Abstract
The utility of abdominal aortic calcification (AAC) for prediction of cardiovascular events (CVEs) in patients with acute coronary syndrome (ACS) remains to be determined. The aim of this prospective study was to determine the predictive value of the abdominal aortic calcification index (ACI), a semi-quantitative measure of AAC, for CVEs in patients with ACS. We evaluated 314 patients with ACS. All patients underwent successful percutaneous coronary intervention to the culprit coronary vessel without in-hospital adverse events. ACI was calculated on non-contrast computed tomography images. CVEs were defined as a composite of cardiovascular death, ACS recurrence, and stroke. During a median follow-up period of 19.1 months, CVEs occurred in 29 patients (9.2%). Multivariable regression analysis after adjustment for age and gender showed a significantly higher baseline ACI in patients with CVEs than in those without [median (interquartile ranges), 42.1 (25.9–60.2) vs. 20.8 (8.8–38.6) %; P = 0.021]. The cutoff value of ACI for prediction of CVEs, estimated by receiver-operating characteristic analysis, was 29.2%, with sensitivity of 76% and specificity of 64% (area under the curve, 0.69). After adjustment for conventional cardiovascular risk factors, Cox analysis showed high ACI (≥29.2%) to be significantly associated with increased risk of CVEs (P = 0.011; hazard ratio, 1.82). Multivariate analysis identified high ACI as an independent predictor of CVEs (P = 0.012; hazard ratio, 1.80). Stepwise forward selection procedure also showed that high ACI was a significant independent determinant of CVEs (P = 0.004; R2, 0.089). Both net reclassification improvement (0.64; P = 0.001) and integrated discrimination improvement (0.04; P < 0.001) improved significantly after the addition of high ACI to conventional risk factors. Evaluation of ACI using CT seems to provide valuable clinical information for proper assessment of mid-term CVEs in patients with ACS after percutaneous coronary intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute coronary syndrome (ACS) is an important clinical challenge based on the associated risk of mortality. The development of revascularization procedures and new medical therapies has improved both the short- and long-term prognoses of such patients [1,2,3,4]. However, the rate of secondary cardiovascular events (CVEs) in patients with ACS remains high [1,2,3,4,5]. Thus, risk stratification is important for accurate assessment of long-term prognosis. Previous studies examined the relationship between abdominal aortic calcification (AAC) and CVEs [6,7,8,9]. In the majority of these studies, the study subjects were recruited from the general population, and AAC was evaluated by lateral lumbar radiography [7,8,9]. Given that computed tomography (CT) scan is more sensitive in detecting AAC compared with lumbar radiography, measurement of AAC by CT scan may be more suitable for assessing the risk of CVEs in high-risk patients. Using non-contrast CT scans, we reported previously the utility of the abdominal aortic calcification index (ACI), a semi-quantitative index of AAC, in the prediction of CVEs in asymptomatic chronic kidney disease patients not on hemodialysis [10]. However, the exact predictive value of ACI for CVEs in patients with ACS has not been determined definitively. The aim of this study was to determine the predictive value of ACI for CVEs in patients with ACS.
Materials and methods
Subjects
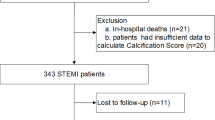
We screened 453 consecutive patients with ACS who underwent successful percutaneous coronary intervention (PCI) to the culprit coronary vessel at our hospital between February 2013 and January 2017. ACS included acute MI and unstable angina pectoris. Acute MI was defined as typical acute chest pain associated with myocardial ischemia and abnormal levels of cardiac biomarkers (> 99th centile of the upper normal limit) [11]. Acute MI was also divided into ST-segment elevation MI (STEMI) and non-ST-segment elevation MI (NSTEMI), based on electrocardiographic findings [12]. Unstable angina pectoris was defined as acute chest pain or worsening chest discomfort with normal cardiac enzyme levels, requiring revascularization. We excluded the subjects with the following conditions: (1) history of in-hospital adverse events, including death, cardiac surgery, cardiac arrest requiring resuscitation, ACS recurrence, or stroke; (2) history of abdominal aortic replacement; (3) presence of other life-threatening diseases; and (4) follow-up period limited to ≤ 6 months. Accordingly, a total of 314 patients were evaluated in this study (Supplemental Fig. 1). The study protocol complied with the Declaration of Helsinki and was approved by the local ethics committee (IRB #2017371). Written informed consent was obtained from each participant.
Measurement of abdominal aortic calcification index
All subjects underwent a 64-slice non-contrast CT scan (Siemens Medical Solutions, Forchheim, Germany) within 2 weeks of PCI. Scanning was performed in the supine position and cranio-caudal direction. Images were obtained from the takeoff of the renal artery to the bifurcation of the aorta into the common iliac arteries at 5-mm intervals. Each cross-section image of the abdominal aorta on each slice was divided into 12 radial segments. Calcification was considered to be present if an area of > 1 mm2 displayed a density of > 130 Hounsfield units. The number of calcified segments on each slice was counted. The ACI was calculated using the following equation (Fig. 1) [10, 13, 14]: ACI (%) = (total score for calcification on all slices)/(number of slices × 12) × 100. AAC was measured semi-quantitatively and independently by two physicians who were both blinded to the clinical characteristics of the patients. The inter- and intra-observer variabilities of ACI correlated well [r = 0.99 (P < 0.001) and r = 0.99 (P < 0.001), respectively]. Details of the procedure were described previously [10, 13].
The method used for calculating abdominal aortic calcification index (%). a Images were obtained from the takeoff of the renal artery to the bifurcation of the aorta into the common iliac arteries at 5-mm intervals. b The cross-section of the abdominal aorta on each slice was divided into 12 radial segments. The number of calcified segments was counted on each slice. c This formula was used to calculate the abdominal aortic calcification index (ACI). d Representative cross-sectional images of the abdominal aorta in patients with 1.9% (d-1), 42.9% (d-2), and 92.6% ACI (d-3). Details were described by Tatami et al. [10]
Data collections
The clinical data of medical history, physical examination, anthropometric measurements, and self-reported questionnaires on lifestyle (e.g., smoking habit) were collected during hospitalization. Left ventricle ejection fraction (LVEF) was measured using transthoracic echocardiography before discharge. Blood pressure was measured at least twice after > 5 min rest in the sitting position before discharge. Venous blood samples (total volume; 10 ml) were also collected in the early morning after overnight fasting before discharge. Fasting plasma glucose level, blood glycosylated hemoglobin content, and serum concentrations of total cholesterol, triglycerides, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, creatinine, and B-type natriuretic peptide (BNP) were measured. The estimated glomerular filtration rate (eGFR) was calculated using a simplified prediction equation derived from that in the Modification of Diet in Renal Disease Study [15] and proposed by the Japanese Society of Nephrology: eGFR (ml/min/1.73 m2) = 194 × [age (years)]−0.287 × [serum creatinine (mg/dl)]−1.094 [× 0.739 for females] [16].
Clinical events were collected from the medical records or telephone interview. Follow-up was censored on September 18, 2018. The endpoint of this study was CVE, which was defined as composite of cardiovascular death, ACS recurrence, and stroke. Cardiovascular death was defined as death due to acute MI, sudden cardiac death, heart failure, stroke, cardiovascular procedure, cardiovascular hemorrhage, or other cardiovascular-related causes [12]. When non-cardiac cause of death could not be identified, it was also considered as cardiac death. ACS recurrence was defined as either recurrent acute MI or unstable angina pectoris. Recurrent MI also included a rise in cardiac biomarker values of > 5 or > 10 times above the 99th centile of the upper normal limit in subjects who underwent additional PCI or bypass graft surgery [12]. Stroke included both ischemic and hemorrhagic types, and was defined as an acute episode of neurological dysfunction caused by focal or global brain, spinal cord, or retinal vascular injury as a result of infarction or hemorrhage [12].
Statistical analysis
Data with normal distribution pattern were expressed as mean ± standard deviation, and those with skewed distribution were expressed as median values (interquartile ranges). Categorical variables were compared by the Chi square test. Comparisons between two groups were conducted by the unpaired Student’s t test (for variables with normal distribution) or by Mann–Whitney U test (for variables with skewed distribution). ACI was compared between patients with and without CVEs by multivariable regression analysis after adjustment for age and gender. Receiver-operating characteristic curve analysis was performed to determine the optimal cutoff value of ACI for the prediction of CVEs. The event-free survival curve was analyzed using Kaplan–Meier method with log-rank test. The relationship between ACI and CVEs was evaluated by Cox analysis adjusted for conventional cardiovascular risk factors (including age, gender, body mass index, hypertension, dyslipidemia, diabetes, current smoking, and eGFR). Cox proportional hazard model was also performed to identify independent predictors of CVEs. Multivariate analysis included all variables measured at baseline that showed P < 0.05 with CVEs in univariate analysis. Stepwise forward selection analysis was also performed to examine the effects of ACI as well as other covariates (P < 0.05 in Cox univariate analysis) on CVEs. In this analysis, the P levels for inclusion in and exclusion from the model were 0.25 and 0.1, respectively. The C-index, net reclassification improvement, and integrated discrimination improvement were calculated to assess whether the accuracy of predicting CVEs would improve after adding ACI into the base model with other risk factors [17, 18]. The base model consisted of conventional risk factors. Finally, the relationships between ACI and clinical characteristics were examined by linear regression analysis. In addition, Cox analysis was performed to evaluate the association of ACI with CVEs after adjustment for each variable related to ACI (P < 0.05). P < 0.05 denoted the presence of a statistically significant difference. Statistical tests were conducted using the JMP software (version 5.1 and 13.0; SAS Institute, Inc., Cary, NC). Delong test for C-index was examined with sample script (https://www.jmp.com/japan/support/faq/stat_3605.shtml).
Results
The baseline characteristics of the study subjects are summarized in Table 1. The median follow-up period was 19.1 months. During the follow-up period, ACS recurrence was diagnosed in 10 patients (STEMI, n = 1; NSTEMI, n = 3; unstable angina pectoris, n = 6), and stroke in 14 (ischemic stroke, n = 10; hemorrhagic stroke, n = 4) (Table 2). Nine patients died and the cause of death was cardiovascular-related (sudden cardiac death, n = 4; heart failure, n = 3; stroke, n = 2). Thus, the rate of CVEs was 9.2% (29 patients) in our cohort. In patients with CVEs, age and the prevalence of previous heart failure and left main coronary artery lesion were significantly higher, whereas body mass index, hemoglobin, and serum concentrations of peak creatine kinase, triglycerides and LDL-cholesterol were lower, compared to patients without (Table 1). Classification of ACS, culprit artery of ACS, and strategy of PCI to culprit vessel were also significantly different between patients with and without CVEs. In patients with CVEs, fewer were treated with acetylsalicylic acid, compared to those without CVEs.
ACI was 22.3 (9.4–42.1) % for the entire group. After adjustment for age and gender, multivariable regression analysis showed that ACI was significantly higher in patients with CVEs, compared to those without [42.1 (25.9–60.2) vs. 20.8 (8.8–38.6) %; P = 0.021, Fig. 2]. Receiver-operating characteristic curve analysis showed a cutoff value of ACI of 29.2% for the prediction of CVEs, with sensitivity of 76% and specificity of 64% [area under the curve 0.69; 95% confidence interval (95% CI) 0.57–0.78] (Supplemental Fig. 2).
Comparison of abdominal aortic calcification index (%) of patients with and without cardiovascular events. Data were expressed as median (interquartile ranges) and analyzed by multivariable regression analysis after adjustment for age and gender. Cardiovascular events were defined as composite of cardiovascular death, recurrent acute coronary syndrome, and stroke
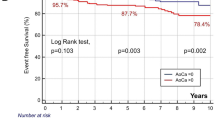
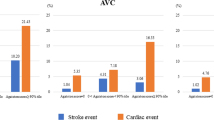
The rate of CVEs was significantly higher in patients with high ACI (≥ 29.2%) than in those with low ACI (< 29.2%) (Table 2). The rates of stroke and cardiovascular deaths were also significantly higher in patients with high ACI. Kaplan–Meier analysis showed significantly worse event-free survival rate in patients with high ACI, compared to those with low ACI (log-rank test, P < 0.001) (Fig. 3). Cox analysis adjusted for conventional cardiovascular risk factors showed that high ACI was significantly associated with CVEs [P = 0.011; hazard ratio (HR) 1.82 (95% CI 1.15–3.02)], stroke [P = 0.024; HR 2.21 (95% CI 1.11–5.02)] and cardiovascular death [P = 0.008; HR 4.13 × 103 (95% CI 1.55–not applicable)], but not with ACS recurrence [P = 0.477; HR 1.37 (95% CI 0.61–3.20)]. In univariate Cox analysis, CVEs were significantly associated with high ACI, age, body mass index, history of heart failure, left main coronary artery lesion, hemoglobin, serum concentrations of LDL-cholesterol, triglycerides, and BNP, and classification of ACS (Table 3). The risk of CVEs was significantly higher in NSTEMI, compared to STEMI [P = 0.015; HR 2.83 (95% CI 1.24–6.34)]. It was lower in patients who had received drug-eluting stents [P = 0.005; HR 0.27 (95% CI 0.11–0.68)] and bare-metal stents [P = 0.028; HR 0.16 (95% CI 0.03–0.81)], compared to those treated by balloon angioplasty. Multivariate analysis identified high ACI as an independent and significant predictor of CVEs. Stepwise forward selection analysis identified high ACI (P = 0.004; R2, 0.089) and history of heart failure (P = 0.044; R2, 0.024) as independent determinants of CVEs. In addition, we evaluated whether the addition of ACI into the base model, together with conventional risk factors, improved the accuracy of prediction of CVEs. The addition of high ACI to conventional risk factors significantly improved both the net reclassification improvement (0.64; P = 0.001) and integrated discrimination improvement (0.04; P < 0.001), but not the C-index [P = 0.279; base model, 0.73 (95% CI 0.62–0.82) vs. model with high ACI, 0.76 (0.63–0.85)].
Kaplan–Meier event-free survival curve for cardiovascular events. The event-free survival rate was significantly worse in patients with high ACI (≥ 29.2%; broken line) than in low ACI (< 29.2%; solid line) (log-rank test, P < 0.001). Cardiovascular events were defined as composite of cardiovascular death, recurrent acute coronary syndrome, and stroke. ACI abdominal aortic calcification index
Finally, we examined the relationships between ACI and clinical characteristics by linear regression analysis. ACI was significantly associated with age (P < 0.001; β = 1.12), male sex (P < 0.001; β = − 6.35), body mass index (P < 0.001; β = − 1.34), hypertension (P < 0.001; β = 5.29), current smoking (P < 0.001; β = − 5.01), atrial fibrillation (P = 0.016; β = 6.04), history of MI (P = 0.002; β = 5.55), heart failure (P = 0.001; β = 6.75), systolic blood pressure (P = 0.023; β = 0.19), diastolic blood pressure (P < 0.001; β = − 0.47), hemoglobin (P < 0.001; β = − 4.92), LDL-cholesterol (P = 0.008; β = − 0.12), HDL-cholesterol (P = 0.005; β = 0.33), BNP (P < 0.001; β = 0.03), eGFR (P < 0.001; β = − 0.29), and LVEF (P = 0.005; β = − 0.38). Cox analysis adjusted for all these variables related to ACI (P < 0.05) demonstrated that high ACI was significantly associated with increased risk of CVEs [P = 0.010; HR 1.84 (95% CI 1.15–3.06)].
Discussion
The present study investigated the association of ACI with mid-term CVEs in patients with ACS who had undergone PCI. Our observational study demonstrated that patients who developed CVEs had significantly higher ACI compared with those who did not. Furthermore, high ACI was an independent and significant predictor of mid-term CVEs, and was associated with stroke and cardiovascular death. In addition, the inclusion of ACI in the prediction model for CVEs improved the accuracy of the model. Previous studies reported the relationship between AAC and CVEs in several populations [6,7,8,9,10, 19, 20]. Furthermore, a meta-analysis of observational studies emphasized the predictive value of AAC for long-term CVEs [6], although the majority of the populations in these studies were individuals from the general population [7, 8]. The same relationship between AAC and CVEs was also reported in patients at high risk, such as those with chronic kidney disease or peripheral artery disease [10, 19, 20]. However, the value of AAC in predicting secondary CVEs in patients with coronary artery disease has not been determined precisely. To the best of our knowledge, the present study is the first report to provide the prognostic value of AAC in patients with ACS.
Vascular calcification is an active and complex process involving numerous events leading to calcium deposition in the arterial wall [21]. These mechanisms include: (1) high serum levels of calcium and phosphate; (2) induction of osteogenesis; (3) inadequate inhibition of the mineralization process; and (4) migration and differentiation of macrophages and vascular smooth muscle cells into osteoclast-like cells [21,22,23,24]. Furthermore, the potential of a genetic mechanism is suggested in the development of vascular calcification [25]. Regardless of the exact mechanism, vascular calcification involves the formation of calcified deposits of hydroxyapatite crystals in the vascular wall. Vascular calcification is classified histopathologically into two categories: intimal and medial calcification [21]. Intimal calcification is associated with atherosclerotic plaques, whereas medial calcification is more widespread in the lower abdominal regions [22]. However, the underlying mechanisms of the relationship between AAC and CVEs have not been fully elucidated. Vascular calcification is associated with certain conventional cardiovascular risk factors [26]. ACI was also related to the same factors. Each segment of the vascular tree (e.g., abdominal aorta, coronary artery, and carotid-cerebral artery) is exposed to the same risk factors of atherosclerosis, and these risk factors can play important roles in the development of atherosclerosis at each segment. Thus, the relationship between AAC and CVEs is potentially influenced by the effects of conventional cardiovascular risk factors on coronary and carotid-cerebral artery, although high ACI was significantly associated with CVEs even after adjustment for those risk factors. In this regard, AAC is significantly associated with both coronary [13] and carotid artery calcification [27]. Patients with severe AAC may have more advanced atherosclerotic lesions in both coronary and carotid-cerebral arteries, resulting in increased risk of CVEs. Hence, AAC may be a surrogate marker of generalized systemic atherosclerosis, including the effects of conventional cardiovascular risk factors. Another explanation is that decreased vascular compliance associated with advanced AAC can lead to increased left ventricle afterload, resulting in left ventricular hypertrophy and heart failure [28, 29]. Decreased vascular compliance is also associated with increased pulse pressure [30], and the latter is reported to be an independent predictor of CVEs [31].
A meta-analysis of several observational studies concluded that AAC correlated with increased risks for coronary events, cerebrovascular events, and all CVEs [6]. A study from the Multi-Ethnics Study of Atherosclerosis showed that both AAC and coronary artery calcification could predict coronary artery disease and CVEs in individuals free of cardiovascular diseases, whereas AAC alone was associated with cardiovascular mortality in the same population [32]. In the present study, AAC was significantly associated with stroke and cardiovascular death, but not with ACS recurrence in patients with ACS. The impact of AAC on secondary coronary events might be relatively small in patients with ACS, although the statistical power of the present study might be insufficient to allow proper evaluation of coronary events due to the small population and short follow-up period. Further studies of larger population samples and long-term follow-up are required to evaluate the exact impact of ACI on secondary coronary artery events in patients with ACS.
Previous studies discussed the relationship between thoracic aortic calcification and CVEs [33,34,35]. However, there were certain differences in the results of these studies. In a general cohort from the Multi-Ethnics Study of Atherosclerosis, thoracic aortic calcification was associated with coronary artery events only in women, independent of coronary artery calcification [33]. In another study involving a sample from the general population, thoracic aortic calcification was also associated with coronary artery events and all-cause mortality, but these relationships diminished following adjustment for coronary artery calcification [34]. In a retrospective small study, Yang et al. [35] found a significant relationship between aortic arch calcification and CVEs in patients with ACS. The prognostic value of thoracic aortic calcification for CVEs remains to be elucidated.
Although the present study identified the prognostic value of AAC in patients with ACS, abdominal CT scan is not a standard imaging procedure for patients with ACS in routine clinical practice. CT scan can potentially detect asymptomatic subclinical disorders. Previous studies indicated that the prevalence of abdominal aortic aneurysm was 2.4 times higher in patients with coronary heart disease than in the general population [36], whereas the incidence of cancer was higher in patients after MI [37]. However, radiation exposure and medical cost should be considered in CT scan. Accurate assessment of risk may be more important in high-risk patients. In this regard, our observations encourage assessment of AAC by abdominal CT scan, especially for patients with ACS and the following features: old age, low body mass index, heart failure, hypo-LDL-cholesterolemia, hyper-triglyceridemia, anemia, and left main coronary artery lesion.
At present, there are no established guidelines for secondary prevention in patients with severe AAC. Several studies showed some protective effects of bisphosphonates against the development of aortic and coronary artery calcification in patients on hemodialysis [38, 39]. However, a recent meta-analysis did not support any clinically important effects of bisphosphonates for cardiovascular events [40]. Given that conventional risk factors affect the development of secondary CVEs in patients with severe AAC, a more aggressive approach towards these risks might be attributable to secondary prevention in patients with severe AAC. Further studies are needed to determine better management protocol for secondary prevention of CVEs in patients with severe AAC.
Lateral lumbar radiography is the traditional tool used to estimate the severity of AAC [6,7,8,9], whereas CT-based scoring methods have been adopted in recent studies [10, 13, 32, 41, 42]. Although the Agatston method was developed originally to evaluate coronary artery calcification [43], it has been also used to assess AAC in several studies [32, 41, 42]. Agatston score is calculated by multiplying each area of interest by a weighted score assigned to the highest density calcification within an individual area [43]. The AAC score derived by the Agatston method was reported not only to be associated with coronary artery disease [41], but also to predict CVEs in individuals free of cardiovascular diseases [32]. While the complexity of this method allows detailed calcification scoring, it often requires software to calculate the score. In the present study, we adopted the ACI, a semi-quantitative measure of AAC, in part due to the simplicity of this method. This score is calculated based on manual counting by physicians without the need for any expensive software. Although this scoring method has the potential to be observer-dependent, the inter- and intra-observer variabilities of ACI correlated well in the present study. Our results showed the prognostic value of ACI on mid-term CVEs in patients with ACS, whereas our previous study demonstrated the predictive value of ACI on long-term CVEs in patients with chronic kidney disease [10]. Another study reported that ACI was also significantly associated with sever coronary artery calcification in patients with chronic kidney disease who were free of coronary artery disease [13]. These findings highlight the importance of ACI as a useful tool for estimation of AAC in various clinical populations.
The present study has several limitations. First, this observational study was conducted in a single center and included a small population sample. Second, evaluation using multi-slice CT did not allow distinction between intimal and medial vascular calcification. Third, we did not compare ACI, a semi-quantitative tool based on non-contrast CT scan, with other quantitative methods by plain X-ray or CT scan. Fourth, radiation exposure should be considered in non-contrast CT scan, although CT scan is more sensitive in detecting vascular calcification than plain X-ray. Fifth, PCI strategy or additional medical therapies might influence mid-term CVEs. The risk of CVEs was significantly higher in patients treated by balloon angioplasty.
In conclusion, ACI was significantly associated with mid-term CVEs in patients with ACS after PCI. The addition of ACI to a model that included traditional risk factors improved the accuracy of prediction of CVEs. Semi-quantitative evaluation of ACC using non-contrast CT scan seems to add valuable information and provide accurate assessment of mid-term CVEs in patients with ACS after PCI.
References
Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, Califf RM, Kong DF, Roe MT (2009) Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation 119:3110–3117
Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T, on behalf of the PACIFIC Investigators (2013) Management and two-year long-term clinical outcome of acute coronary syndrome in Japan. Prevention of AtherothrombotiC Incidents Following Ischemic Coronary Attack (PACIFIC) Registry. Circ J 77:934–943
Schwartz GG, MD, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T, for the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators (2001) Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285:1711–1718
Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Nakamura M (2014) Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome. The PRASFIT-ACS study. Circ J 78:1684–1692
Piironen M, Ukkola O, Huikuri H Havulinna AS, Koukkunen H, Mustonen J, Ketonen M, Lehto S, Airaksinen J, Kesäniemi YA, Salomaa V (2017) Trends in long-term prognosis after acute coronary syndrome. Eur J Prev Cardiol 24:274–280
Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ (2012) Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart 98:988–994
Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA (2001) Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 103:1529–1534
van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC (2004) Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam study. Circulation 109:1089–1094
Schousboe JT, Taylor BC, Kiel DP, Ensrud KE, Wilson KE, McCloskey EV (2008) Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J Bone Miner Res 23:409–416
Tatami Y, Yasuda Y, Suzuki S, Ishii H, Sawai A, Shibata Y, Ota T, Shibata K, Niwa M, Morimoto R, Hayashi M, Kato S, Maruyama S, Murohara T (2015) Impact of abdominal aortic calcification on long-term cardiovascular outcomes in patients with chronic kidney disease. Atherosclerosis 243:349–355
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD: the writing group on behalf of the joint ECS/ACCF/AHA/WHF task force for the universal definition of myocardial infarction (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60:1581–1598
Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, J. Cross JT, Drozda JP, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander JE, Krumholz H, Labarthe DR, Long JB, Mascette AM, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Werf FV, Weiner BH, Weintraub WS (2013) 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (writing committee to develop acute coronary syndromes and coronary artery disease clinical data standards). Circulation 127:1052–1089
Takayama Y, Yasuda Y, Suzuki S, Shibata Y, Tamai Y, Shibata K, Niwa M, Sawai A, Morimoto R, Kato S, Ishii H, Maruyama S, Murohara T (2016) Relationship between abdominal aortic and coronary calcification as detected by computed tomography in chronic kidney disease patients. Heart Vessels 31:1030–1037
Taniwaki H, Ishimura E, Tanabe T, Tsujimoto Y, Shioi A, Shoji T, Inaba M, Inoue T, Nishizawa Y (2005) Aortic calcification in haemodialysis patients with diabetes mellitus. Nephrol Dial Transpl 20:2472–2478
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; for the modification of diet in renal disease study group (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, on behalf of the collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172
Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y (2007) Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis 49:417–425
Parr A, Buttner P, Shahzad A, Golledge J (2010) Relation of infra-renal abdominal aortic calcific deposits and cardiovascular events in patients with peripheral artery disease. Am J Cardiol 105:895–899
Karwowski W, Naumnik B, Szczepański M, Myśliwiec M (2012) The mechanism of vascular calcification—a systematic review. Med Sci Monit 18:RA1-RA11
Rocha-Singh KJ, Zeller T, Jaff MR (2014) Peripheral artery calcification: Prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 83:E212–E220
Sage AP, Tintut Y, Demer LL (2010) Regulatory mechanisms in vascular calcification. Nat Rev Cardiol 7:528–536
Giachelli CM (2004) Vascular calcification mechanisms. J Am Soc Nephrol 15:2959–2964
Demer LL, Tintut Y (2014) Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol 34:715–723
Chen NX, Moe SM (2012) Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep 14:228–237
Friedlander AH, El Saden SM, Hazboun RC, Chang TI, Wond WK, Garrett NR (2015) Detection of carotid artery calcification on the panoramic images of post-menopausal females is significantly associated with severe abdominal aortic calcification: a risk indicator of future adverse vascular events. Dentomaxillofac Radiol 44:20150094. https://doi.org/10.1259/dmfr.20150094
Rajkumar C, Cameron JD, Christophidis N, Jennings GL, Dart AM (1997) Reduced systemic arterial compliance is associated with left ventricular hypertrophy and diastolic dysfunction in older people. J Am Geriatr Soc 45:803–808
Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH (1999) Increased pulse pressure and risk of heart failure in the elderly. JAMA 281:634–639
Raij L, Gonzalez-Ochoa AM (2011) Vascular compliance in blood pressure. Curr Opin Nephrol Hypertens 20:457–464
Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P (2018) Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc 7:e007621. https://doi.org/10.1161/JAHA.117.007621
Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, Cohoon KP, Srikanthan P, Watson KE, Wong ND (2014) Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 34:1574–1579
Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R (2011) Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 215:196–202
Kälsch H, Lehmann N, Berg MH, Mahabadi AA, Mergen P, Möhlenkamp S, Bauer M, Kara K, Dragano N, Hoffmann B, Moebus S, Schmermund A, Stang A, Jöckel KH, Erbel R (2014) Coronary artery calcification outperforms thoracic aortic calcification for the prediction of myocardial infarction and all-cause mortality: the Heinz Nixdorf Recall Study. Eur J Prev Cardiol 21:1163–1170
Yang TL, Huang CC, Huang SS, Chiu CC, Leu HB, Lin SJ (2017) Aortic arch calcification associated with cardiovascular events and death among patients with acute coronary syndrome. Acta Cardiol Sin 33:241–249
Hernesniemi JA, Vänni V, Hakala T (2015) The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. J Vasc Surg 62:232–240
Malmborg M, Christiansen CB, Schmiegelow MD Torp-Pedersen C, Gislason G, Schou M (2018) Incidence of new onset cancer in patients with a myocardial infarction—a nationwide cohort study. BMC Cardiovasc Disord 18:198
Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K, Aoki T, Nihei H (2004) Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis 44:680–688
Hashiba H, Aizawa S, Tamura K, Kogo H (2006) Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Ther Apher Dial 10:59–64
Kim DH, Rogers JR, Fulchino LA, Kim CA, Solomon DH, Kim SC (2015) Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS ONE 10:e0122646
An C, Lee HJ, Lee HS, Ahn SS, Choi BW, Kim MJ, Chung YE (2014) CT-based abdominal aortic calcification score as a surrogate maker for predicting the presence of asymptomatic coronary artery disease. Eur Radiol 24:2491–2498
Tullos BW, Sung JH, Lee JE, Criqui MH, Mitchell ME, Taylor HA (2013) Ankle-brachial index (ABI), abdominal aortic calcification (AAC), and coronary artery calcification (CAC): The Jackson Heart Study. Int J Cardiovasc Imaging 29:891–897
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplemental Figure 1.
Flow diagram of the patient recruitment process applied in the present study. We screened 453 consecutive patients with ACS who underwent successful PCI to the culprit coronary vessel. Furthermore, 139 were excluded. Accordingly, a total of 314 patients were evaluated in this study. ACS, acute coronary syndrome; PCI, percutaneous coronary intervention (JPG 71 kb)

Supplemental Figure 2.
Receiver operating characteristic curve analysis of abdominal aortic calcification index for cardiovascular events. Cardiovascular events were defined as composite of cardiovascular death, recurrent acute coronary syndrome, and stroke. Area under the curve was 0.69 (95% confidence interval, 0.57-0.78). The cut-off value of ACI was 29.2% with sensitivity of 76% and specificity of 64%. ACI, abdominal aortic calcification index (JPG 57 kb)
Rights and permissions
About this article
Cite this article
Oishi, H., Horibe, H., Yamase, Y. et al. Predictive value of abdominal aortic calcification index for mid-term cardiovascular events in patients with acute coronary syndrome. Heart Vessels 35, 620–629 (2020). https://doi.org/10.1007/s00380-019-01527-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01527-6