Abstract
Objective
To investigate the feasibility, image quality and radiation dose for high-pitch dual-source CT angiography (CTA) of the whole aorta without ECG synchronisation.
Methods
Each group of 40 patients underwent CTA either on a 16-slice (group 1) or dual-source CT device with conventional single-source (group 2) or high-pitch mode with a pitch of 3.0 (group 3). The presence of motion or stair-step artefacts of the thoracic aorta was independently assessed by two readers.
Results
Subjective and objective scoring of motion and artefacts were significantly reduced in the high-pitch examination protocol (p < 0.05). The imaging length was not significantly different, but the imaging time was significantly (p < 0.001) shorter in the high-pitch group (12.2 vs. 7.4 vs. 1.7 s for groups 1, 2 and 3). The ascending aorta and the coronary ostia were reliably evaluable in all patients of group 3 without motion artefacts as well.
Conclusion
High-pitch dual-source CT angiography of the whole aorta is feasible in unselected patients. As a significant advantage over regular pitch protocols, motion-free imaging of the aorta is possible without ECG synchronisation. Thus, this CT mode bears potential to become a standard CT protocol before trans-catheter aortic valve implantation (TAVI).
Key Points
• High-pitch CT angiography without ECG synchronisation can provide motion-free aortic imaging
• High-pitch CTA could become the standard protocol before trans-catheter aortic valve implantation
• Without ECG-gating, there is no need for special preparation of the patient
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The continuing advances in CT technology have provided ongoing opportunities to improve image quality and to discover new imaging applications for use in clinical practice every day. As the detector collimation has nowadays reached sub-millimetre levels in state-of-the-art Multi-Detector CT (MDCT), the focus in recent years has been on speeding up image acquisition time by increasing the number of detector rows to increase imaging coverage per rotation. With the introduction of the second generation of dual-source CT approximately 1.5 years ago, increasing pitch above the traditional technical limit of 1.5 as with single-source CT has been introduced as a different approach to reduce image acquisition time and to increase anatomical coverage [1]. Recently some scientific papers investigating this new high-pitch DSCT technique for different clinical applications were published [2–6]. Most of them focussed on ECG-synchronised imaging of the heart or the great thoracic vessels in the ‘triple-rule-out’ clinical setting, whereas literature on non-ECG-synchronised applications is scarce.
Computed Tomographic Angiography (CTA) is an accurate method for diagnosing abnormalities of the aorta (e.g. dissection or aneurysm) and to follow-up patients after surgery or endovascular aortic repair. However, throughout the anatomical proximity of the heart, motion artefacts, especially of the ascending aorta, are common without the use of electrocardiographic (ECG) synchronisation. Such artefacts can cause diagnostic difficulties, occasionally leading to misdiagnosis by simulating double-contours in the ascending aorta that might be misinterpreted as type A dissection [7]. Thus, the use of ECG-synchronised protocols is mandatory for reliable motion-free imaging of the ascending aorta. Although in recent studies high-pitch ECG-gated dual-source CTA of the chest has shown its feasibility for clinical routine [8], the use of ECG is still more time consuming in terms of patient preparation and setting optimal CT parameters.
Hence, the main goal of this work was to investigate whether motionless imaging of the thoracic and ascending aorta can be achieved in patients undergoing high-pitch dual-source CTA for the whole body aorta. Further, because an advantage in dose compared with conventional protocols has been described for this novel technique, we compared CT dose parameters in totally three different examination protocols that are in use in clinical routines in our department.
Materials and methods
Patients and CT protocols
The study was performed as a single-centre, observer-blinded study. The institutional review board approved the study, and written informed consent was obtained by all patients. Data of consecutive unselected patients who underwent clinically indicated CT of the whole aorta without requiring ECG synchronisation on a 16-slice or dual-source CT device between January 2010 and January 2011 were analysed.
There were three groups of patients, each consisting of 40 individuals. Group 1 underwent CTA on a conventional 16-slice CT device (Sensation 16, Siemens Healthcare, Forchheim, Germany) with a pitch of 1.2, collimation of 16 × 1.5 mm, rotation time of 0.5 s, tube potential of 120 kV and 190 reference mAs. Group 2 was examined on a dual-source CT system operated in regular single-source mode functioning as a 128-slice CT (Definition Flash, Siemens, Forchheim, Germany) with a pitch of 1.2, collimation of 128 × 0.6 mm, rotation time of 0.5 s, tube potential of 120 kV and 210 reference mAs. Group 3 was examined on the same CT but in dual-source mode with a pitch of 3.0, rotation time of 0.28 s, tube potential of 100 kV and 184 reference mAs (Table 1). Automatic exposure control was used in all groups (CAREdose 4D, Siemens). The patients ranged in age from 23 years to 91 years (mean 68.0 years +/− 14.4), and there were 77 men (age range, 23–90 years; mean age 63.2 years +/− 13,2) and 43 women (age range, 36–91 years; mean age 68.1 years +/− 14.1). The median age in group 1 was 68.5 years (36–83), in group 2 it was 69.0 years (23–90) and in group 3 it was 64.0 years (27–86) with no significant differences between the groups (p > 0.2). Data were acquired in craniocaudal direction in deep inspiratory breath-hold. The imaging range extended from the upper thorax aperture to the inguinal ligaments.
Contrast enhancement was achieved by injecting a fixed amount of 90 mL of iodinated contrast material (iodine concentration of 400 mg/mL, Imeron 400, Bracco Imaging, Konstanz, Germany) followed by a 50 mL saline chaser. The bolus was injected through an 18–20 G intravenous access on the patient’s forearm at a flow of 4–5 mL/s using a double-syringe electronic power injector (Injektron CT2, Medtron, Saarbruecken, Germany). CTA was automatically started utilising the bolus tracking technique at the level of the descending thoracic aorta after a trigger threshold of 140 HU was reached. The start delay was set to 5 s in groups 1 and 2, and 15 s in group 3.
Transverse images were reconstructed at 1.5-mm slice thickness with 1.0-mm increments using a medium-soft convolution kernel (B30f), a matrix size of 512 × 512 and a CTA window (centre: 100 HU; width: 700 HU). Further, for a quick overview transverse 5.0 mm slices with 5.0 mm increments were reconstructed. For 3D evaluation, we further routinely reconstruct coronal and parasagittal (“candy cane view”) images at 2 mm slice thickness with 2 mm increments.
Image analysis
The total examination time for the CTA series was recorded in seconds. As measures of objective image quality several region-of-interest (ROI) measurements were performed by one radiologist with 2 years of experience of CT on a regular PACS workstation (Centricity 4.2, General Electric Healthcare, Dornstadt, Germany) using a circle tool: Background Noise (BN) was determined as the standard deviation of air measured presternally in front of the patient at the level of the ascending aorta; further, attenuation along the aorta at different anatomical levels (ascending aorta, descending thoracic aorta, abdominal aorta at the level of the celiac trunk, aortic bifurcation and common femoral artery on both sides), in the right and left ventricle and in the pulmonary trunk was measured to determine the contrast bolus geometry among the three groups. Based on these measurements, signal-to-noise ratio (SNR) was determined according to the following equation: \( {\text{SNR}} = {\text{Attenuation}}/{\text{BN}} \).
To calculate the contrast-to-noise ratio (CNR), we measured attenuation and standard deviation (SD) of the gluteus maximus muscle (ROImuscle) compared with the attenuation of the aorta at the celiac trunk. CNR was then calculated as: \( {\text{CNR}} = \left( {{\text{ROIaorta}} - {\text{ROImuscle}}} \right)/{\text{image noise}} \). To minimise bias from single measurements, we calculated the average of four measurements for each ROI.
A figure of merit (FOM) was calculated as the ratio of the CNR² to dose (expressed as CTDIvol) for the different CT protocols. The FOM enables the assessment of CNR change independent of the tube current–time product and effective dose [9, 10].
Subjective image quality rating was conducted in a blinded fashion by two independent radiologists. Concerning motion artefacts we primarily investigated the thoracic aorta as most of the motion artefacts are typically located here near the beating heart. A special focus was set on the possibility of depicting the aortic annulus, measuring the distance between the aortic annulus plane and the coronary ostia. Another important item was the presence of motion artefacts observable as double contours in the ascending aorta mimicking type A dissections. Image quality was rated using a scale of 1–5 (1 = excellent, 2 = good, 3 = moderate, 4 = fair, 5 = poor).
Additionally, to analyse the pathologies of the whole aorta (including descending aorta, the pelvic region down to the femoral arteries) we included the factor of “overall quality”. This factor was assessed in the same way by applying a 5-point scale (1 = excellent; 5 = poor) for the depiction of lesions (e.g. aneurysm, dissection etc.). Studies that did not meet at least a score of “5” concerning the overall quality were considered to be non-diagnostic and are instantly repeated in our clinical routine. Therefore, subjective image quality rating in this evaluation does not include the category “non-diagnostic study”.
Radiation exposure
For the estimation of patient dose, we recorded the volume CT dose index (CTDIvol in mGy) and dose length product (DLP in mGy*cm) from the patient protocol, which is automatically generated at the end of an examination and stored in the PACS of our department.
Statistical analysis
All statistical analyses were performed computer-based by using dedicated software (BiAS 9.07, Epsilon Verlag, Frankfurt, Germany). Continuous variables were expressed as median +/− standard deviations and categorical variables as frequencies or percentages. We used the Kruskal–Wallis Test to compare the study population, image noise, -quality, -attenuation and dose values. A Cohen’s kappa analysis was performed to determine inter-observer agreement for subjective image quality scoring.
Results
All CT examinations were deemed diagnostic. There was no need to repeat a single examination during clinical routine because of unsatisfying aortic enhancement or missed bolus. There were 23 patients with Stanford type B dissection, 27 patients with aortic aneurysm (16 abdominal aortic aneurysm, 10 thoracic aortic aneurysm and 1 combined thoracic-abdominal aneurysm), 34 patients after endovascular repair using stent grafts, 5 patients with acute arterial bleeding, 1 patient with Leriche’s syndrome and 30 patients with no findings.
Mean imaging time was significantly shorter in group 3 with a median of 1.7 s (1.1–1.9 s) compared with group 1 (median: 12.2 s; range: 7.7–14.0 s) and group 2 (median 7.4 s; range: 6.1–8.4 s) with no significant differences in imaging length (Table 2).
The median BMI was, except between group 2 and 3, not statistically significant different between the three groups (Table 2).
Median CTDIvol and DLP were, as expected, significantly lower in group 3 compared to both other groups (Fig. 1, Table 3).
Concerning image noise as an objective image quality parameter, the high-pitch protocol had significantly (p < 0.01) more image noise compared with the 16-slice and the 128-slice group (Table 4). The median arterial attenuation of the aorta at the celiac trunk was in the high-pitch protocol significantly higher compared to both other groups (Table 4). From these data, a median SNR for the aorta at the level of the celiac trunk was calculated (Table 4).
The figure of merit revealed the highest values for the high-pitch protocol followed by the 128-slice and 16-slice protocol, the difference between groups 1 and 3 remained marginal, just below the level of significance, p = 0.06 (Fig. 2, Table 4).
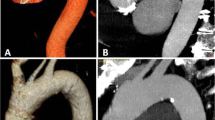
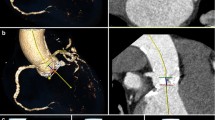
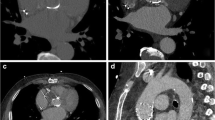
Heartbeat-related artefacts occurred in 33/40 patients in group 1, 30/40 patients in group 2 and in 0/40 patients in group 3 (Figs. 3 and 4). Thus, concerning the motion artefacts of the ascending aorta, there was a manifest improvement in image quality using the high-pitch protocol (Figs. 5 and 6). In this group, exclusion of type A dissection was reliably possible in every patient. Comparing the other factors like overall quality, movement artefacts or differentiation of the coronary ostia, the high-pitch protocol was superior to the conventional single-source protocols (p < 0.05; Table 4). Inter-observer agreement was good with a kappa value of 0.89.
Contrast bolus geometry was measured by following the contrast media through the vessels, beginning at the right ventricle, ending at both femoral arteries as shown in Fig. 7. Concerning the graph and the image analysis, the heart was caught in the wash-out phase in the high-pitch group. The contrast media timing using a delay of 15 s in this group demonstrated a good attenuation of the whole aorta by simultaneously reducing artefacts in the ascending aorta. Compared to the single-source protocols, the peak of attenuation in the high-pitch protocol occurred more towards the lower aortic and the pelvic region. The arterial attenuation along the z-axis was more homogenous in both single-source groups with a small peak in the ascending aorta.
Discussion
High-pitch angiography of the body aorta with modern thin-section CT devices (MDCT) currently represents the most widely accepted test of choice to rule out aortic dissection, rupture, aneurysm, and for follow-up after endovascular repair or surgery. However, motion artefacts especially in the ascending aorta might cause problems in identifying type A dissections confidently. Hence, ECG-synchronised CT protocols are used when the ascending aorta is the focus of interest. ECG-gated imaging of the thoracic aorta commonly uses the same retrospective approach [2, 4, 5, 8]. However, retrospective ECG-gating, even when ECG-controlled tube current modulation is applied, is associated with a high radiation dose because of low pitch values resulting in overlapping of data acquisition that is needed for a complete image reconstruction. Prospectively ECG-triggered sequences, especially in high-pitch protocols, can substantially reduce the estimated radiation exposure while providing the same diagnostic image quality as retrospective ECG-gated helical CT angiography [2, 8, 11]. However, the use of ECG-synchronisation is more time consuming in daily routine, because the ECG leads need to be fixed, the patient’s heart rate needs to be stable and ideally low often making beta-blockade necessary. For us, the high table speed at a pitch of 3.0 was an idea to get the whole aorta examined in one single session without ECG-gating and without movement artefacts. The table speed at a pitch of 3.0 is about 411 mm/s resulting in a median total acquisition time of 1.7 s. The very high table feed virtually “freezes” the movement of the heart and that affects the image quality especially in the ascending aorta; there were no artefacts attributable to motion of the heart or other movement artefacts in the high-pitch group. Subjective image analysis did not reveal a missing contrast bolus or even abnormalities that could not be analysed. Image quality was rated as excellent or very good in every case.
Recently, an interesting paper concerning non-ECG-gated imaging of the thoracic aorta was published [12]. The study demonstrates that the motion free evaluation of the ascending aorta was possible in 95% of the cases using a non-ECG-gated high-pitch protocol. This meets with the results we observed. As interventional approaches for the replacement of the aortic valve (trans-catheter aortic valve implantation; TAVI) are becoming more common in clinical routine, there is not only the need for a motion-free ascending aorta to evaluate both coronary ostia and the aortic annulus [13, 14]. There is also the need for information about the access path for the interventional procedure. Information about the calibres of the femoral arteries, vessel kinking, or degree of arteriosclerotic changes and stenoses is essential. The CT protocol introduced in this study bears the potential to achieve these goals in one single examination with one single contrast bolus injection.
Concerning the contrast bolus timing, the empirically chosen start delay of 15 s after reaching a 140 HU threshold in the descending aorta proved its robustness. The heart was hit in the wash-out phase while the main contrast bolus was in the abdominal aorta. Compared with the high-pitch protocol, the contrast distribution along the z-axis in the conventional examinations was more homogeneous (Fig. 2). Through the lower pitch, the CT follows the contrast bolus, whereas with the high pitch the whole aorta ideally needs to be “just there” at the right time, since literally a snap-shot of the aorta is taken. When we look at the bolus geometry, the peak of attenuation occurred at the level of the femoral arteries in the high-pitch group. We think that the examination could be started a bit earlier; a start delay of about 10–12 s should be appropriate. We also think, that there is a potential with this novel CT mode to reduce the total amount of contrast material volume, since the fear of losing the contrast bolus because the CT machine is not fast enough is not justified. Elevating the trigger threshold and shortening the start delay together with more sophisticated contrast injection protocols may facilitate a whole body CTA with 50 mL of contrast material. Alternatively, these parameters could be modified to enable double-rule-out imaging of pulmonary embolism and aortic dissection.
Discussing radiation exposure, it was up to 59% lower using the high-pitch mode compared with the single-source modes. However, this has primarily only little to do with the high-pitch mode per se, since we used real-time online tube current output modulation (CAREdose) also in the high-pitch group. The tube potential of 100 kV is the main factor for this finding [2, 3, 8, 15]. If we were to adjust the examination parameters to the same image-noise levels and hence tube potential settings in all three groups, we would be very likely to observe the same radiation exposure [16]. On the other hand side, the goal of automated exposure control (AEC) software is, to maintain a constant level of image quality independent of the patients’s body habitus. AEC typically achieves that goal by adjusting the effective tube current and thus photon flux. But every X-ray tube has an upper peak output limit, above which the total applicable energy is capped. This may result in artificially lower dose values in bigger patients and deterioration of image quality in these cases. However, to investigate this effect, a dedicated study is necessary.
To compensate for the uneven tube parameters between the groups, figure of merit calculations were performed. Here we observed that the high-pitch protocol was the most dose-efficient protocol from all three. After corrections for dose and image quality, the paramount advantage of non-ECG-gated high-pitch CTA of the aorta is the ultrafast examination time that suppresses motion artefacts.
Limitations
Firstly, the second smaller detector of the dual-source system covers a field of view of only 33 cm [1]. Thus, especially in big and overweighed patients, parts of the subcutaneous structures may be cropped. However, this does not affect the centrally located arteries. Secondly, the patients’ heart rate was not recorded. Therefore, we cannot make a definite statement, if our observations are true only up to a certain heart rate. However, since we examined an unselected patient cohort including emergency cases as well as follow-up patients, it is likely that the patient population showed a wide range of resting heart rates from bradycardia to tachycardia. Nevertheless, this issue should be investigated in a future project. Thirdly, the maximum energy that can be applied to the patient in high-pitch mode is capped. At pitches of 3.0 and 100 kV, the tube current output is limited to 184 reference mAs. This results in “underradiation” of bigger patients in whom the automated exposure control software requires a higher tube output to maintain a constant image quality as expressed by noise. Indeed, image noise was significantly higher in the high-pitch group compared to the single-source groups. X-ray tubes with higher output capacity are mandatory to overcome this issue.
Conclusion
High-pitch dual-source CTA without ECG synchronisation effectively avoids motion artefacts of the aorta, especially of the ascending aorta related to conventional single-source CT protocols. With the use of the same amount of contrast media, the high-pitch protocol exhibited sufficient contrast attenuation and a significantly shorter image acquisition time. Thus, this novel CT mode bears potential to become a standard CT protocol before trans-catheter aortic valve implantation (TAVI) or in uncooperative, restless patients under emergency conditions.
References
Flohr TG, Leng S, Yu L, Allmendinger T, Bruder H, Petersilka M, Eusemann CD, Stierstorfer K, Schmidt B, McCollough CH (2009) Dual-source spiral CT with pitch up to 3.2 and 75 ms temporal resolution: image reconstruction and assessment of image quality. Med Phys 36(12):5641–5653
Lell M, Marwan M, Schepis T, Pflederer T, Anders K, Flohr T, Allmendinger T, Kalender W, Ertel D, Thierfelder C, Kuettner A, Ropers D, Daniel WG, Achenbach S (2009) Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol 19(11):2576–2583
Weustink AC, Neefjes LA, Kyrzopoulos S, van Straten M, Neoh Eu R, Meijboom WB, van Mieghem CA, Capuano E, Dijkshoorn ML, Cademartiri F, Boersma E, de Feyter PJ, Krestin GP, Mollet NR (2009) Impact of heart rate frequency and variability on radiation exposure, image quality, and diagnostic performance in dual-source spiral CT coronary angiography. Radiology 253(3):672–680
Goetti R, Feuchtner G, Stolzmann P, Desbiolles L, Fischer MA, Karlo C, Baumueller S, Scheffel H, Alkadhi H, Leschka S (2010) High-pitch dual-source CT coronary angiography: systolic data acquisition at high heart rates. Eur Radiol 20(11):2565–2571
Sommer WH, Schenzle JC, Becker CR, Nikolaou K, Graser A, Michalski G, Neumaier K, Reiser MF, Johnson TR (2010) Saving dose in triple-rule-out computed tomography examination using a high-pitch dual spiral technique. Invest Radiol 45(2):64–71
Schell B, Bauer RW, Lehnert T, Kerl JM, Hambek M, May A, Vogl TJ, Mack MG (2010) Low-dose computed tomography of the paranasal sinus and facial skull using a high-pitch dual-source system-First clinical results. Eur Radiol 21(1):107–112
Qanadli SD, El Hajjam M, Mesurolle B, Lavisse L, Jourdan O, Randoux B, Chagnon S, Lacombe P (1999) Motion artifacts of the aorta simulating aortic dissection on spiral CT. J Comput Assist Tomogr 23(1):1–6
Blanke P, Bulla S, Baumann T, Siepe M, Winterer JT, Euringer W, Schafer AO, Kotter E, Langer M, Pache G (2010) Thoracic aorta: prospective electrocardiographically triggered CT angiography with dual-source CT–feasibility, image quality, and dose reduction. Radiology 255(1):207–217
Nakaura T, Awai K, Oda S, Funama Y, Harada K, Uemura S, Yamashita Y (2011) Low-kilovoltage, high-tube-current mdct of liver in thin adults: pilot study evaluating radiation dose, image quality, and display settings. AJR Am J Roentgenol 196(6):1332–1338
Schindera ST, Nelson RC, Mukundan S Jr, Paulson EK, Jaffe TA, Miller CM, DeLong DM, Kawaji K, Yoshizumi TT, Samei E (2008) Hypervascular liver tumors: low tube voltage, high tube current multi-detector row CT for enhanced detection–phantom study. Radiology 246(1):125–132
Roos JE, Willmann JK, Weishaupt D, Lachat M, Marincek B, Hilfiker PR (2002) Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology 222(1):271–277
Karlo C, Leschka S, Goetti RP, Feuchtner G, Desbiolles L, Stolzmann P, Plass A, Falk V, Marincek B, Alkadhi H, Baumuller S (2011) High-pitch dual-source CT angiography of the aortic valve-aortic root complex without ECG-synchronization. Eur Radiol 21(1):205–212
Masson JB, Kovac J, Schuler G, Ye J, Cheung A, Kapadia S, Tuzcu ME, Kodali S, Leon MB, Webb JG (2009) Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv 2(9):811–820
Zahn R, Schiele R, Kilkowski C, Klein B, Schwarz AK, Zeymer U, Lehmann A, Cornelius B, Horack M, Saggau W, Werling C (2011) Transcatheter aortic valve implantation (TAVI)TAVI): a new therapeutic option for patients with severe symptomatic aortic stenosis who are not suitable or at high risk for surgical valve replacement. Dtsch Med Wochenschr 135(33):1589–1595
Goetti R, Baumuller S, Feuchtner G, Stolzmann P, Karlo C, Alkadhi H, Leschka S (2010) High-pitch dual-source CT angiography of the thoracic and abdominal aorta: is simultaneous coronary artery assessment possible? AJR Am J Roentgenol 194(4):938–944
Zhang C, Zhang Z, Yan Z, Xu L, Yu W, Wang R (2010) 320-row CT coronary angiography: effect of 100-kV tube voltages on image quality, contrast volume, and radiation dose. Int J Cardiovasc Imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beeres, M., Schell, B., Mastragelopoulos, A. et al. High-pitch dual-source CT angiography of the whole aorta without ECG synchronisation: Initial experience. Eur Radiol 22, 129–137 (2012). https://doi.org/10.1007/s00330-011-2257-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2257-5