Abstract
Objective
Evaluation of the intimal flap visibility comparing 2nd and 3rd generation dual-source high-pitch CT.
Methods
Twenty-five consecutive patients with aortic dissection underwent CT angiography on a second and third generation dual-source CT scanner using prospective ECG-gated high-pitch dual-source CT acquisition mode. Contrast material, saline flush and flow rate were kept equal for optimum comparability. The visibility of the intimal flap as well as the delineation of the different vascular structures was evaluated.
Results
In 3rd generation dual-source high-pitch CT we could show a significant improvement of intimal flap visibility in aortic dissection. Especially, the far end of the dissection membrane could be better evaluated in 3rd generation high-pitch CT, reaching statistical significance (P < 0.01).
Conclusion
3rd Generation high-pitch CT angiography shows a better delineation of the aortic intimal flap in a small patient cohort, especially in the far ends of the dissection membrane. This might be due to higher tube power in this CT generation. However, to generalise these findings larger trials are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aortic dissection is a potentially fatal clinical emergency. Prevalence has been reported from 0.5 to 2.95 per 100,000/year with a mortality of 3.25–3.6 per 100,000/year [1]. Survival rates in the acute stage of Stanford type A and B dissection (DeBakey I–III) are very low with a fatality rate of up to 20 % before the hospital is reached [1–3]. Imaging in the emergency setting is vital for initial assessment [4]. Conservative therapy is the treatment of choice in the majority of Stanford type B dissections while Stanford type A dissections are referred for surgical or interventional treatment in nearly all cases [5]. Repeated imaging to exclude aortic rupture by assessment of the aortic dissection and the aortic diameter is an essential element when monitoring patients under conservative treatment [4, 6]. Available imaging modalities for follow-up of type B aortic dissections include computed tomography (CT), magnetic resonance imaging (MRI), ultrasound and conventional angiography [5]. Several technical advances have pre-dispositioned CT angiography amongst these. Current generation dual-source CT scanners are capable of pitch values above 3 [7], which makes full length aortic scanning in sub-second levels feasible [8–12]. The primary advantage of this technique is the ability to virtually freeze motion. Dual-source high-pitch acquisition is especially beneficial in the evaluation of the thoracic aorta where heart and aortic motion artefacts can mimic aortic dissection [10, 13]. As third generation dual-source CT scanners are now available, image acquisition in high-pitch mode has become increasingly widespread in clinical routine.
The objective of our study was therefore to evaluate the influence of a new CT scanner generation on the delineation of the aortic dissection membrane using 2nd and 3rd generation dual-source CT imaging for the follow-up of confirmed aortic dissection.
Materials and methods
Subjects
This study was performed using a single-centre, observer-blinded, retrospective design. The local ethics committee approved this study, and informed consent was waived because of the retrospective nature of the study. All primary patients who underwent clinically indicated CTA of the entire aorta for follow-up of aortic dissection from October 2013 to October 2014 were included in this study.
This patient cohort consisted of 25 individuals (Table 1). From these, 50 CT studies were included in this analysis. Each patient had received two scans: the first scan on a second generation dual-source CT (SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany), and the second scan on a third generation dual-source CT (SOMATOM Force; Siemens Healthcare, Forchheim, Germany). The time period between both examinations was set by the referring clinician and ranged from 6 to 12 months. For the analysis, two groups were defined depending on the CT scanner used (Table 2).
CT protocols
Group 1 underwent CTA on a 2nd generation dual-source CT device operated in prospective ECG-gated dual-source high-pitch mode (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany) with a pitch of 3.2, collimation of 2 × 128 × 0.6 mm using z-flying focal spot, and rotation time of 0.28 s.
Group 2 was examined on a 3rd generation dual-source CT device operated in prospective ECG-gated dual-source high-pitch mode (SOMATOM Force, Siemens Healthcare, Forchheim, Germany) with a pitch of 3.2, collimation of 2 × 192 × 0.6 mm using z-flying focal spot, and rotation time of 0.25 s.
All protocol parameters other than CT scanner model were kept constant between the two groups (Table 1). Automatic tube current modulation as well as automated tube potential selection was used in all groups (CareDose4D and CarekV, Siemens Healthcare, Forchheim, Germany). Image acquisition was performed in the craniocaudal direction in deep inspiratory breath-hold. The scan length included the entire chest, abdomen and pelvis in all cases (from superior thoracic aperture to the inguinal ligaments). All patients were examined for follow-up of their aortic dissection under conservative treatment.
Contrast medium volume was kept constant between both groups at 90 mL of iodinated contrast material (iodine concentration: 400 mg/mL, Imeron 400, Bracco Imaging, Konstanz, Germany) followed by a 50 mL saline chaser bolus. An 18–20 G intravenous access on the patient’s forearm was used for contrast injection with a flow of 4 mL/s using a double-syringe power injector (Injektron CT2, Medtron, Saarbruecken, Germany). CTA was automatically triggered by the bolus-tracking technique; the ROI was placed in the descending thoracic aorta at the height of the pulmonary trunk and the trigger threshold was set at 200 HU. The start delay was set to 7 s in both groups.
Transverse images were reconstructed at 0.75 mm slice thickness with 0.5 mm increment, a matrix size of 512 × 512 and a CTA window (centre: 100 HU; width: 700 HU). Furthermore, for a quick overview, transverse 5.0 mm slices with 5.0 mm increments were reconstructed. For 3D evaluation, coronal and parasagittal reformations at 2 mm slice thickness with 2 mm increments were reconstructed. Group 1 images were reconstructed using a medium-soft convolution kernel in filtered back projection (B30f). Group 2 images were reconstructed using a medium-soft convolution kernel in filtered back projection technique (Bv 36) [14].
Image analysis
Objective image analysis
Measures of objective image quality were performed by a radiologist with 5 years of experience in CT angiography on a commercially available PACS workstation (Centricity 4.2, General Electric Healthcare, Munich, Germany). Several region-of-interest (ROI) measurements were drawn using a circle tool (ascending aorta, descending aorta, aorta at celiac trunk, femoral artery). Image noise was determined as the standard deviation of air measured presternally at the level of the ascending aorta. Based on these measurements, the signal-to-noise ratio (SNR) was determined according to the following equation: SNR = attenuation/image noise.
Subjective image analysis
Subjective image quality rating was conducted by two independent radiologists (with 4 and 5 years’ experience in reading CTA examinations) in a blinded fashion. This rating followed a 5-point Likert-scale for each parameter (5 = excellent; 4 = good; 3 = moderate; 2 = fair; 1 = unacceptable). The parameter ‘overall image quality’ was used to grade the depiction of aortic pathologies (e.g. false/true lumen, thrombosis). For evaluation of the intimal flap, we evaluated, based on the quality of the images, the reader’s ability to rule out type A and B dissections, to define the start- and end-point of each dissection, as well as to visually delineate the intimal flap at mid distance between these two points. The rating further included visualisation of the coronary ostia for analysis of movement artefacts (5 = no artefacts; 4 = minimal artefacts; 3 = moderate artefacts; 2 = extensive artefacts; 1 = severe artefacts).
Radiation exposure
CT dose index (CTDIvol; in mGy) was recorded from the patient protocol, which is automatically generated at the end of each examination. All protocols were adjusted to similar kV/ref. mAs settings using automated dose control software (Table 1).
Statistical analysis
All statistical analyses were performed using dedicated software (Stata/IC 13.1, StataCorp LP, Texas, USA). Continuous variables are expressed as median and range; categorical variables are expressed as frequencies or percentages. The Wilcoxon signed rank test was used to compare image noise, attenuation and dose values. A P < 0.05 was defined as statistically significant. A Cohen’s kappa analysis was performed to determine inter-observer agreement for subjective image quality scoring.
Results
All CT examinations were of diagnostic image quality; no examinations had to be excluded from analysis. Contrast enhancement was rated as sufficient in all patients.
Objective image quality
Median SNR of the aorta at the level of the coeliac trunk was 36.6 for group 1 and 41.2 for group 2 (P = 0.03) (Table 4). Image noise in group 1 was significantly higher than in group 2 (P = 0.002).
Subjective image quality
Visual comparison of the intimal flap revealed a significant difference in the visualisation of the proximal as well as the distal intimal flap region (Table 3). In the central region of the aortic dissection membrane image quality was comparable between both groups (P = 0.2). Differentiation between type A and type B dissections was possible in all patients. For each patient, Stanford classification of the dissection was equal in both examinations. There were no motion artefacts present (Table 3).
Inter-observer agreement was good with a Cohen’s κ value of 0.77.
Radiation exposure
Parameters of radiation exposure only reached statistically significant differences for CTDIvolume (Table 4). Group 2 had a median CTDIvolume of 3.81 mGy compared to 4.85 mGy in Group 1. Imaging time and imaging length were comparable in both groups and did not reach statistical significance (Table 4).
Discussion
In our study, we showed a significant improvement of image quality in evaluation of the proximal and distal ends of the intimal flap using 3rd generation dual-source high-pitch CT.
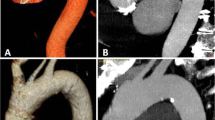
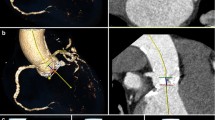
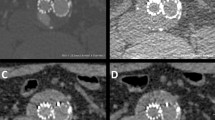
This improvement of intimal flap visualisation is directly related to the reader’s ability to judge progression of the intimal tear. This was the case both proximally and distally to the extent present in the previous CT study. This is one of the most important features in follow-up imaging of aortic dissection. We found improvements in image quality that were especially noticeable on the distal end of the dissection membrane (Figs. 1, 2, 3). As image reconstruction did not differ, we think that the main driver in this setting is the higher tube output in the new 3rd generation CT scanner compared to the 2nd generation CT scanner [15].
Left: Group 2 (SOMATOM Force); Right: Group 1 (Definition Flash). The dissection membrane in the left carotid artery has not been described in the first radiologic report (right hand side), because it was not visible. Imaging on 3rd generation dual-source CT has clearly visualised the dissection membrane (left hand side)
In clinical routine a verification of the intimal flap along the patient z-axis is one of the main questions of the referring clinician. To date, it is not described how detailed the intimal flap description is at need and if a small dissection, in the end, really changes the therapy. Larger trials are needed to focus on these questions.
Radiation dose was found to be roughly constant, while automated tube current modulation and automated tube potential selection (Care kV, Siemens Healthcare, Forchheim, Germany) were enabled in both groups (image quality parameters in Table 1). The dose modulation software selects the tube potential as well as the tube current according to the patients’ habitus. Because the examined patients were the same in both groups, we consider this assessment of radiation dose valid, as far as can be evaluated with a population group of this size. Third generation computed tomography is a relatively new technology that has been available since late 2013. Therefore, only a few comparable clinical observations for coronary heart CT exist to date [16, 17]. Gordic et al. reported a radiation dose of 3.2 mSV for a thoraco-abdominal examination, with a coronary CT angiography protocol at a pitch of 3.2 imaged with 120 kVp using automated tube potential selection [16]. Compared to second generation dual-source CT, Meyer et al. found a significant reduction of radiation exposure as well as contrast material using 70 keV as well as dual-source high-pitch CT for the imaging of cardiac vessels [17]. In this study by Meyer et al. the radiation dose reduction is likely to be a result of the adjustment of the tube potential to 70 keV.
In our data, contrast enhancement was adequate in all cases within our study. Both groups reached a median vascular enhancement above 300 HU. This is in line with enhancement values of vessel enhancement reaching 200 HU or higher reported in the literature [18–20].
In this study, a threshold of 200 HU was used, since a lower threshold will result in higher delay-times. There are two common strategies for the timing of contrast bolus: either the test-bolus or the bolus-tracking method is used [21, 22]. In previous evaluations, a contrast material enhancement threshold of 50 HU was determined to be adequate for diagnostic image quality [18]. However, as ongoing developments have led to increased imaging speeds [18], there has been a trend considering the higher attenuation thresholds necessary for adequate vessel enhancement in CT angiography. The bolus-tracking technique, however, remains a gold standard for imaging of the aorta from the results of this study, since it is both easy to use and does not require a second injection of contrast material.
Limitations
We are aware of several limitations of this study. First, we used a qualitative image scoring system to evaluate the aortic intimal flap. This evaluation is subject to potential observer bias. To balance this potential bias, we conducted the evaluation with two experienced radiologists. As a further measure, both readers were blinded to the date and relevant details of the examinations. The resulting inter-observer agreement was good, suggesting that observer bias was low.
Another limitation is the retrospective approach; however, this observational study might be a first step for further evaluations.
Finally, due to the fast table movement, the CT tube operates at a tube current close to its absolute capacity. This is the case, especially in 2nd generation dual-source high-pitch imaging [15]. In 3rd generation dual-source high-pitch CT, both X-ray tubes are capable of generating a peak tube current of 1300 mA. According to protocol output, however, the capacity of tube output was not reached in either examination.
Conclusion
Third generation high-pitch dual-source CT significantly improves delineation of proximal and distal ends of the intimal flap in aortic dissections. However, to really verify if a better delineation leads to a change in clinical treatment, larger trials are needed.
References
Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA (2001) Diagnosis and management of aortic dissection. Eur Heart J 22(18):1642–1681. doi:10.1053/euhj.2001.2782
Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE (1970) Management of acute aortic dissections. Ann Thorac Surg 10(3):237–247
Debakey ME, Henly WS, Cooley DA, Morris GC Jr, Crawford ES, Beall AC Jr (1965) Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 49:130–149
Golledge J, Eagle KA (2008) Acute aortic dissection. Lancet 372(9632):55–66. doi:10.1016/S0140-6736(08)60994-0
Apostolakis E, Baikoussis NG, Georgiopoulos M (2010) Acute type-B aortic dissection: the treatment strategy. Hellenic J Cardiol 51(4):338–347
Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, Smith DE, Suzuki T, Fattori R, Llovet A, Froehlich J, Hutchison S, Distante A, Sundt T, Beckman J, Januzzi JL Jr, Isselbacher EM, Eagle KA (2007) Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 357(4):349–359. doi:10.1056/NEJMoa063232
De Zordo T, von Lutterotti K, Dejaco C, Soegner PF, Frank R, Aigner F, Klauser AS, Pechlaner C, Schoepf UJ, Jaschke WR, Feuchtner GM (2012) Comparison of image quality and radiation dose of different pulmonary CTA protocols on a 128-slice CT: high-pitch dual-source CT, dual energy CT and conventional spiral CT. Eur Radiol 22(2):279–286. doi:10.1007/s00330-011-2251-y
Goetti R, Feuchtner G, Stolzmann P, Desbiolles L, Fischer MA, Karlo C, Baumueller S, Scheffel H, Alkadhi H, Leschka S (2010) High-pitch dual-source CT coronary angiography: systolic data acquisition at high heart rates. Eur Radiol 20(11):2565–2571
Wuest W, Anders K, Schuhbaeck A, May MS, Gauss S, Marwan M, Arnold M, Ensminger S, Muschiol G, Daniel WG, Uder M, Achenbach S (2012) Dual-source multidetector CT-angiography before transcatheter aortic valve implantation (TAVI) using a high-pitch spiral acquisition mode. Eur Radiol 22(1):51–58. doi:10.1007/s00330-011-2233-0
Beeres M, Schell B, Mastragelopoulos A, Herrmann E, Kerl JM, Gruber-Rouh T, Lee C, Siebenhandl P, Bodelle B, Zangos S, Vogl TJ, Jacobi V, Bauer RW (2012) High-pitch dual-source CT angiography of the whole aorta without ECG synchronisation: initial experience. Eur Radiol 22(1):129–137. doi:10.1007/s00330-011-2257-5
Lell M, Marwan M, Schepis T, Pflederer T, Anders K, Flohr T, Allmendinger T, Kalender W, Ertel D, Thierfelder C, Kuettner A, Ropers D, Daniel WG, Achenbach S (2009) Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual-source CT: technique and initial experience. Eur Radiol 19(11):2576–2583. doi:10.1007/s00330-009-1558-4
Bolen MA, Popovic ZB, Tandon N, Flamm SD, Schoenhagen P, Halliburton SS (2012) Image quality, contrast enhancement, and radiation dose of ECG-triggered high-pitch CT versus non-ECG-triggered standard-pitch CT of the thoracoabdominal aorta. AJR Am J Roentgenol 198(4):931–938. doi:10.2214/AJR.11.6921
Karlo C, Leschka S, Goetti RP, Feuchtner G, Desbiolles L, Stolzmann P, Plass A, Falk V, Marincek B, Alkadhi H, Baumuller S (2011) High-pitch dual-source CT angiography of the aortic valve-aortic root complex without ECG-synchronization. Eur Radiol 21(1):205–212. doi:10.1007/s00330-010-1907-3
Gordic S, Morsbach F, Schmidt B, Allmendinger T, Flohr T, Husarik D, Baumueller S, Raupach R, Stolzmann P, Leschka S, Frauenfelder T, Alkadhi H (2014) Ultralow-dose chest computed tomography for pulmonary nodule detection: first performance evaluation of single energy scanning with spectral shaping. Invest Radiol 49(7):465–473. doi:10.1097/RLI.0000000000000037
Beeres M, Bauer R, Kerl J, Vogl T, Lee C (2015) Energy limits in second generation high-pitch dual-source CT: comparison in an upper abdominal phantom. J Clin Imaging Sci 5(1):2. doi:10.4103/2156-7514.150441
Gordic S, Husarik DB, Desbiolles L, Leschka S, Frauenfelder T, Alkadhi H (2014) High-pitch coronary CT angiography with third generation dual-source CT: limits of heart rate. Int J Cardiovasc Imaging 30(6):1173–1179. doi:10.1007/s10554-014-0445-5
Meyer M, Haubenreisser H, Schoepf UJ, Vliegenthart R, Leidecker C, Allmendinger T, Lehmann R, Sudarski S, Borggrefe M, Schoenberg SO, Henzler T (2014) Closing in on the K edge: coronary CT angiography at 100, 80, and 70 kV-initial comparison of a second- versus a third-generation dual-source CT system. Radiology 140244. doi:10.1148/radiol.14140244
Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256(1):32–61. doi:10.1148/radiol.10090908
Cademartiri F, Nieman K, van der Lugt A, Raaijmakers RH, Mollet N, Pattynama PM, de Feyter PJ, Krestin GP (2004) Intravenous contrast material administration at 16-detector row helical CT coronary angiography: test bolus versus bolus-tracking technique. Radiology 233(3):817–823. doi:10.1148/radiol.2333030668
Fleischmann D (2005) How to design injection protocols for multiple detector-row CT angiography (MDCTA). Eur Radiol 15(Suppl 5):E60–E65
Kerl JM, Lehnert T, Schell B, Bodell B, Beeres M, Jacobi V, Vogl TJ, Bauer RW (2011) Intravenous contrast material administration at high-pitch dual-source CT pulmonary angiography: test bolus versus bolus-tracking technique. Eur J Radiol. doi:10.1016/j.ejrad.2011.09.018
Weininger M, Barraza JM, Kemper CA, Kalafut JF, Costello P, Schoepf UJ (2011) Cardiothoracic CT angiography: current contrast medium delivery strategies. AJR Am J Roentgenol 196(3):W260–W272. doi:10.2214/AJR.10.5814
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No funding was received for this work. The authors declare no potential conflict of interest.
Ethical standards
The local ethics committee approved this study, and informed consent was waived because of the retrospective nature of the study. All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Beeres, M., Bucher, A.M., Wichmann, J.L. et al. Improved visual delineation of the intimal flap in Stanford type A and B dissections at 3rd generation dual-source high-pitch CT angiography. Radiol med 121, 573–579 (2016). https://doi.org/10.1007/s11547-016-0634-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0634-5