Abstract
In the Canary Islands, a number of old Andean potato introductions have been maintained by farmers and are cultivated there since the sixteenth century. Genetic resistance is an inexpensive way to control the main pests and diseases of potato and avoids the use of phytochemicals or other costly protective measures. In this study, we have analysed eight Solanum tuberosum subsp. andigena and S. tuberosum subsp. tuberosum accessions, representing old potato varieties from Tenerife Island, for their resistance levels to Phytophthora infestans, Globodera rostochiensis, Globodera pallida and Pectobacterium atrosepticum. New resistance sources against P. infestans in leaves and P. atrosepticum were found, as well as partial resistance to both nematode species. The results suggest the potential exploitation of the cultivar Venezolana Negra in breeding programmes in order to improve pest and disease resistance of potato.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potato (Solanum tuberosum L.) is the fourth most important crop in the world after corn, rice and barley (Faostat 2014). This crop represents also a major and basic food crop in many countries and produces a higher amount of energy and protein per hectare than any other food crop, including cereals (Singh 2008). Potato was first cultivated 6000 to 10,000 years ago around the Titicaca Lake, Peru (Spooner and Hetterscheid 2004). According to Cook and Cook (1998), the first written references for potatoes were in 1552 who named them “turma de tierra (earth tuber)” and mentioned the local Quetchua name “Papa”. Caspar Bahuin was the first to describe potato in 1596, already 157 years before Carl von Lenné (1753; Species Plantarum). However, he used a slightly different name, S. tuberosum esculentum.
There were several introductions into Europe around the same time. John Hawkins reported loading potatoes in Venezuela on March 28, 1565. Popular legend has long credited Sir Walter Raleigh as the first bringing the potato to England, but history suggests Sir Francis Drake as a more likely candidate, in 1586, after battling the Spaniards in the Caribbean. According to Ruiz de Gordoa (1981), potatoes were sent from Cuzco to the Spanish King Felipe II in 1565, but they could have been introduced even earlier (1562) into the Canary Islands (Ruiz de Galarreta and Ríos 2008).
The first records of cultivated potato outside South America were from Gran Canaria (Canary Islands) in 1567 (Hawkes and Francisco-Ortega 1993; Rios et al. 2007) and shortly thereafter from mainland Spain in 1573 (Hawkes and Francisco-Ortega 1992; Romans 2005; Ames and Spooner 2008). In 1574, potato appeared in Tenerife Island (Lobo-Cabrera 1988). During the time of the colonies, the Canary Islands were the first entry point into Europe for goods from South America, including novel plant species such as potato. Modern potato cultivars are very different from this original material, but in the Canary Islands, a number of Andean introductions have been maintained and were cultivated since the mid-sixteenth century (Rios et al. 2007). They were classified for the first time by Zubeldia et al. (1955) into the following species, S. tuberosum subsp. andigena, S. tuberosum subsp. tuberosum and Solanum chaucha. Rodríguez (2000) made a basic characterization of 14 local cultivars from Tenerife Island: Azucena Blanca, Azucena Negra, Bonita Blanca, Bonita Colorada, Bonita Llagada, Bonita Ojo de Perdiz, Bonita Negra, Colorada, Torrenta, Borralla, Mora, Negra and Andina Blanca y Andina Negra. Ríos (2002) characterized morphologically 52 accessions of Canarian landraces obtaining similar results to those described by Zubeldia et al. (1955).
Based on this study, he argues that 11 of these cultivars have typical characteristics of subsp. andigena and two, Mora and Borralla, have features of both subspecies. Barandalla et al. (2006) and Ruiz de Galarreta et al. (2007) analysed with SSR markers the relationship among local cultivars from Tenerife and La Palma islands, respectively. Ruiz de Galarreta et al. (2011) performed also a joint analysis of local cultivars from mainland Spain and from the Canary islands in order to detect potential relationships at the molecular level. All these studies revealed some particularities of certain Canary cultivars which might exist also with respect to disease and pest resistances.
The potato is sensitive to a wide range of pests and diseases, but within landraces, specific resistance against certain pathogens has been found (Ruiz de Galarreta et al. 1998; Ritter et al. 2008). Late blight caused by the oomycete Phytophthora infestans is considered the most important potato pathogen worldwide and can destroy a susceptible potato crop within 1 week, especially under favourable climatic conditions. However, under optimal weather conditions, the pathogen may complete several infection cycles a week on a susceptible host, with control failure leading to rapid epidemics and crop loss (Cooke et al. 2012).
After a latent period as short as 3 days (Flier and Turkensteen 1999), new sporangia are formed and spread to infect new plants. The fast and efficient spread, infection and colonization of the host plant gives the potential of destroying all above ground parts of a crop within a week (Andersson 2007).
The infection affects all vegetative parts of the plant including the tubers, requiring frequent fungicide applications for its control. The co-existence of two mating types and sexual reproduction increases the chance of developing resistance to fungicides such as metalaxyl. The majority of varieties grown in Europe and North America are susceptible to late blight (Flier et al. 2003). Several resistance screenings have been performed in Solanum wild species (Hawkes 1994). Quantitative resistance does not confer absolute protection, and qualitative or vertical resistance is highly race-specific and becomes rapidly ineffective due to changes in the pathogen population (Forbes et al. 2005).
Potato cyst nematodes Globodera pallida Stone and Globodera rostochiensis Woll. represent also important potato pests (Evans and Trudgill 1992). Initial screenings of tuber-bearing Solanum species identified resistance to G. rostochiensis in S. tuberosum ssp. andigena and Solanum vernei (Castelli et al. 2003).
Tuber soft rot caused by Pectobacterium atrosepticum (syn. Erwinia carotovora subsp. atroseptica van Hall), generates significant crop losses in seed and ware potato production, both in the field and during storage. Diseased seed tubers are the primary infection source, but spores can survive also in the soil and can be spread by irrigation water (Egúsquiza 2000). Resistance sources are present in several tuber-bearing Solanum species (Allefs et al. 1995).
This paper reports on the response of old potato cultivars from Tenerife Island (Spain), in controlled infection experiments with P. infestans, G. rostochiensis, G. pallida and P. atrosepticum in order to identify potentially new resistant sources and to extend the present gene pool of exploitable resistances.
Materials and Methods
Plant Material
Eight Solanum genotypes representing old potato cultivars from Tenerife Island (Spain) were evaluated. The accessions, previously characterized by Ríos (2002) and Ruiz de Galarreta et al. (2011), were obtained from the germplasm collection of the Center for the Conservation of Biodiversity from Tenerife (CCBAT). Three commercial cultivars belonging to the species S. tuberosum (Bintje, Desiree and Spunta) were selected as susceptible controls. Table 1 shows the accession code, common name and taxonomic assignment for each accession.
Resistance Evaluation
P. infestans
The resistance against P. infestans in leaves was evaluated using the method of Vleeshouwers et al. (1999). The MP 324 strain used for inoculations has mating type A1 and a high level of aggressiveness in foliage. It was provided by the Plant Breeding and Acclimatization Institute (IHAR) from Poland and has all virulence genes except R5, R8 and R9. Isolates were first inoculated on tuber slices of the susceptible potato cv. Bintje and incubated in the dark at 18 °C for 5–7 days. When sporulating mycelium was present, small tufts of mycelium were placed in 9-cm Petri dishes containing rye agar medium. Inoculum for infection was prepared by dipping sporulating plates in 4 ml of water, filtering the crude suspension through cheesecloth and adjusting the concentration to 4.104 sporangia ml−1. The experiment was conducted with three replicates per genotype, inoculating five leaflets of each Canarian cultivar and the susceptible control cv. Bintje. The leaflets were collected from each of the two youngest fully expanded leaves of each plant and kept on moist paper in transparent boxes with the abaxial surface upwards. After the discs were inoculated with a 40-μl drop of spore suspension and incubated for 5 days in a climate chamber at 18 °C with an 18-h light period, the estimation of leaf resistance was made according to the percentage of the infected leaflet disc surface, as recorded after 5 days of incubation, when the leaves of the control variety were fully infected. The area under the total disease progress curve (AUDPC) was calculated using the trapezoidal integration method and transformed into the relative area under the disease curve (rAUDPC) (Fry 1978). The AUDPC was calculated as
where x i = proportion tissue affected at the ith observation, t = time (days) after inoculation at the ith observation and n = total number of observations. Values for AUDPC were normalized by dividing the AUDPC by the total area of the graph (=the number of days from inoculation to the end of the observation period). The normalized AUDPC is referred to as relative AUDPC. Units for AUDPC are days-proportion, and relative rAUDPC is a percentage value.
Whole-tuber inoculation experiments were performed using tubers from all cultivars and the susceptible control. For this purpose, tubers were dug by hand to minimize wounding, immediately transferred to the laboratory and washed. Five undamaged tubers were inoculated using the same isolate MP324 and were subsequently incubated at 18 °C with an 18-h light period. Tuber resistance against P. infestans was determined according to the methods described by Flier et al. (2001). After 2 weeks of storage, tubers were visually examined for the presence of tuber blight symptoms, and the percentage of infected area per tuber was calculated. Diseased tubers were cut longitudinally, and disease severity was scored as described by Flier et al. (2003) for each individual tuber. The following scale was used: 0 = no symptoms, 1 = <2.5% of cut area with symptoms, 2 = 2.5–10%, 3 = 10–25%, 4 = 25–50% and 5 = >50% of cut area with symptoms. Values for AUDPC were also calculated and normalized.
Accessions without infection or with infection not exceeding 10% on any leaf and tuber surface were defined as resistant, whereas accessions with 10 to 20% of infected leaf surface were defined as partially resistant. Genotypes exceeding 20% of infected surface were considered susceptible.
G. rostochiensis and G. pallida
For evaluating the resistance against nematodes G. rostochiensis (pathotype Ro1,4) and G. pallida (pathotype Pa2/3), a bioassay was performed in the greenhouse as a pot test following the methodology described by Ruiz de Galarreta et al. (1998). Five tubers and three replicates with a total of 15 tubers per cultivar were planted individually into pots for the evaluation of G. rostochiensis and G. pallida, respectively. The infected soil contained 30 cysts per pot of G. rostochiensis and G. pallida in two sets, separately. The cysts of G. pallida Pa 2/3 were collected in 2012 from Bañares (Alava, Basque Country, Spain). For the test on G. rostochiensis, the cysts came from infected fields of Urturi (Alava, Basque Country, Spain) and maintained in NEIKER-Tecnalia. Desiree was used as a susceptible control variety in each assay.
Plants were grown at 20 °C for 10 weeks, and the accessions and controls were scored 10 weeks after inoculation. Resistance levels were classified as fully resistant (accessions with an average cyst counts of ≤5), partial resistant (accessions with average cyst counts between 5 and 20) or susceptible (accessions with an average cyst counts of ≥20 cysts) according to Rousselle-Bourgeois and Mugniery (1995). Resistant genotypes were planted again into pots containing soil with 30 cysts to confirm their resistance.
P. atrosepticum
Resistance levels to the bacteria P. atrosepticum were determined as described by Foolad et al. (2002). Tubers were washed thoroughly in running tap water, and the surface was disinfected by dipping in 90% ethanol for 3 min before use. All tests were performed with strain VC7004/CR3 from the NEIKER culture collection. Bacterial suspensions in sterile distilled water were prepared from cultures grown on solid King’s B medium at 27 °C for 24 h. The concentrations of the stock suspensions were calculated from optical density readings of 350 nm and adjusted to 2 × 108 cells ml−1. The actual number of colony-forming units (CFU) per millilitre of inoculum suspension was determined by plating serial dilutions of each suspension on King’s B plates and counting visually the number of colonies formed after 24 h of incubation at 27 °C.
Inoculations were performed according to Lapwood and Gans (1984) with minor modifications. Each tuber was cut longitudinally, and a cylindrical well (5 mm in diameter, 5 mm deep) was extracted from the middle of each half tuber 2 h before inoculation. Half tubers were placed on filter paper in black plastic containers. Six half tubers of each potato genotype were inoculated with 200 μl of suspension and incubated in a chamber for 6 days at 20 °C in constant darkness. The area under the total disease progress curve (AUDPC) was calculated. Genotypes were classified into resistant, partial resistant and susceptible based on the infected surface as described for P. infestans. In each batch of inoculations, the susceptible control cultivar Spunta was included.
Results
Foliar resistance levels against our late blight isolate varied considerably among the potato cultivars that were evaluated. Venezolana Negra (S. tuberosum × S. andigena) and cv. Moras (S. tuberosum) showed partial resistance to the P. infestans strain with an rAUDPC mean value of 0.120 and 0.342 (Fig. 1a). In contrast, the susceptible control Bintje had a value of 0.912. All other cultivars showed rAUDPCs comparable with that of cv. Bintje, ranging from 0.729 to 0.896. Moreover, the most resistant cv. Venezolana Negra revealed also the lowest standard deviation of AUDPC values (0.009).
All cultivars were susceptible to tuber inoculations of P. infestans with the exception of cv. Bonita Colorada (S. andigena) which showed an rAUDPC of 0.110 and was considered as a partial resistant cultivar (Fig. 1b). No relationships between P. infestans resistance in tubers and leaves were observed.
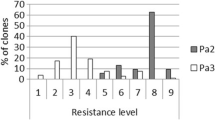
With respect to G. rostochiensis, all cultivars were susceptible with cyst numbers ranging from 156.3 to 203.1 (Table 2). With respect to G. pallida (Pa2/3), partial resistance in cvs. Bonita Ojo de Perdiz (S. andigena). Azucena Negra (S. andigena) and cv. Venezolana Negra (S. tuberosum × S. andigena) with average cyst counts of 13.1 ± 2.3, 15.3 ± 1.4 and 17.1 ± 2.5, respectively. In contrast, the susceptible control Desiree had an average of 189.3 ± 12.1 cysts. These genotypes were planted again into pots containing soil with 30 cysts, and we confirmed their partial resistance detected.
We found total resistance to P. atrosepticum in cv. Bonita Colorada and cv. Terrenta, both S. andigena species, showing 0% infection (Fig. 1c). On the other hand, four cultivars Azucena Negra, Bonita Ojo Perdiz, Venezolana Negra and Moras showed partial resistance ranging from 0.025 to 0.067 rAUDPC (Fig. 1c). The susceptible cv. Spunta showed an rAUDPC value of 0.980, similar to that of the susceptible cv. Peluca Rosada with 0.958.
Table 3 summarizes the resistances found in all evaluated cultivars. The multiple resistance of the cv. Venezolana Negra with resistance to P. infestans in leaves and partial resistance to G. pallida and P. atrosepticum are notable.
Discussion
The potato crop is affected by many pests and diseases that cause substantial damages in the field, losses during storage or affects the quality of the tubers. Managing these pathogens requires crop rotations, utilization of pesticides and other practices that are costly to farmers or environmentally unsafe. However, it may be also possible to confer resistance by transferring a naturally found resistance gene to potato cultivars of interest by crossings or somatic hybridizations, if this is possible, or using transgenic approaches.
On the other hand, native potato species are cultivated under harsh environmental conditions where modern potato cultivars cannot compete. They have been locally selected by the Andean farmers in order to provide their subsistence. Some old introductions of native potato species exist also in Europe. In the Canary Islands, the cvs. Azucena Negra or Peluca Rosada are highly appreciated, among others. Despite its low productivity, the high market prices make it a lucrative crop. Some of these cultivars have been evaluated for their nutritional composition (Rodríguez et al. 2012), but there are no published studies about their resistance levels to the main potato pests and diseases. Ruiz de Galarreta et al. (1998) found resistances against P. infestans, in some Solanum wild species such as Solanum bulbocastanum, Solanum circaeifolium, Solanum demissum, Solanum phureja, Solanum pinnatisectum and Solanum stoloniferum. In the present work, we have found partial foliar resistance to P. infestans in the cv. Venezolana Negra and partial tuber resistance in Bonita Colorada. The level of tuber infection was not found to be related to foliar blight resistance, which is in agreement with earlier studies from Wastie (1991).
Gabriel et al. (2008) found a negative correlation between foliar and tuber disease in five diploid potato progenies with segregating resistance levels. Spielman et al. (1992) reported that the sporulation capacity of isolates measured in a growth chamber was closely correlated with the AUDPC measured in a small field experiment. Forbes et al. (2005) found broad geographical stability of quantitative resistance to late blight in a set of potato genotypes from S. tuberosum in international trials at several locations. Further studies will be necessary for evaluating field resistance to P. infestans in our Canarian cultivars.
Resistance against G. pallida Pa2/3 has been reported in specific accessions of Solanum berthaultii, Solanum gourlayi, Solanum brevicaule, Solanum multidissectum and S. vernei (Turner 1989; Kreike et al. 1994). Also, Castelli et al. (2003) evaluated the Commonwealth Potato Collection for resistance to G. pallida and G. rostochiensis and found clones with high levels of resistance. Vallejos (1994) found partial resistance to G. pallida, in a native potato variety sipancachi (S. andigena). Similar results were obtained in the present work, with cvs. Azucena Negra and Bonita Ojo Perdiz both belonging to S. andigena. Also, Estrada (2000) suggested that the maintained sources to improve resistance to Globodera sp. should include accessions from S. vernei, Solanum spegazzini and S. andigena.
Since antibiotics are generally not permitted for crop protection purposes, chemical control of bacterial soft rot in potato is not possible. Therefore, genetic resistance is the best method to control this disease. Sources of resistance are present in several tuber-bearing species of Solanum (Rousselle-Bourgeois and Priou 1995), which have been exploited in prebreeding and breeding programmes. However, the introgression of the resistance genes into commercial cultivars is difficult because of incompatibility with S. tuberosum, complex inheritance and linkage drag of unwanted traits from the resistant parent (Ahmet et al. 2004). Quantitative variation has been observed (Lyon 1989), suggesting that resistance to soft rot is primarily of the polygenic, non-specific type. In our study, we have found resistance in one cv. belonging to S. andigena Bonita Colorada. These results agree with those described by Ritter et al. (2008) who also detected resistances in native potatoes from S. andigena such as Pulu and Puca Quitish.
In general, the results obtained in our work demonstrate that within the gene pool of Solanum still numerous, in part unexploited, resistances are available which could favourably be used for potato breeding by introducing them into new cultivars. However, it will be necessary to know the type of resistance and how they are inherited in each case. Molecular techniques including linkage mapping and QTL analysis would allow to identify markers for qualitative or quantitative resistance genes which could be used in marker-assisted selection.
References
Ahmet L, Stevenson WR, Helgeson JP, Jiang J (2004) Transfer of tuber soft rot and early blight resistances from Solanum brevidens into cultivated potato. Theor Appl Genet 109:249–254
Allefs SJHM, Florack DEA, Hoogendoom C, Willem J, Stiekema WJ (1995) Erwinia soft rot resistance of potato cultivars transformed with a gene construct coding for antimicrobial peptide cecropin B is not altered. Am Potato J 72:437–445
Ames M, Spooner DM (2008) DNA from herbarium specimens settles a controversy about origins of the European potato. Am J Bot 95:252–257
Andersson B (2007) Sexual reproduction in Phytophthora infestans—epidemiological consequences. Doctoral thesis. Swedish University of Agricultural Sciences, 31p
Barandalla L, Ruiz de Galarreta JI, Ríos D, Ritter E (2006) Molecular analysis of local potato cultivars from Tenerife Island using microsatellite markers. Euphytica 152:283–291
Castelli L, Ramsay G, Bryan G, Neilson SJ, Phillips MS (2003) New sources of resistance to the potato cyst nematodes Globodera pallida and G. rostochiensis in the Commonwealth Potato Collection. Euphytica 129:377–386
Cook PA, Cook ND (1998) The discovery and conquest of Peru: chronicles of the new world encounter. Duke University Press, Durham
Cooke DEL, Cano LM, Raffaele S, Bain RA, Cooke LR, Etherington GJ, Deahl KL, Farrer RA, Gilroy EM, Goss EM, Grunwald NJ, Hein I, Maclean D, McNicol JW, Randall E, Oliva RF, Pel MA, Shaw DS, Squires JN, Taylor MC, Vleeshouwers VG, Birch PR, Lees AK, Kamoun S (2012) Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog 8:1–14
Egúsquiza BR (ed) (2000) La papa: producción, transformación y comercialización. Prisma, Lima Perú, p 192
Estrada N (2000) La biodiversidad en el mejoramiento genético de la papa. In: Hardy B, Martínez E (eds.) Universidad de La Molina, Perú, p 372
Evans K, Trudgill DL (1992) Pest aspects of potato production. Part 1. The nematode pest of potatoes. In: Harris PM (ed) The potato crop, 2nd edn. Chapman and Hall, London, pp 438–475
Faostat (2014) Available in http://faostat.fao.org [16 May 2014].
Flier WG, Turkensteen LJ (1999) Foliar aggressiveness of Phytophthora infestans in three potato growing regions in the Netherlands. Eur J Plant Pathol 105:381–388
Flier WG, Turkensteen LJ, Van Den Bosch TBM, Vereijken PFG, Mulder A (2001) Differential interaction of Phytophthora infestans on tubers of potato cultivars with different levels of blight resistance. Plant Pathol 75:133–136
Flier WG, Van Den Bosch GBM, Turkensteen LJ (2003) Stability of partial resistance in potato cultivars exposed to aggressive strains of Phytophthora infestans. Plant Pathol 52:326–337
Foolad R, Zhang P, Khan AA, Nino-Liu D, Lin Y (2002) Identification of QTLs for early blight (Alternaria solani) resistance in tomato using backcross populations of a Lycopersicon esculentum x L. hirsutum cross. Theor Appl Genet 104:945–958
Forbes GA, Chacon MG, Kirk HG, Huarte MA, Van Damme M, Distel S, Mackay GR, Stewart HE, Lowe R, Duncan JM, Mayton HS, Fry WE, Andrivon D, Ellissèche D, Pellé R, Platt HW, Mackenzie G, Tarn TR, Colon LT, Budding DJ, Lozoya-Saldaña H, Hernandez-Vilchis A, Capezio S (2005) Stability of resistance to Phytophthora infestans in potato: an international evaluation. Plant Pathol 54:364–372
Fry WE (1978) Quantification of general resistance of potato cultivars and fungicide effects for integrated control of potato late blight. Phytopathology 68:1650–1655
Gabriel J, Hernández M, Plata G, Barandalla L, López R, Sánchez I, Ruiz de Galarreta JI, Ritter E (2008) Utilization of molecular markers (SSRs and cDNAs) for screening known QTLs for late blight (Phytophthora infestans) resistance in potato. Proceedings of the 18th EUCARPIA general congress, Valencia, Spain. 9-12 September, 2008. pp. 274-278
Hawkes JG (1994) Origins of cultivated potatoes and species relationships. In: Bradshaw JE, Mackay GR (eds) Potato genetics. CAB International, Wallingford, pp 3–42
Hawkes JG, Francisco-Ortega J (1992) The potato in Spain during the late 16th century. Econ Bot 46:86–97
Hawkes JG, Francisco-Ortega J (1993) The early history of the potato in Europe. Euphytica 70:1–7
Kreike CM, De Koning JRA, Vinke JH, Van Ooijen JW, Stiekema WJ (1994) Quantitatively-inherited resistance to Globodera pallida is dominated by one major locus in Solanum spegazzinii. Theor Appl Genet 88:764–769
Lapwood DH, Gans PT (1984) A method for assessing the field susceptibility of potato cultivars to blackleg (Erwinia carotovora subsp. atroseptica). Ann Appl Biol 104:315–320
Lobo-Cabrera M (1988) El comercio canario europeo bajo Felipe II. Viceconsejería de Cultura y Deportes del Gobierno de Canarias y Secretaría Regional de Turismo. Cultura e Emigracao de Governo Regional da Madeira, Funcha
Lyon GD (1989) The biochemical basis of resistance of potatoes to soft rot Erwinia spp.—a review. Plant Pathol 38:313–339
Ríos D (2002) Caracterización morfológica y ecofisiológica de un grupo de cultivares locales de papas de Tenerife. Tesis Doctoral. Universidad de Santiago de Compostela. 273 pp
Rios D, Ghislain M, Rodriguez F, Spooner D (2007) What is the origin of the European potato? Evidence from Canary Island landraces. Crop Sci 47:1271–1280
Ritter E, Barandalla L, López R, Ruiz de Galarreta JI (2008) Exploitation of exotic, cultivated Solanum germplasm for breeding and commercial purposes. Potato Res 51:301–311
Rodríguez C (2000) Características morfológicas de catorce variedades de papa tradicionales de Tenerife. Bachelor’s Tesis. Centro Superior de Ciencias Agrarias. Universidad de Laguna. Tenerife
Rodríguez B, Hernandez L, Rios D, Hernan L, Luna N, Rodriguez EM, Diaz C (2012) Differentiation of potato cultivars experimentally cultivated based on their chemical composition and by applying linear discriminant analysis. Food Chem 133:1241–1248
Romans A (2005) The potato book. Frances Lincoln, London
Rousselle-Bourgeois F, Mugniery D (1995) Screening tuber-bearing Solanum spp. for resistance to Globodera rostochiensis Rol Well. and G. pallida Pa2/3 Stone. Potato Res 38:241–249
Rousselle-Bourgeois F, Priou S (1995) Screening tuber-bearing Solanum spp. for resistance to soft rot caused by Erwinia carotovora ssp. atroseptica (van Hall) Dye. Potato Res 38:111–118
Ruiz de Galarreta JI, Ríos DJ (2008) Variedades de Patata y Papas Españolas. Neiker-Tecnalia, Vitoria- Gasteiz
Ruiz de Galarreta JI, Carrasco A, Salazar A, Barrena I, Iturritxa E, Marquinez R, Legorburu FJ, Ritter E (1998) Wild Solanum species as resistance sources against different pathogens of potato. Potato Res 41:57–68
Ruiz de Galarreta JI, Barandalla L, Lorenzo R, Gonzalez J, Rios D, Ritter E (2007) Microsatellite variation in potato landraces from the island of the La Palma. Span J Agric Res 5:186–192
Ruiz de Galarreta JI, Barandalla L, Rios D, Lopez R, Ritter E (2011) Genetic relationships among local potato cultivars from Spain using SSR markers. Genet Resour Crop Evol 58:383–395
Ruiz de Gordoa QJ (1981) La patata de siembra en Álava. Libreria Arias Montano, Badajoz
Singh HP (2008) Policies and strategies conducive to potato development in Asia and the Pacific Region. In: Papademetriou MK (ed) Workshop to Commemorate the International Year of Potato (Proceedings). FAO, Bangkok, pp 19–29
Spielman LJ, Mcmaster BJ, Fry WE (1992) Relationships among measurements of fitness and disease severity in Phytophthora infestans. Plant Pathol 41:317–324
Spooner DM, Hetterscheid WLA (2004) Origin of the modern cultivated potato. Am J Potato Res 82:90–91
Turner SJ (1989) New sources of resistance to potato cyst-nematodes in the Common Wealth Potato Collection. Euphytica 42:145–153
Vallejos N (1994) Prueba de clones promisorios de papa por su resistencia a la verruga Synchytrium endobióticum, al nematodo quiste Globodera spp. y nematodo rosario Nacobbus aberrans. Tesis de Grado para obtener el Título de Ingeniero Agrónomo. Universidad Mayor de San Simón. Cochabamba, Bolivia. 95 pp
Vleeshouwers VGAA, Van Dooijeweert W, Keizer LCP, Sijpkes L, Govers F, Colon L (1999) A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. Eur J Plant Pathol 105:241–250
Wastie RL (1991) Resistance to powdery scab of seedling progenies of Solanum tuberosum. Potato Res 34:249–252
Zubeldia A, López-Campos G, Sañudo-Palazuelos A (1955) Estudio, descripción y clasificación de un grupo de variedades primitivas de patata cultivadas en las Islas Canarias. Vida Agrícola 33:991–1006
Acknowledgements
This work was financed by INIA RTA2013-00006-C03-1, the Cabildo of Tenerife and the Basque Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alor, N., López-Pardo, R., Barandalla, L. et al. New Sources of Resistance to Potato Pathogens in old Varieties of the Canary Islands. Potato Res. 58, 135–146 (2015). https://doi.org/10.1007/s11540-015-9293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-015-9293-5