015) Aortic root, not valve, calcification correlates with coronary artery calcification in patients with severe aortic stenosis: a two-center study. Atherosclerosis 243(2):631–637
Rivera JJ, Nasir K, Katz R, Takasu J, Allison M, Wong ND, Barr RG, Carr JJ, Blumenthal RS, Budoff MJ (2009) Relationship of thoracic aortic calcium to coronary calcium and its progression [from the Multi-Ethnic Study of Atherosclerosis (MESA)]. Am J Cardiol 103(11):1562–1567
Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R (2011) Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 215(1):196–202
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Schoepf receives research support from Astellas, Bayer, Bracco, GE, Medrad, and Siemens. Drs. Schoepf, De Cecco, and Varga-Szemes are consultants for Guerbet. All other authors have no conflicts of interest to disclose.
Christian Tesche is an exchange visiting scholar supported by a grant from the Fulbright Visiting Scholar Program of the U.S. Department of State, Bureau of Educational and Cultural Affairs (ECA). This single-center study was approved by the local Institutional Review Board and written informed consent was obtained from all individual patients included in the study. The study was performed in compliance with HIPAA regulations. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tesche, C., De Cecco, C.N., Stubenrauch, A. et al. Correlation and predictive value of aortic root calcification markers with coronary artery calcification and obstructive coronary artery disease. Radiol med 122, 113–120 (2017). https://doi.org/10.1007/s11547-016-0707-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0707-5